Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores
Figures
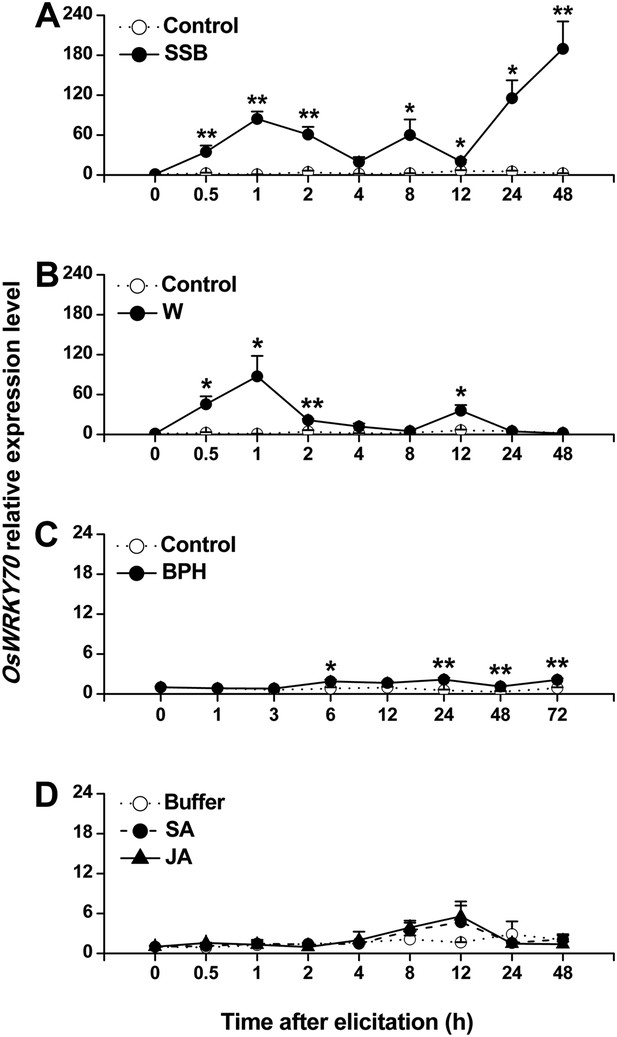
Expression of OsWRKY70 in rice after different treatments.
Mean transcript levels (+SE, n = 3–4) of OsWRKY70 in rice plants that were treated with either rice striped stem borer (SSB) (A), mechanically wounded (B), rice brown planthopper (BPH) (C), jasmonic acid (JA), salicylic acid (SA), or a buffer (50 mM phosphate buffer, pH = 8.0) (Buffer) (D). Controls correspond to non-manipulated plants. Transcript levels were analyzed by QRT-PCR. Asterisks indicate significant differences in transcript levels between treatments and controls (*, p < 0.05; **, p < 0.01; Student's t-test).
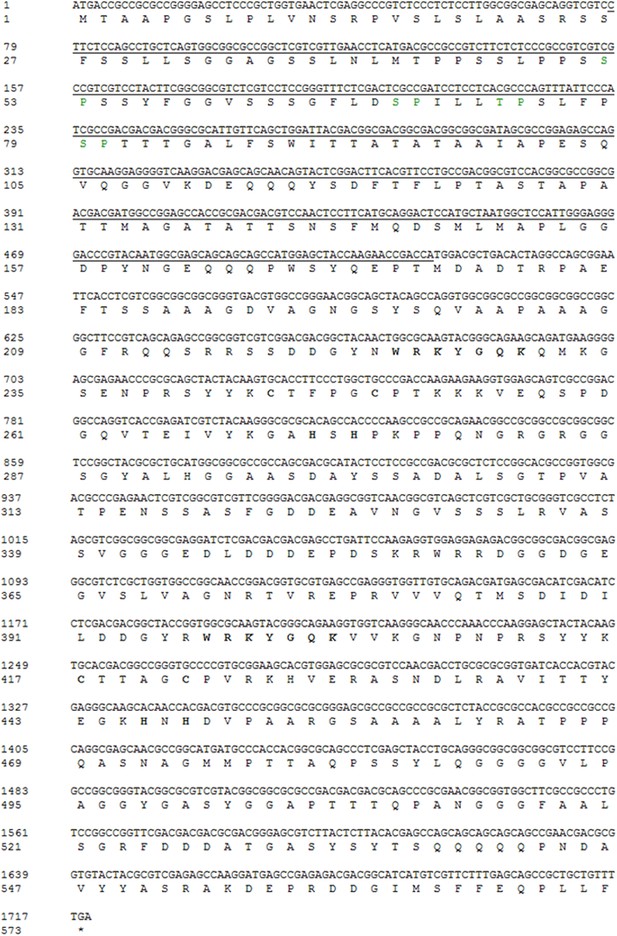
Nucleotide and amino acid sequence of OsWRKY70.
SP cluster (green), WRKY domain and Zinc fingure (bold) were shown. The underline sequence was used for RNAi construction.
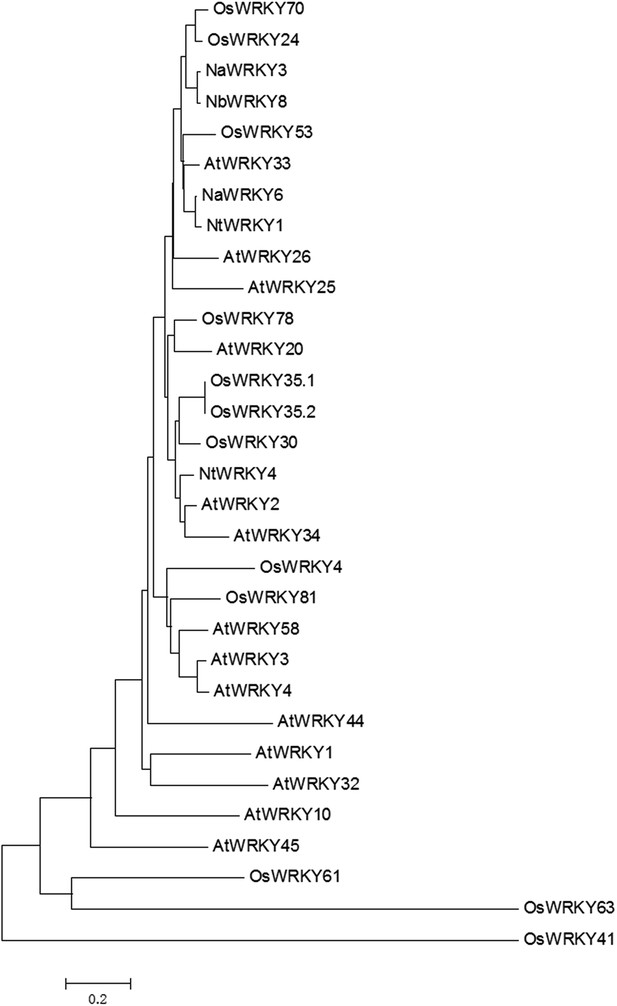
Phylogenetic relationships of Group Ⅰ type WRKY genes from different species.
Neighbor-joining phylogenetic trees were produced using MEGA3.1 based on multiple sequence alignments made with ClustalX. Selected proteins accession numbers are as follows: Arabidopsis thaliana (At, Tigr ID): AtWRKY1 (At02g04880), AtWRKY2 (At02g30250), AtWRKY3 (At02g03340), AtWRKY4 (At01g13960), AtWRKY10 (At01g55600), AtWRKY20 (At04g26640), AtWRKY25 (At02g30250), AtWRKY26 (At05g07100), AtWRKY32 (At04g30935), AtWRKY33 (At02g38470), AtWRKY34 (At04g26440), AtWRKY44 (At02g37260), AtWRKY45 (At03g01970), AtWRKY58 (At03g01080); Oryza sativa (Os, Tigr ID): OsWRKY4 (Os03g55164), OsWRKY24 (Os01g61080), OsWRKY30 (Os08g38990), OsWRKY35.1 (Os04g39570.1), OsWRKY35.2 (Os04g39570.2), OsWRKY41 (Os11g45924), OsWRKY53 (05g27730), OsWRKY61 (Os11g45850), OsWRKY63 (Os11g45920), OsWRKY70 (Os05g39720), OsWRKY78 (Os07g39480), OsWRKY81 (Os12g02400); Nicotiana attenuate (Na, NCBI ID): NaWRKY3 (AY456271), NaWRKY6 (AY456272); Nicotiana benthamiana (Nb, NCBI ID): NbWRKY8 (AB445392); Nicotiana tabacum (Nt, NCBI ID): NtWRKY1 (AF096298), NtWRKY4 (AF193771).
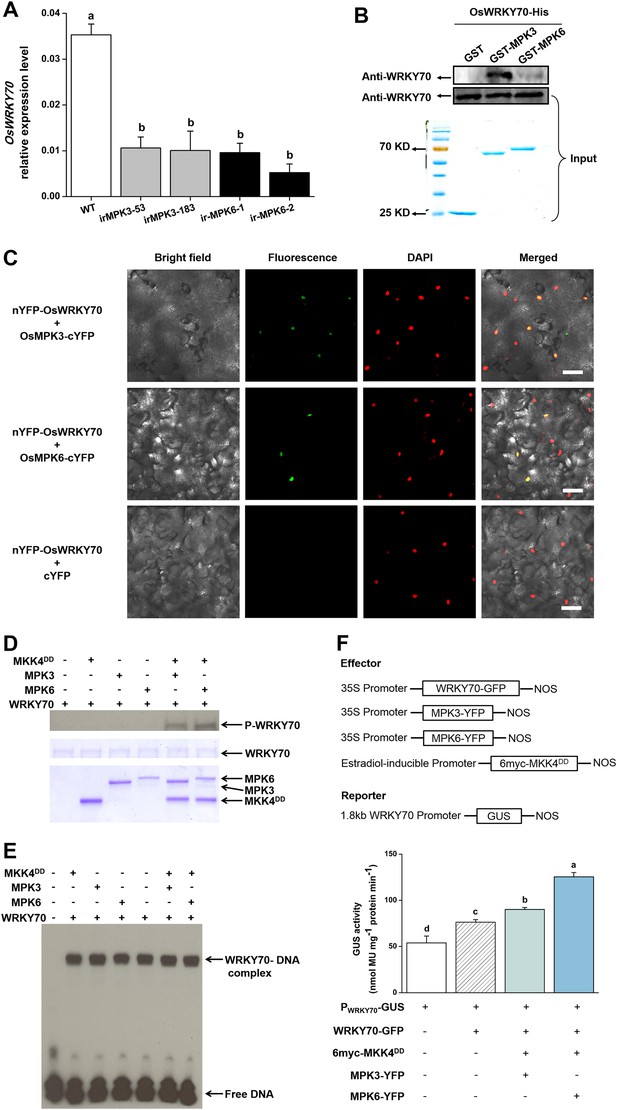
Interactions between OsWRKY70 and OsMPK3/6.
(A) Mean transcript levels (+SE, n = 5) of OsWRKY70 in transgenic lines with silencing of OsMPK3 (irMPK3 lines, irMPK3-53, and irMPK3-183) or OsMPK6 (irMPK6 lines, irMPK6-1, and irMPK6-2) after infested by SSB for 1 hr. (B) In vitro interaction assays between OsWRKY70 and OsMPK3 or OsMPK6. GST, GST-MPK3, and GST-MPK6 purified proteins were incubated with WRKY70-His as indicated. WRKY70-His input and pulled-down fractions were analyzed by immunoblotting using anti-WRKY70 antibody (top). Input proteins were monitored by Coomassie blue staining (bottom). This experiment was repeated 3 times with similar results. (C) In vivo bimolecular fluorescence complementation interaction assays between OsWRKY70 and OsMPK3 or OsMPK6. Fluorescence was observed from complementation of the N-terminal part of the YFP fused with OsWRKY70 (nYFP-OsWRKY70) with OsMPK3 or OsMPK6 fused with the C-terminal part of the YFP (OsMPK3-cYFP or OsMPK6-cYFP) and co-localized with DAPI stains in the nuclear compartment of tobacco leaf cells. No fluorescence was observed when nYFP-OsWRKY70 was co-expressed with unfused cYFP. Scale bar, 50 μm. (D) In vitro phosphorylation of OsWRKY70 by OsMPK3/6. The phosphorylated form of OsWRKY70 (P-WRKY70) was detected by using Phos-tag Biotin BTL-104 (top). Input proteins, including OsWRKY70-His (WRKY70), GST-OsPMK3 (MPK3), GST-OsPMK6 (MPK6), and His-OsMKK4DD (MKK4DD) were monitored by Coomassie blue staining. (E) Assays for W-box binding activity of OsWRKY70. GST-OsMPK3 or GST-OsMPK6 was activated by a constitutively active form of OsMKK4, His-OsMKK4DD. BS65 containing two W-boxes was used as the probe. (F) Assays for transactivation activity of OsWRKY70. Leaves of N. benthamiana were agroinfiltrated with the indicated constructs. 24 hr later, leaves were injected with 10 mM 17-β-estradiol and were incubated for 12 hr. Total protein was extracted and GUS activities were subsequently quantified. Eight plants were used for each treatment. Letters indicate significant differences among different lines (A) or treatments (F) (p < 0.05, Duncan's multiple range test).
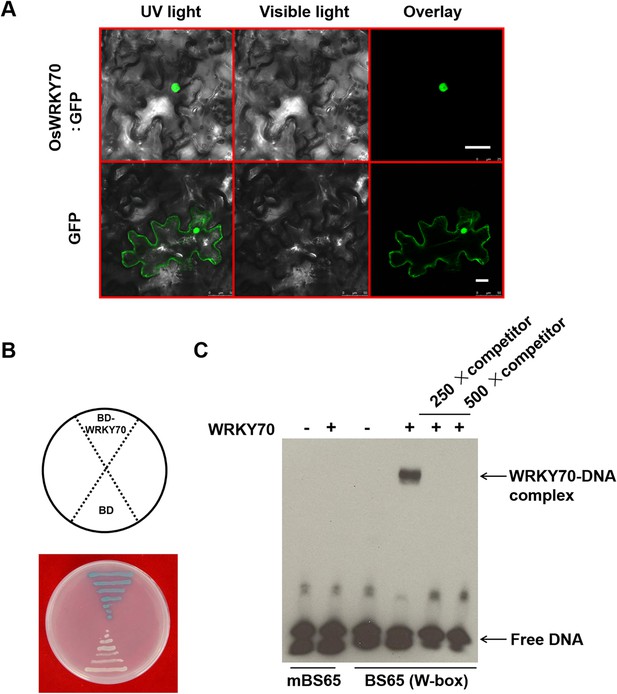
Subcellular localization, DNA-binding ability, and transcriptional activation activity of OsWRKY70.
(A) Subcellular localization of OsWRKY70. N. benthamiana cells were transformed with GFP and OsWRKY70:GFP. After incubation for 48 hr, the transformed cells were observed under a confocal microscope. The photographs were taken in UV light, visible light, and in combination (overlay), respectively. Scale bar, 25 μm. (B) Transcriptional activation activity of OsWRKY70 in yeast cells. Y187 yeast cells containing pGBKT7 and pGBKT7-WRKY70, respectively, were plated on SD medium (−Trp) containing X-α-gal at 30°C for 12 hr and then the color was observed. (C) W-box binding ability of OsWRKY70 analyzed by electrophoretic mobility shift assay (EMSA). The recombinant OsWRKY70 protein can bind the W-box sequence BS65 but not to the mutant probe mBS65. Competition experiments were performed using unlabeled BS65 as a competitor in a 250-fold molar excess. This experiment was repeated twice with similar results.
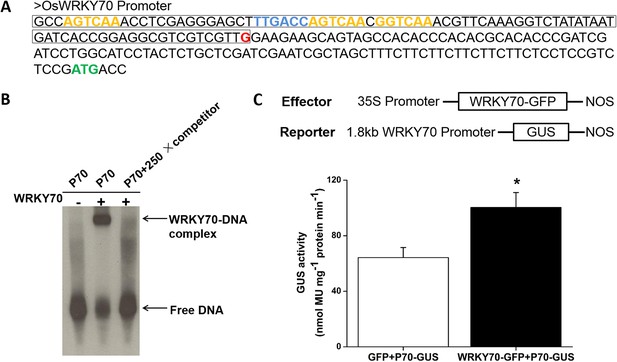
Self-activation of OsWRKY70.
(A) Partial sequence of OsWRKY70 promoter. Green highlights represent the translation initiation sites. Red highlights represent the transcription start sites. Blue and orange highlights represent W-boxes in sense and antisense strands, respectively. The framed sequence was used for EMSA assay. (B) In vitro EMSA assay for the binding activity of WRKY70 to the minimal WRKY70 promoter (P70). Competition experiments were performed using unlabeled BS65 as a competitor in a 250-fold molar excess. (C) In vivo transient assay for transactivation activity of OsWRKY70. The WRKY70 promoter (1.8 kb):GUS and 35S:WRKY70-GFP were transiently expressed in N. benthamiana leaf cells. 2 days after infiltration, samples were harvested and GUS activities were quantified. 35S:GFP was used as a control. Eight plants were used for each treatment. Asterisks indicate significant differences in WRKY70-GFP-expressed plants compared with GFP controls (*, p < 0.05; Student's t-test).
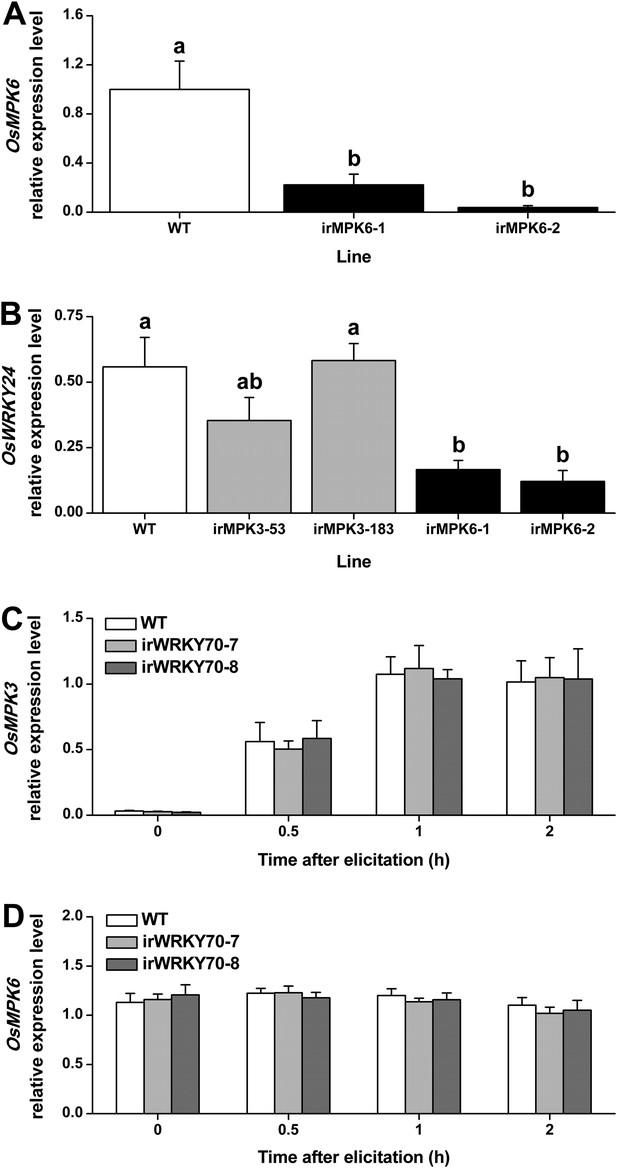
Transcript levels of OsMPK6, OsWRKY24, OsMPK3, and OsMPK6 in different transgenic lines.
(A) Mean expression levels (+SE, n = 6) of OsMPK6 in OsMPK6 silenced lines (irMPK6-1 and irMPK6-2). Samples used for QRT-PCR were from plant stems that were infested by SSB for 1 hr. (B) Mean transcript levels (+SE, n = 5) of OsWRKY24 in irMPK3 (irMPK3-53, irMPK3-183) and irMPK6 lines after infestation by SSB for 1 hr. (C, D) Mean transcript levels (+SE, n = 5) of OsMPK3 (C) and OsMPK6 (D) in irWRKY70 lines after infestation by SSB for 1 hr. Letters indicate significant differences among different lines (p < 0.05, Duncan's multiple range test).
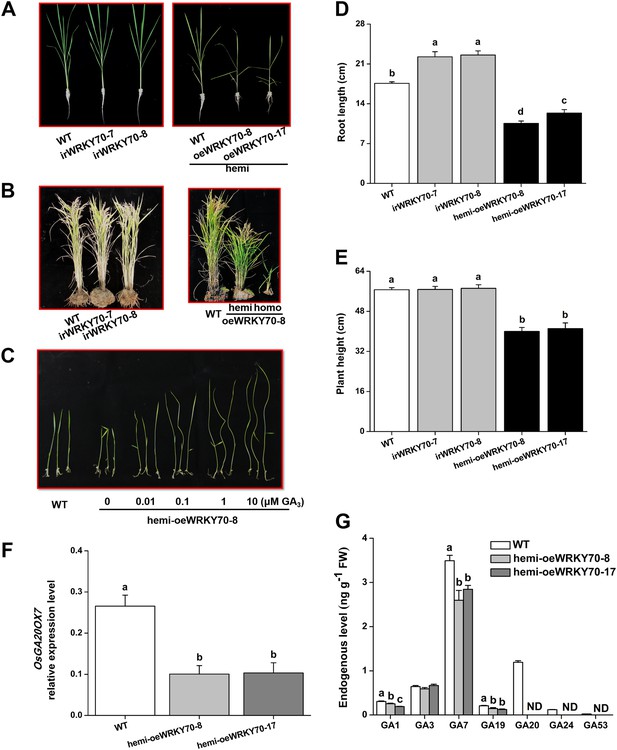
Altering OsWRKY70 expression affects GA levels and plant growth.
(A, B) Growth phenotypes of OsWRKY70 transgene lines (irWRKY70 lines, irWRKY70-7 and irWRKY70-8, and oeWRKY70 and hemi-oeWRKY70 lines, oeWRKY70-8, hemi-oeWRKY70-8 and hemi-oeWRKY70-17) and wild-type (WT) plants at tillering stage (A) and heading stage (B). (C) 10-day-old seedlings of WT and hemi-oeWRKY70-8 lines whose seeds were surface sterilized and placed on 1/2 Murashige and Skoog agar medium containing GA3 (minimum purity > 99%, Sigma, St Louis, MO) at various concentrations. This experiment was repeated 3 times with similar results. (D, E) Root length (D) and plant height (E) of transgenic lines with silencing (irWRKY70) or overexpressing (hemizygous lines, hemi-oeWRKY70 lines) of OsWRKY70 and WT plants at tillering stage. (F) Mean transcript levels (+SE, n = 5) of OsGA20ox7 in hemi-oeWRKY70-8, hemi-oeWRKY70-17, and WT plant. (G) Mean levels (+SE, n = 3) of gibberellins (GAs), including GA1, GA3, GA7, GA19, GA20, GA24, and GA53, in hemi-oeWRKY70-8, hemi-oeWRKY70-17, and WT plants. Letters indicate significant differences among different lines (p < 0.05, Duncan's multiple range test).
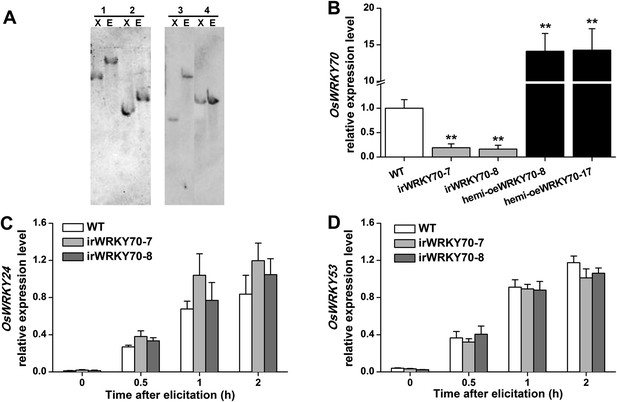
OsWRKY70 transgenic lines and levels of OsWRKY70, OsWRKY24, and OsWRKY53 transcripts in the transgenic lines and WT plants.
(A) Southern blot analysis of transgenic lines with silencing (irWRKY70 lines, irWRKY70-7, and irWRKY70-8) or overexpressing (hemizygous lines, hemi-oeWRKY70 lines, hemi-oeWRKY70-8, and hemi-oeWRKY70-17) of OsWRKY70. Genomic DNA was digested with XbaⅠ(X) and EcoRⅠ(E). The Blot was hybridized with a probe specific for gus reporter gene. All transgenic lines have a single insertion of the transgene. Lane 1, irWRKY70-7; lane 2, irWRKY70-8; lane 3, hemi-oeWRKY70-8; lane 4, hemi-oeWRKY70-17. (B) Mean expression levels (+SE, n = 6) of OsWRKY70 in irWRKY70, hemi-oeWRKY70, and WT plants at 1 hr after infestation by SSB. (C, D) Mean transcript levels (+SE, n = 5) of OsWRKY24 (C) and OsWRKY53 (D) in irWRKY70, hemi-oeWRKY70, and WT plants at different time after infestation by SSB. Asterisks indicate significant differences in irWRKY70 and hemi-oeWRKY70 compared with WT plants (**, p < 0.01; Student's t-test).
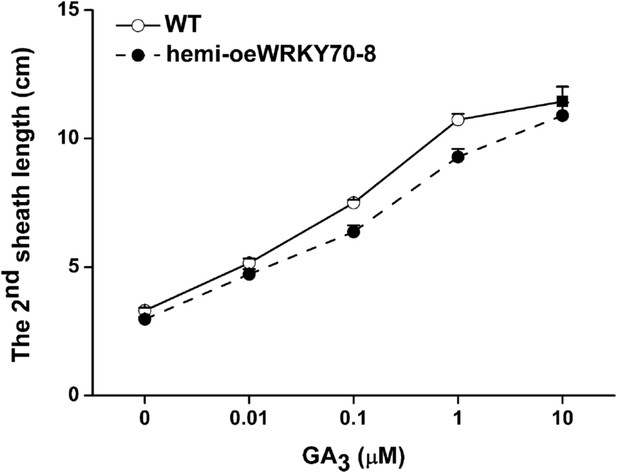
Elongation of the second leaf sheath in hemi-oeWRKY70-8 and WT plants in response to GA3.
WT and hemi-oeWRKY70-8 seeds were surface sterilized and placed on 1/2 Murashige and Skoog agar medium containing GA3 (minimum purity > 99%, Sigma) at various concentrations. The length of the second leaf sheaths was measured 10 days later.
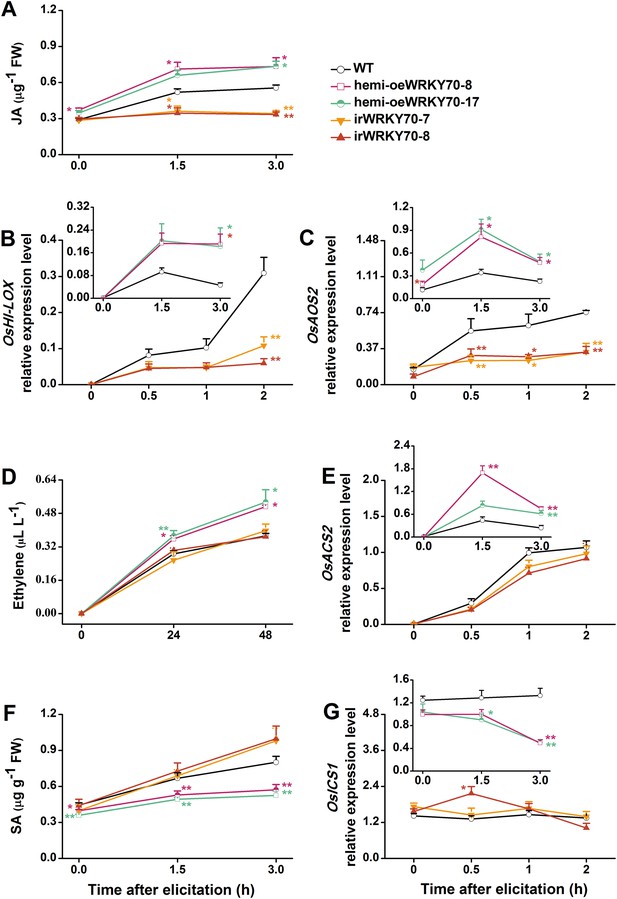
OsWRKY70 mediates SSB-elicited JA, SA, and ET accumulation.
Mean levels (+SE, n = 5–10) of JA (A), ET (D), and SA (F), and mean expression levels (+SE, n = 5) of OsHI-LOX (B), OsAOS2 (C), OsACS2 (E), and OsICS1 (G) in irWRKY70, hemi-oeWRKY70, and WT plants that were individually infested by a third-instar SSB larva. Asterisks indicate significant differences in irWRKY70, hemi-oeWRKY70 compared with WT plants (*, p < 0.05; **, p < 0.01; Duncan's multiple range test).

W-box elements in promoter regions of OsHI-LOX, OsICS1, OsAOS2, OsACS2, and OsGA20ox7.
Green highlights represent the translation initiation sites. Red highlights represent the transcription start sites that were predicted by BDGP (http://www.fruitfly.org/seq_tools/promoter.html). Dark gray highlights and gray highlights represent W-box (C/TTGACT/C) and W-box like (TGACT/C) motif, respectively, which were predicted by PLACE (http://www.dna.affrc.go.jp/htdocs/PLACE/).
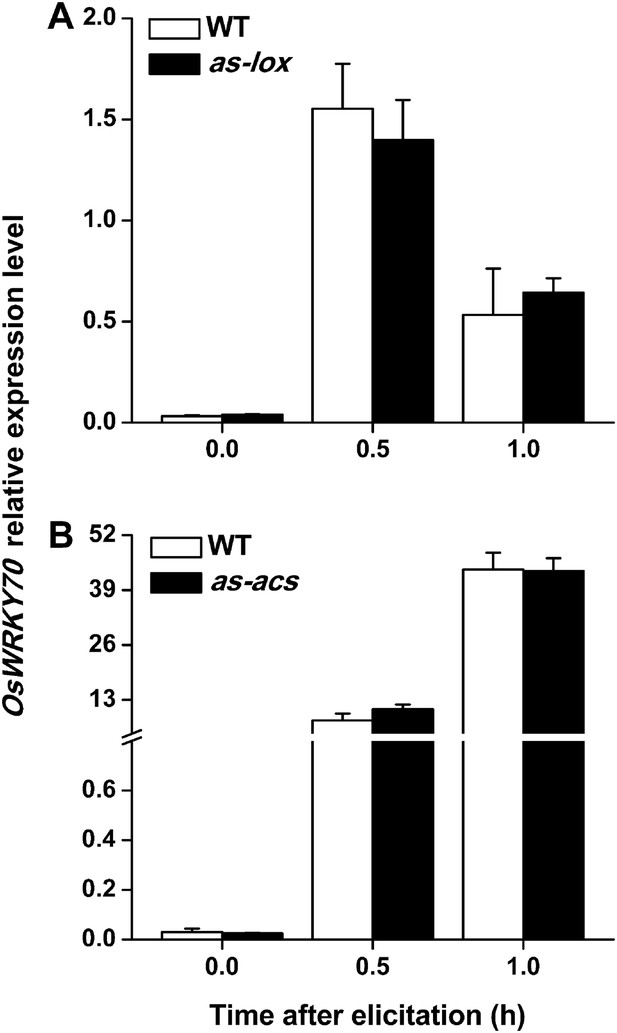
Levels of OsWRKY70 transcripts in WT plants and transgenic lines with impaired JA (as-lox) and ethylene (as-acs) biosynthesis.
Mean transcript levels (+SE, n = 5) of OsWRKY70 in transgenic lines with impaired JA (A, as-lox) and ethylene (B, as-acs) biosynthesis and WT plants after they were infested by SSB.
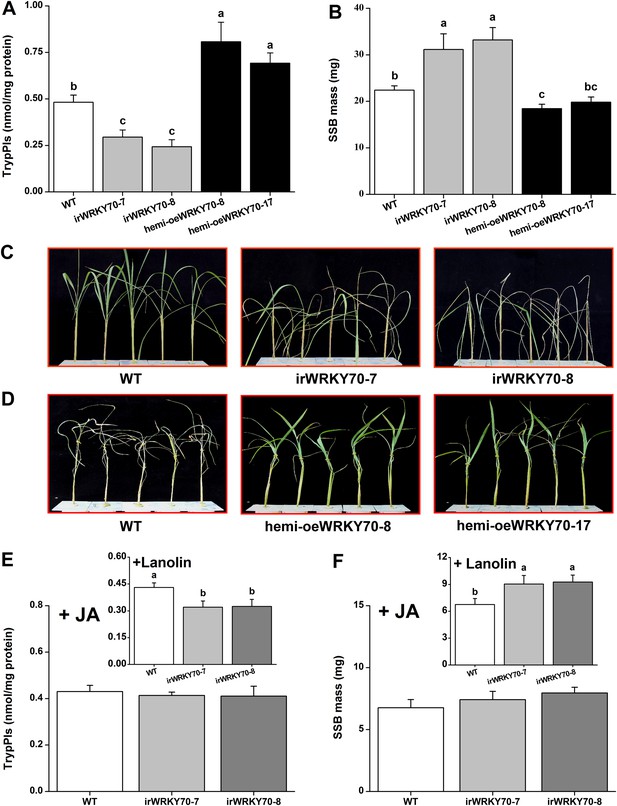
OsWRKY70 positively regulates resistance in rice to SSB.
(A) Mean Trypsin protease inhibitor (TrypPI) activities (+SE, n = 6) in irWRKY70, hemi-oeWRKY70, and WT plants that were individually infested by a third-instar SSB larva for 3 days. (B) Mean larval mass (+SE, n = 50) of SSB that fed on irWRKY70, hemi-oeWRKY70, and WT plants for 14 days. (C, D) Damaged phenotypes of irWRKY70 (C), hemi-oeWRKY70 (D), and WT plants that were individually infested by a third-instar SSB larva for 8 days (n = 10). This experiment was repeated twice with similar results. (E) Mean activities (+SE, n = 6) of TrypPIs in irWRKY70 and WT plants that were individually treated either 100 μg JA in 20 μl of lanolin paste (JA) or with 20 μl of pure lanolin (insert) for 24 hr, followed by SSB feeding for 3 days; (F) Mean larval mass (+SE, n = 50) of SSB 12 days after fed on irWRKY70 and WT plants that were individually treated either 100 μg JA in 20 μl of lanolin paste (JA) or with 20 μl of pure lanolin (insert) for 24 hr. Letters indicate significant differences among different lines (p < 0.05, Duncan's multiple range test).
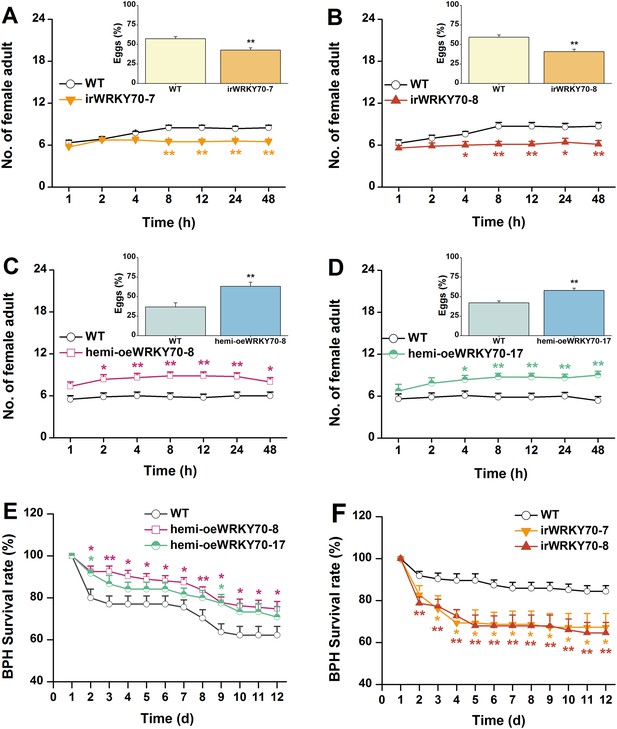
OsWRKY70 negatively regulates resistance of rice to BPH.
(A–D) Mean number of female BPH adults per plant (+SE, n = 8) on pairs of plants (WT vs irWRKY70-7, irWRKY70-8, hemi-oeWRKY70-8, and hemi-oeWRKY70-17, respectively), 1–48 hr after pairs were exposed. Inserts: mean percentage (+SE, n = 8) of BPH eggs per plant on pairs of plants as started above, 48 hr after the release of BPH. (E, F) Mean survival rate (+SE, n = 10) of BPH nymphs that fed on irWRKY70, hemi-oeWRKY70, or WT plants 1–12 days after the start of feeding. Asterisks indicate significant differences in irWRKY70, hemi-oeWRKY70 compared with WT plants (*, p < 0.05; **, p < 0.01; Student's t-test [A-D] or Duncan's multiple range test [E, F]).
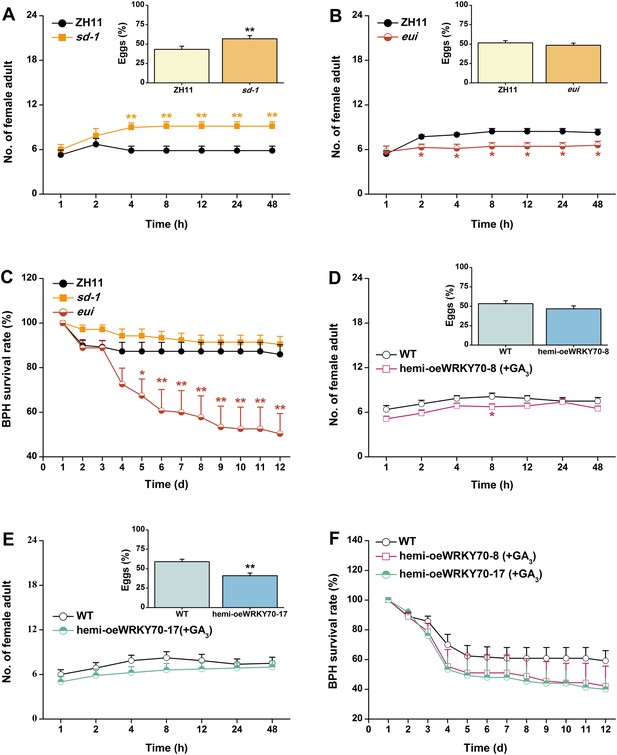
The GA-signaling pathway positively regulates rice resistance to BPH.
(A, B) Mean number of adult female BPH per plant (+SE, n = 8) on pairs of plants (WT (ZH11) vs sd-1 and eui, respectively), 1–48 hr after pairs were exposed. Inserts: mean percentage (+SE, n = 8) of BPH eggs per plant on pairs of plants as started above, 48 hr after the release of BPH. (C) Mean survival rate (+SE, n = 10) of BPH nymphs that fed on sd-1, eui lines, or WT (ZH11) plants 1–12 days after the start of feeding. (D, E) Mean number of female BPH adults per plant (+SE, n = 8) on pairs of plants, a WT plant that was grown in a nutrient solution without GA3 vs a hemi-oeWRKY70-8 (D) or hemi-oeWRKY70-17 (E) plant that was grown in a nutrient solution with GA3 at a concentration of 1 μM for 24 hr, 1–48 hr after pairs were exposed. Inserts: mean percentage (+SE, n = 8) of BPH eggs per plant on pairs of plants as started above, 48 hr after the release of BPH. (F) Mean survival rate (+SE, n = 10) of BPH nymphs that fed on WT plants that were grown in a nutrient solution without GA3 or hemi-oeWRKY70 lines (hemi-oeWRKY70-8 and hemi-oeWRKY70-17) that had been grown in a nutrient solution with GA3 at a concentration of 1 μM for 24 hr, 1–12 days after the start of feeding. Asterisks indicate significant differences in mutants compared with WT plants (*, p < 0.05; **, p < 0.01; Student's t-test [A, B, D, E] or Duncan's multiple range test [C]).
Additional files
-
Supplementary file 1
(A) Primers used for cloning of full-length or partial cDNAs of target genes in this study. (B) Primers and probes used for QRT-PCR of target genes.
- https://doi.org/10.7554/eLife.04805.019





