An intracellular anion channel critical for pigmentation
Figures
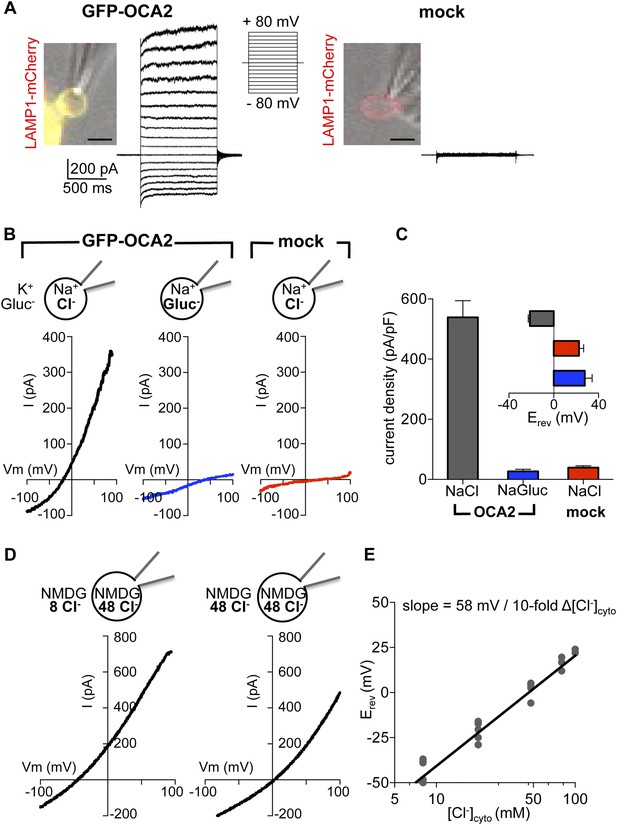
OCA2 contributes to an endolysosomal chloride current with ion channel properties.
(A) Heterologous GFP-OCA2 localized to LAMP1-mCherry-positive late endosomes and lysosomes (endolysosomes) individually dissected from AD293 cells for patch-clamp experiments (scale bar = 5 μm). Whole-endolysosomal currents elicited by voltage steps between −80 mV and +80 mV in a representative endolysosome expressing GFP-OCA2 or in an endolysosome from a mock-transfected cell. (B) Representative whole-endolysosome current–voltage (I–V) relationships in response to voltage ramps. Outwardly rectifying IOCA2 was nearly abolished and its reversal potential (Erev) shifted in the positive direction when luminal Cl− was substituted for gluconate (Gluc−), similar to currents measured from mock-transfected endolysosomes containing a Cl−-based luminal solution. (C) Average current densities (pA/pF) measured at 100 mV. Inset: Average Erev (±s.e.m., n = 6–9 endolysosomes for each condition). (D) In a representative OCA2-expressing endolysosome, Erev was dependent on cytoplasmic [Cl−] ([Cl−]cyto). NMDG-based solutions were used to inhibit endogenous cation permeabilities. (E) IOCA2 Erev varied linearly with [Cl−]cyto when [Cl−]luminal was kept at 48 mM. Each point represents one endolysosome at the [Cl−]cyto indicated on the x-axis.
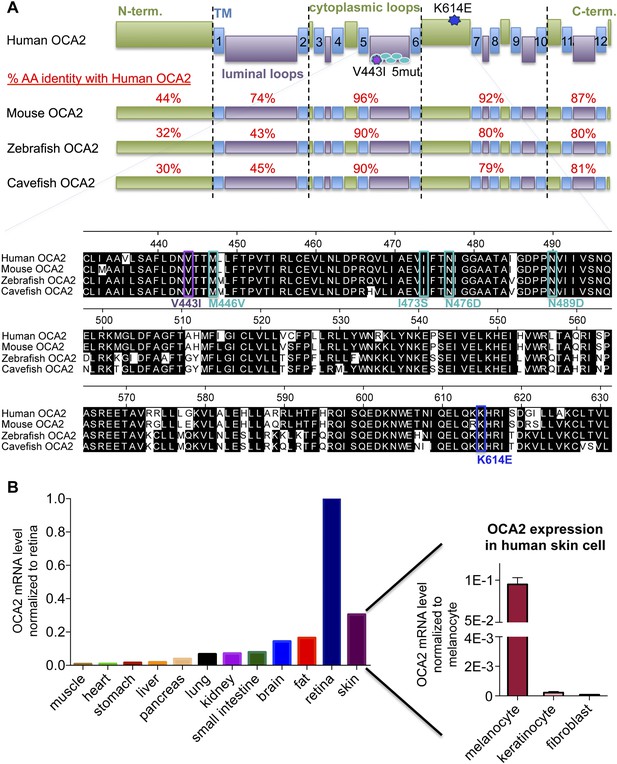
OCA2 is a highly conserved transmembrane protein expressed in pigment cells.
(A) Predicted membrane topology and homology of OCA2 from human, mouse, zebrafish and cavefish. OCA2 sequences were aligned using MegAlign (DNASTAR). Accession numbers of OCA2 sequences used in alignments generated using Clustal W in MegAlign (DNASTAR): NP-000266.2 (human), NP-068679.1 (mouse), XP_695807.5 (zebrafish), ABB29299.1 (cavefish). Amino acid percent identity was determined using Protein BLAST (http://blast.ncbi.nlm.nih.gov) for the indicated regions. TM = transmembrane domain, AA = amino acid. Inset: OCA2 alignment showing conserved regions with albinism-associated mutations used in this study (K614E in dark blue, V443I in purple, 5mut in light blue). (B) OCA2 mRNA expression profile in human tissue. OCA2 expression levels were highest in retina, consistent with OCA2 expression in the densely pigmented retinal pigment epithelium (RPE). Human skin had the second highest levels of OCA2 mRNA, due to high expression in melanocytes and very low levels in keratinocytes and skin fibroblasts, consistent with OCA2 expression in pigment cells. (n = 4 experiments).
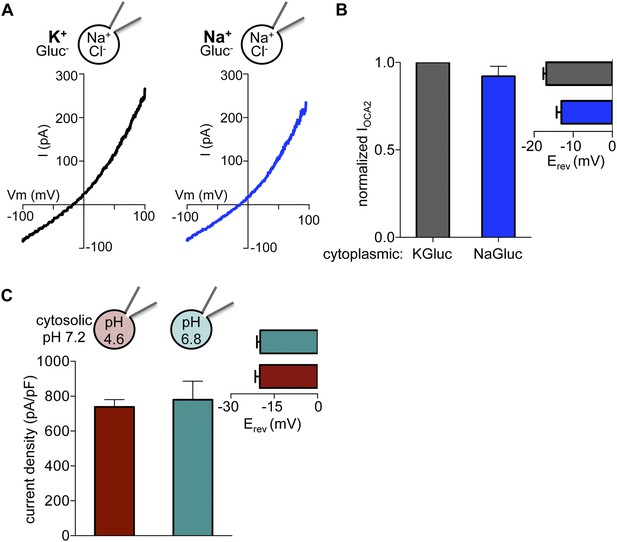
IOCA2 is not mediated by K+ flux or regulated by pH.
(A) In the same endolysosome, IOCA2 was not significantly different when cytoplasmic K+ was substituted with Na+. (B) The IOCA2 amplitude at 100 mV or Erev was not significantly different when cytoplasmic K+ was substituted with Na+ (±s.e.m., n = 3 endolysosomes). (C) IOCA2 current density (pA/pF) measured in endolysosomes expressing OCA2 was similar with a luminal pH of 4.6 or 6.8. Inset: Average Erev (±s.e.m., n = 4 endolysosomes per condition).
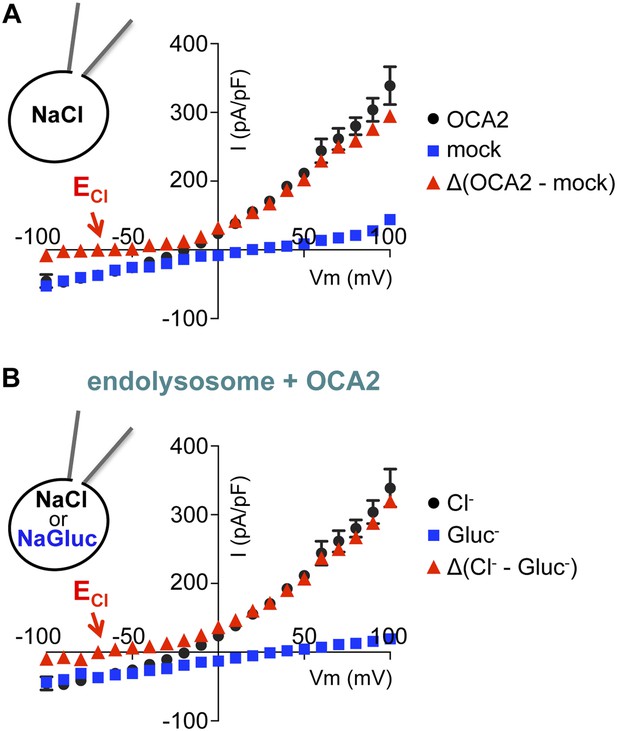
IOCA2 is mediated by Cl− flux.
Average IOCA2 current densities (pA/pF) were calculated at the indicated voltages. Currents recorded from mock-transfected AD293 endolysosomes (A) or endolysosomes expressing OCA2 in the presence of luminal Gluc− (B) were subtracted from currents measured from OCA2-expressing endolysosomes in the presence of luminal Cl−. Δ(OCA2 − mock) and Δ(luminal NaCl − NaGluc) currents have an Erev near ECl (ECl = Nernst potential for Cl−; ±s.e.m., n = 5 endolysosomes per condition).
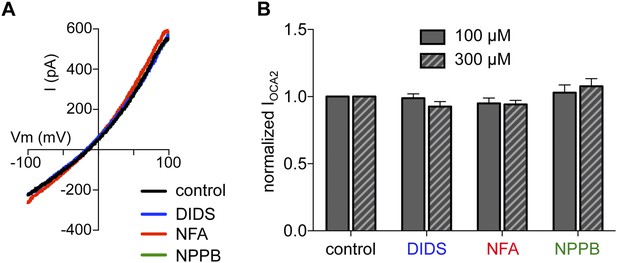
Cl− channel blockers DIDS, NFA, and NPPB do not affect IOCA2.
(A) In a representative endolysosome IOCA2 was not affected by Cl− channel inhibitors 4,4′-diisothiocyanato-2,2′-stilbenedisulfonic acid disodium salt (DIDS), niflumic acid (NFA), or 5-Nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) (each at 100 μM), compared to control (DMSO vehicle). (B) The average current amplitude of IOCA2 normalized to control conditions (<1% DMSO) was similar when DIDS, NFA, or NPPB was bath applied at 100 μM or 300 μM (n = 3–4 endolysosomes for each). DIDS blocks several members of the Chloride Channel (ClC) family, Ca2+-activated Cl− channels (CaCC), Maxi Cl− channels, and volume regulated Cl− channels (VRAC); NFA blocks several ClC members, VRAC, and CaCC; NPPB inhibits several ClC members, Maxi Cl−, VRAC, and cystic fibrosis transmembrane conductance regulator (CFTR). Pharmacological profile information was obtained from the International Union of Basic and Clinical Pharmacology (IUPHAR, http://www.iuphar-db.org).
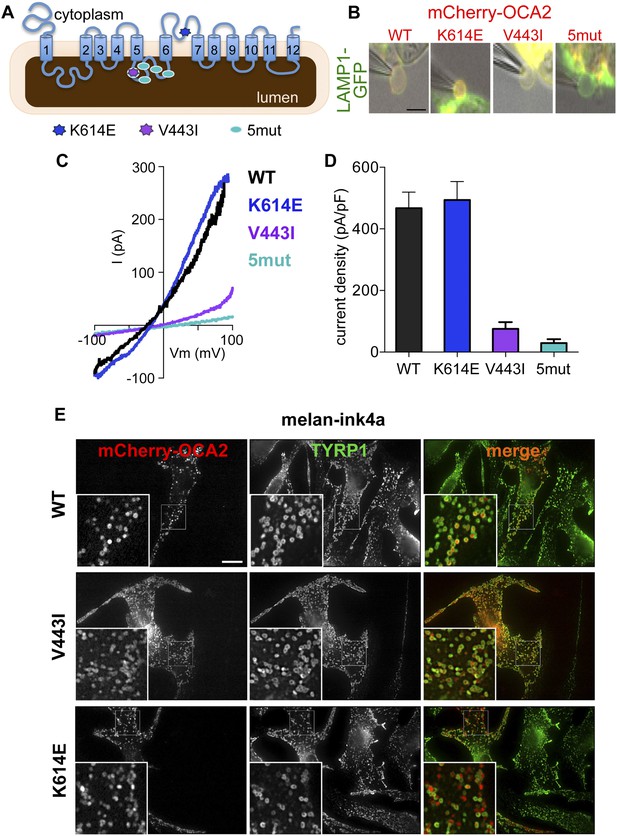
Effect of OCA2 disease-associated mutations on IOCA2.
(A) Predicted topology of OCA2 with albinism-associated mutations: K614E (dark blue) in a conserved cytoplasmic loop, V443I (purple) in a highly conserved luminal loop, and 5mut (light blue) consisting of 5 point mutations (V443I, M446V, I473S, N476D, N489D) in the same luminal loop. (B) mCherry-tagged WT, K614E, and V443I OCA2 localized to LAMP1-GFP-positive isolated endolysosomes (merged in yellow), while 5mut did not (scale bar = 5 μm). (C) Representative I–V relationships measured in response to voltage ramps from endolysosomes expressing WT, K614E, V443I, or from endolysosomes isolated from cells expressing 5mut OCA2. (D) Average IOCA2 current densities (pA/pF) measured at 100 mV (±s.e.m., n = 4–8 endolysosomes for each condition). (E) Melan-ink4a melanocytes expressing mCherry-tagged (red) WT, V443I, or K614E and stained with anti-tyrosinase-related protein one (TYRP1) antibodies (green). Insets, 3× magnification of boxed regions. Merged images (right) show that WT, V443I, and K614E OCA2 variants localize primarily to TYRP1-positive compartments (scale bar = 10 μm).
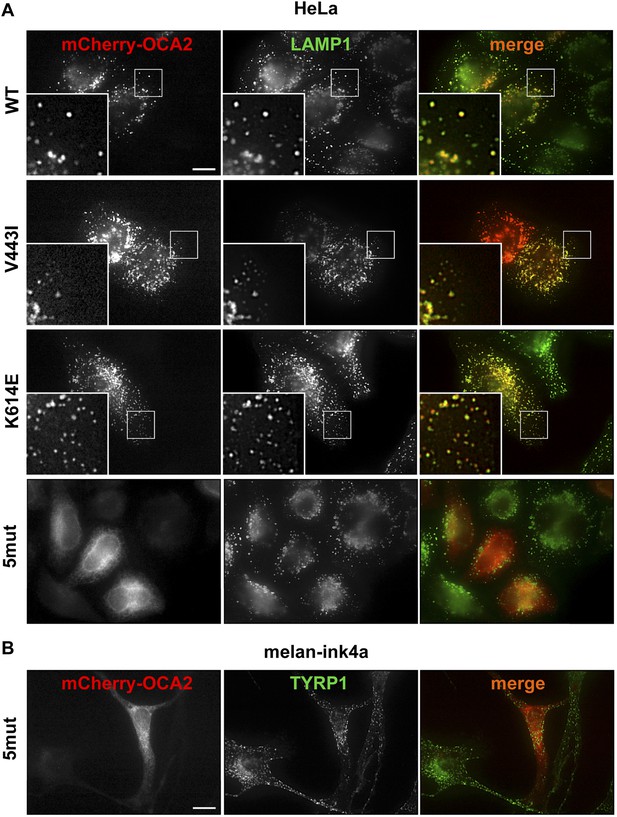
Localization of wild-type (WT), K614E, V443I, and 5mut OCA2.
(A) HeLa cells expressing mCherry-tagged WT, K614E, V443I, or 5mut OCA2 (red) and stained with LAMP1 antibodies (green). All OCA2 variants, except 5mut, localized primarily to LAMP1-positive compartments (orange in merged images) (scale bar = 10 μm). (B) Melan-ink4a melanocytes expressing 5mut OCA2 tagged with mCherry (red) and stained with anti-TYRP1 antibodies show that 5mut OCA2-mCherry does not localize to TYRP1-positive compartments (green) in melanocytes (scale bar = 10 μm).
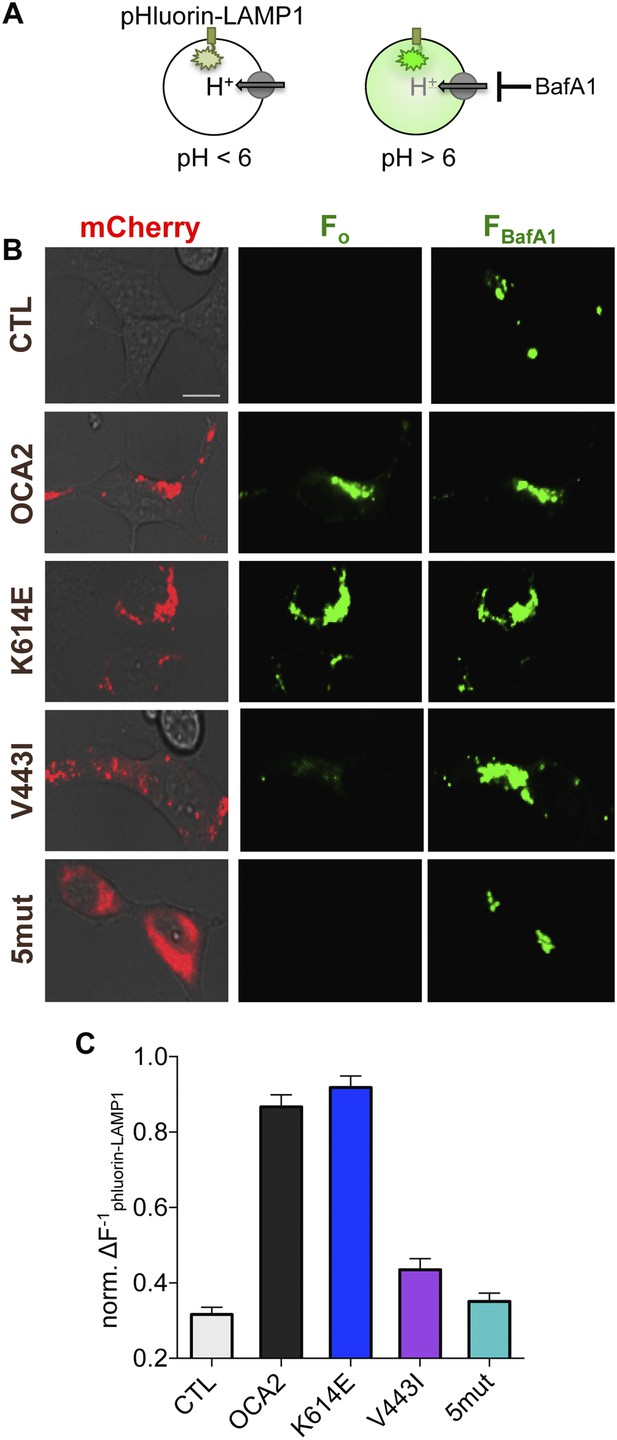
OCA2 expression regulates organellar pH.
(A) Ecliptic pHluorin-LAMP1 fluoresces when luminal pH > 6, but not when pH < 6. The V-ATPase inhibitor bafilomycin A1 (BafA1, 2 μM) was used to neutralize acidic endolysosomes and detect pHluorin-LAMP1 expression. (B) Endolysosomes from mock-transfected control (CTL) AD293 cells expressing pHluorin-LAMP1 were not fluorescent at baseline (Fo) but brightly fluoresced after BafA1 treatment (FBafA1). Endolysosomes coexpressing pHluorin-LAMP1 and mCherry-tagged WT or K614E OCA2 were fluorescent prior to BafA1 treatment (Fo), indicating that expression increased pH. Endolysosomes that coexpressed pHluorin-LAMP1 and V443I were dim at baseline (Fo), similar to CTL and mislocalized 5mut, representing an acidic lumen, and pHluorin-LAMP1 fluorescence increased upon BafA1 treatment (FBafA1) (scale bar = 10 µm). (C) Fluorescence intensity following BafA1 treatment was compared with baseline fluorescence to determine relative baseline acidity in enodolysosomes from CTL cells, expressing WT, K614E, V443I, OCA2 or from cells expressing mislocalized 5mut. Bars represent average normalized change in pHluorin-LAMP1 fluorescence (±s.e.m., n = 3 independent experiments, each experiment calculated as the average of n = 78–214 endolysosomes).
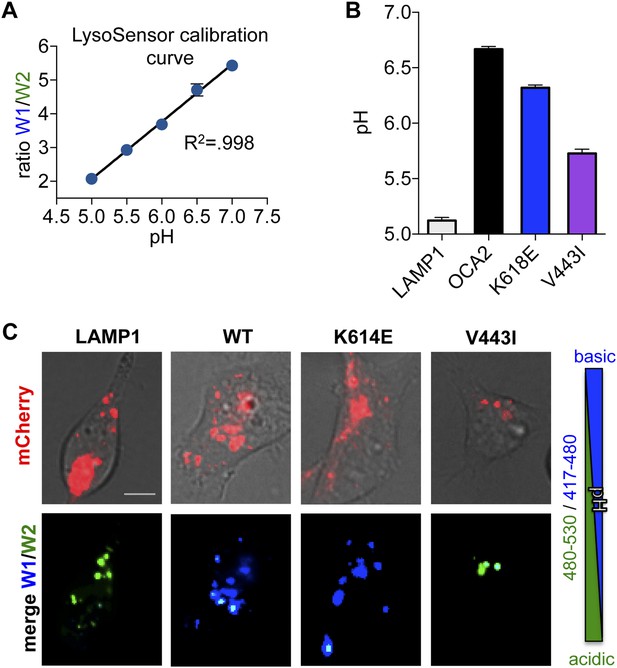
LysoSensor pH measurements.
(A) Lysosensor calibration curve determined from the emission ratio W1 (417–480 nm)/W2 (490–530 nm) at 405 nm excitation for pH values between 5 and 7. Representative curve from one experiment (±s.e.m., n = 231–350 endolysosomes). (B) Predicted pH values based on LysoSensor calibration in LAMP1-expressing control endolysosomes (CTL) compared with endolysosomes expressing WT, K614E, or V443I OCA2 (±s.e.m., n = 3 independent experiments, each experiment calculated as the average of n = 109–635 endolysosomes). (C) Right: Color scale used for Lysosensor. Acidic endolysosomes are shown in green and more neutral ones in blue. Left: AD293 cells loaded with Lysosensor and expressing LAMP1-mCherry (used as a control) or mCherry-tagged WT, K614E, V443I OCA2. Endolysosomes expressing LAMP1-mCherry are green, suggesting that they have acidic pH. Endolysosomes expressing WT or K614E, but not V443I, shift their pH to more neutral values as shown by the blue color (scale bar = 10 μm).
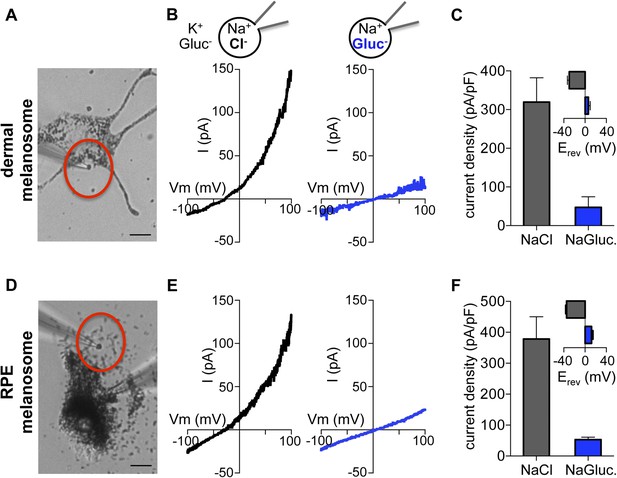
Direct recording from melanosomes identifies an endogenous Cl− current (Imelano).
(A) Dermal macromelanosomes were individually dissected from Oa1−/− melanocytes and patch-clamped. A NaCl-based luminal solution and KGluc-based cytoplasmic solution were used for recordings (scale bar = 10 μm). (B) Representative I–V relationships exhibit a Cl−-dependent outwardly rectifying current (Imelano) with a negative Erev. Substituting luminal Cl− for Gluc− reduced the current amplitude and shifted Erev of Imelano. (C) Average current densities (pA/pF) measured at 100 mV. Inset: Average Erev (±s.e.m., n = 4–5 dermal melanosomes for each condition). (D) Patch-clamp experiments using freshly isolated bullfrog RPE melanosomes (scale = 10 μm). (E) Representative whole-RPE melanosome current–voltage (I–V) relationships in response to voltage ramps. Outwardly rectifying Imelano was reduced and Erev shifted in the positive direction when luminal Cl− was substituted for Gluc−. (F) Average current densities (pA/pF) measured at 100 mV. Inset: Average Erev (±s.e.m., n = 5 RPE melanosomes for each condition).
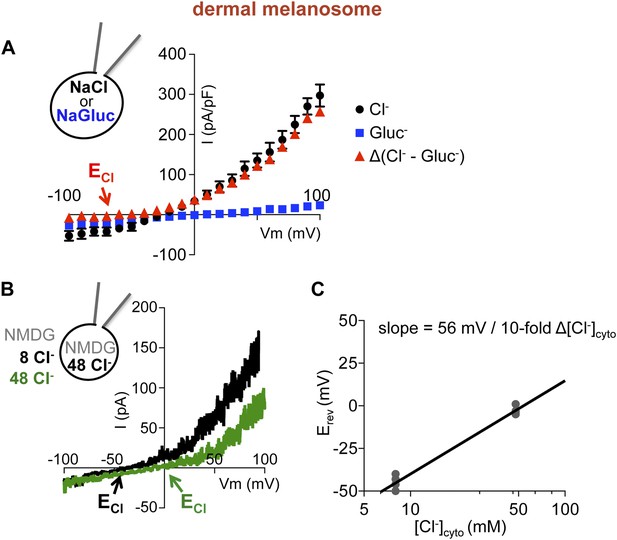
Imelano Erev is dependent on the Cl− concentration gradient.
(A) Average current densities (pA/pF) at the indicated voltages were subtracted to calculate the Cl− component of Imelano. Δ(luminal NaCl − NaGluc) current has an Erev near ECl (ECl = Nernst potential for Cl−; ±s.e.m., n = 4 melanosomes per condition). (B) In a representative dermal melanosome, Erev was dependent on [Cl−]cyto. NMDG-based solutions were used to inhibit endogenous cation permeability. (C) Imelano Erev as a function of [Cl−]cyto, when [Cl−]luminal was kept at 48 mM. Each point represents a melanosome at the indicated [Cl−]cyto on the x-axis. Assuming linear Erev dependence on [Cl−]cyto, the slope of the fitted line is 56 mV/10-fold Δ[Cl−]cyto.
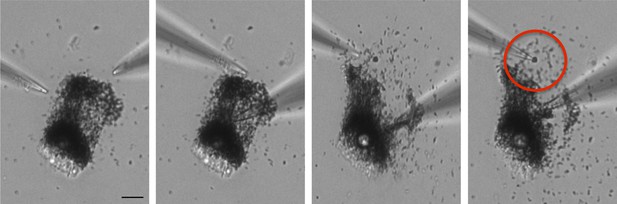
RPE melanosome dissection.
Melanosomes were dissected from freshly isolated bullfrog RPE cells using two glass pipettes. The sequence of images shows one pipette used to hold the non-adherent cell and one to dissect the cell to isolate individual melanosomes (red circle) for patch-clamp experiments (scale = 10 μm).
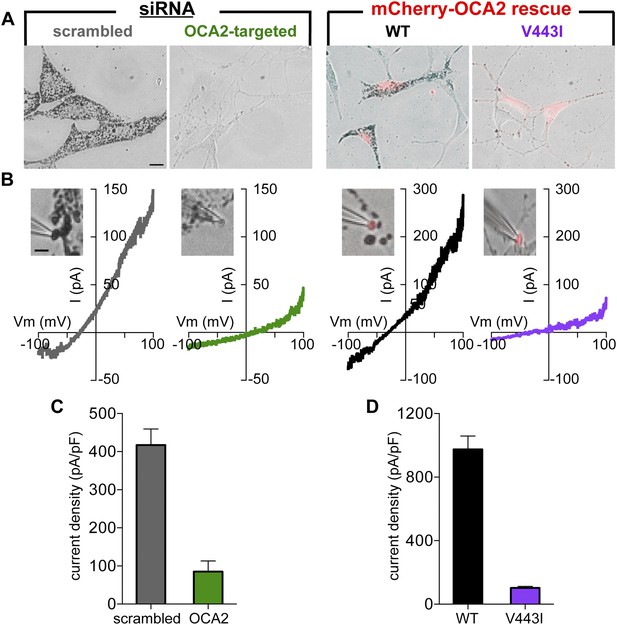
OCA2 is required for Imelano and pigmentation.
(A) Representative images of Oa1−/− melanocytes expressing scrambled or OCA2-targeted siRNA, the latter of which significantly decreased melanin content. Pigmentation was restored by transfection with mCherry-tagged WT OCA2, but not with the mCherry-tagged V443I mutant (scale bar = 10 μm). (B) Representative I–V relationships from melanosomes from cells expressing scrambled siRNA, OCA2-targeted siRNA, and rescued with WT or V443I OCA2. Insets: images of the individual melanosomes used for the shown recordings (scale bar = 3 μm). (C) Average Imelano current densities (pA/pF) measured at 100 mV in melanosomes from cells expressing scrambled siRNA or OCA2-targeted siRNA (±s.e.m., n = 4 melanosomes per condition). (D) Average current densities (pA/pF) measured at 100 mV in melanosomes expressing WT or V443I OCA2 from cells expressing OCA2-targeted siRNA (±s.e.m., n = 3–4 melanosomes per condition).
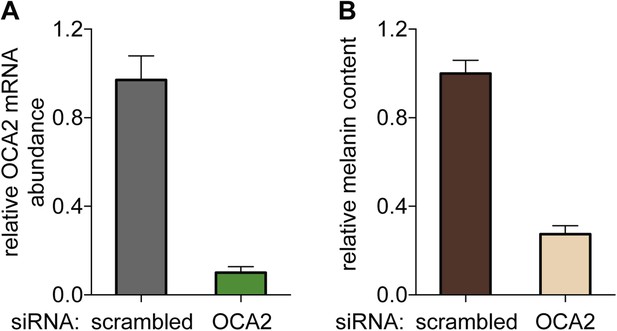
OCA2-targeted siRNA reduces OCA2 mRNA and melanin content in Oa1−/− melanocytes.
(A) OCA2 mRNA levels in melanocytes expressing OCA2-targeted siRNA was reduced by ∼90% compared with scrambled siRNA-expressing melanocytes (±s.e.m., n = 3 experiments). (B) Melanin content of melanocytes expressing OCA2-targeted siRNA was ∼73% lower than that of scrambled siRNA-expressing melanocytes (±s.e.m., n = 3 experiments).
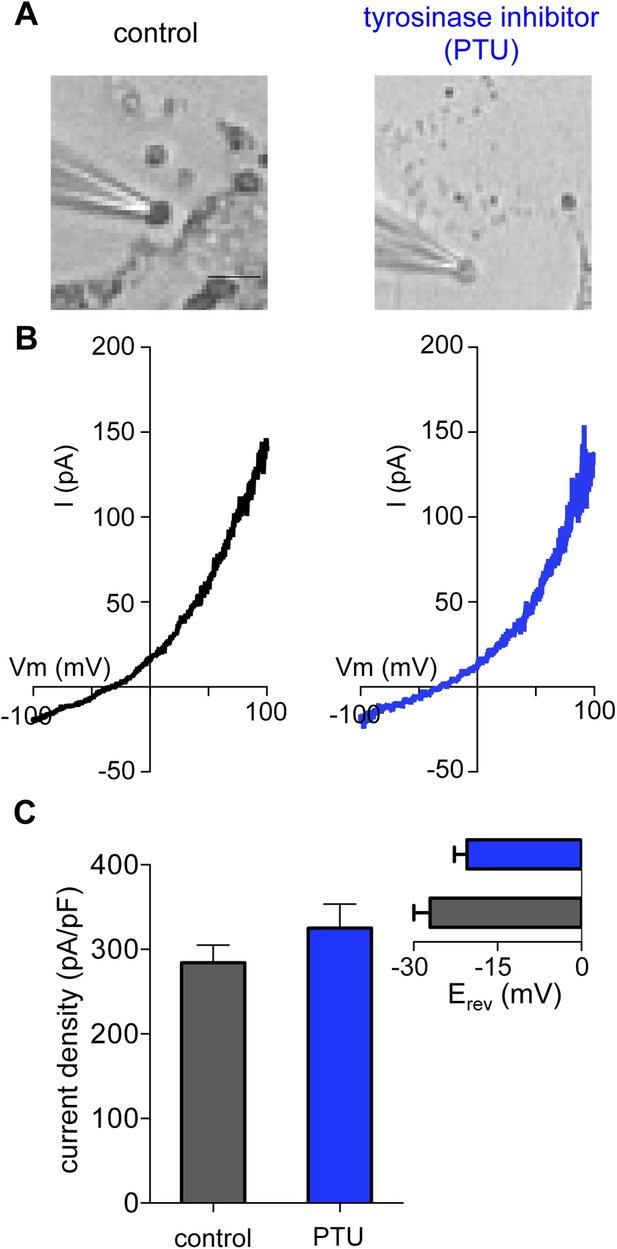
Melanin is not required for Imelano.
(A) Representative images of melanosomes from Oa1−/− melanocytes treated with the tyrosinase inhibitor phenylthiourea (PTU, 300 μM) have significantly reduced pigment (scale bar = 3 μm). (B) A similar Imelano was measured from melanosomes of PTU-treated and control Oa1−/− melanocytes. (C) Average current densities (pA/pF) measured at 100 mV. Inset: Average Erev (±s.e.m., n = 5 melanosomes per condition).
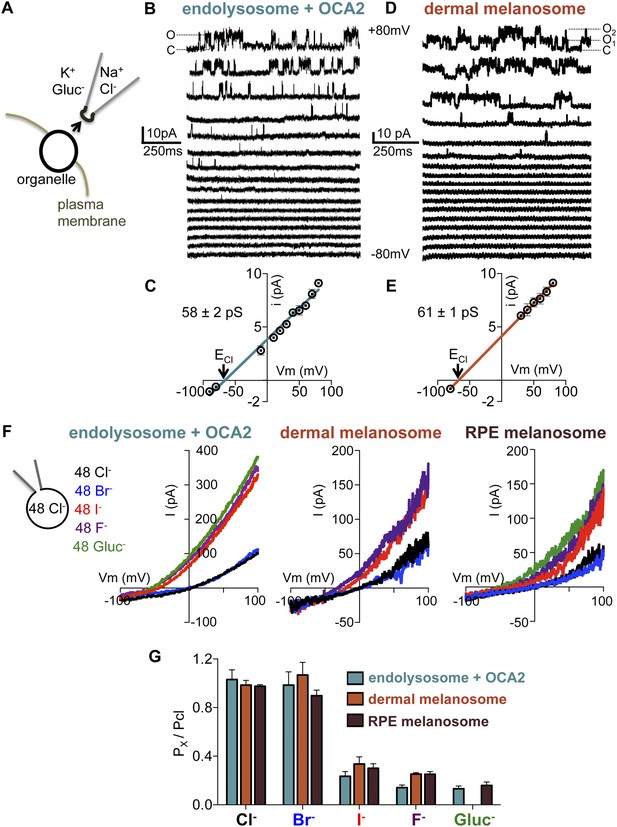
Endolyosomal IOCA2 has the same properties as melanosomal Imelano.
(A) Cytoplasmic-side-out patches were excised from partially dissected organelles to carry out single-channel recordings. (B) Representative single-channel currents recorded in response to voltage steps between −80 mV and +80 mV from a patch excised from an OCA2-expressing endolysosome (O indicates the open state and C the closed state of the channel). (C) Average single-channel current amplitudes from patches excised from OCA2-expressing endolysosomes (±s.e.m., n = 4 patches) (ECl = Nernst potential for Cl−). (D) Single-channel currents from a representative patch excised from a dermal melanosome that contains at least two channels (O1 indicates one open channel, O2 indicates two channels open simultaneously, and C both channels closed). (E) Average single-channel current amplitudes from patches excised from dermal melanosomes (±s.e.m., n = 3 patches). (F) In a representative OCA2-expressing endolysosome, dermal melanosome, and RPE melanosome, currents were selective for Cl− and Br−, but not I−, F−, or Gluc−. In the same organelle, currents were recorded with 48 mM luminal NMDG-Cl while substituting symmetrical concentrations of cytoplasmic anions in an NMDG-based solution. (G) IOCA2 permeability ratios based on Erev measurements (±s.e.m., n = 3–7 organelles for each condition).
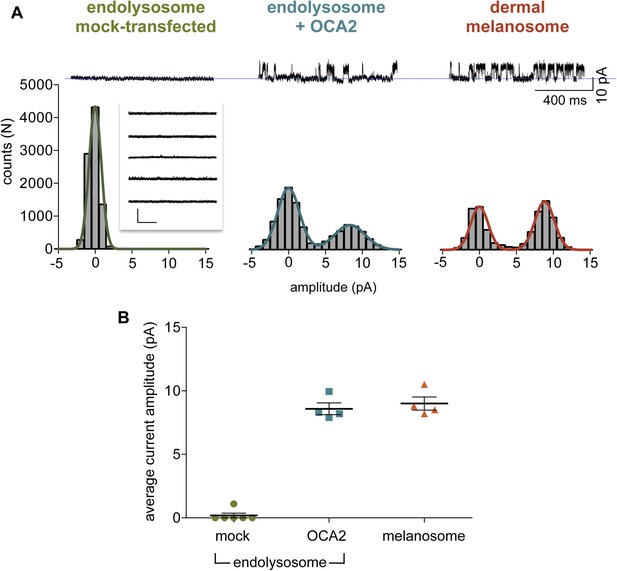
OCA2-mediated single-channel current characteristics.
(A) Top: Representative single-channel currents recorded at 80 mV from cytoplasmic-side-out patches excised from an endolysosome isolated from a mock-transfected AD293 cell, OCA2-expressing endolysosome, and dermal melanosome. Bottom: Associated amplitude histograms. Inset: None of the endolysosome patches from mock-transfected cells exhibited significant single-channel activity (scale = 10 pA, 250 ms). (B) Average maximal current amplitudes at 80 mV calculated from the Gaussian fit of amplitude histograms of individual membrane patches excised from mock-transfected, OCA2-expressing endolysosomes, or dermal melanosomes (±s.e.m., n = 4–6 patches for each condition, i = 0.18 ± 0.18 pA for mock-transfected AD293 endolysosomes, 8.59 ± 0.46 pA for OCA2-expressing endolysosomes, 9.00 ± 0.51 pA for dermal melanosomes).





