Synaptotagmin 1 directs repetitive release by coupling vesicle exocytosis to the Rab3 cycle
Figures
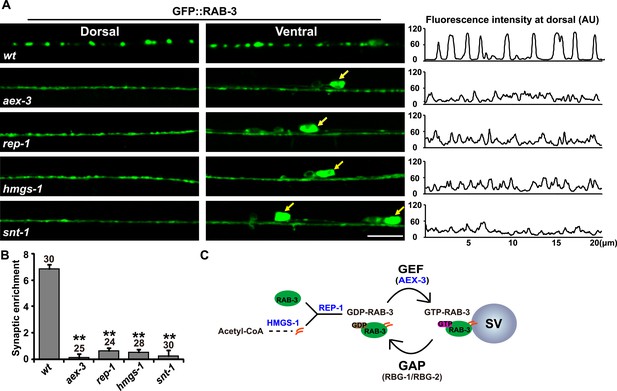
RAB-3 synaptic vesicle association requires SNT-1.
(A) Punctate distribution of GFP::RAB-3 in C. elegans motor neurons in wild-type animals (top). The GFP::RAB-3 puncta become diffuse in aex-3, rep-1, hmgs-1, and snt-1 mutants (lower panels). Yellow arrows indicate the cell bodies along the ventral cord. A representative line-scanning image for each genotype is shown in the right panel. (B) Quantification of the synaptic enrichment in wild-type, aex-3, rep-1, hmgs-1, and snt-1 animals. Data are presented as mean ± SD; **p < 0.01. (C) Schematic representation of the RAB-3/SV association and dissociation cycle. Scale bar, 5 µm.
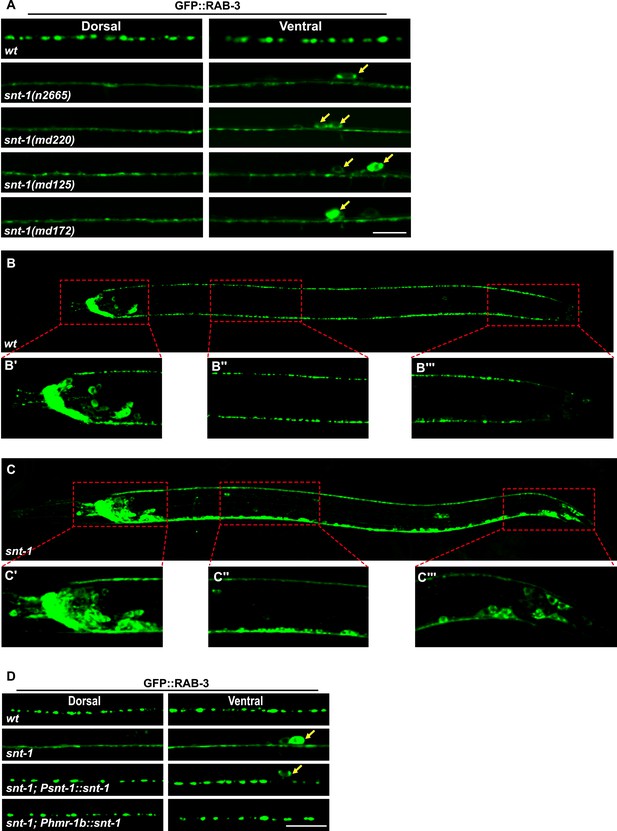
snt-1 is require for RAB-3 synaptic vesicle localization.
(A) Multiple snt-1 alleles show the diffuse GFP::RAB-3 phenotype. White arrows indicate the cell bodies. (B) GFP::RAB-3 expressed pan-neuronally under the control of the Prab-3 promoter displays punctate distribution. (C) GFP::RAB-3 is diffuse in all Prab-3-expressing cells. Red boxed areas are enlarged in panels underneath the image taken from a whole animal (B′, B″, B‴, C′, C″ and C‴). (D) The diffuse GFP::RAB-3 phenotype in snt-1 mutants is rescued by expressing wild-type snt-1 gene pan-neuronally using the Psnt-1 promoter or in DD, VD, and AS neurons using the Phmr-1 promoter. Scale bar, 5 µm.
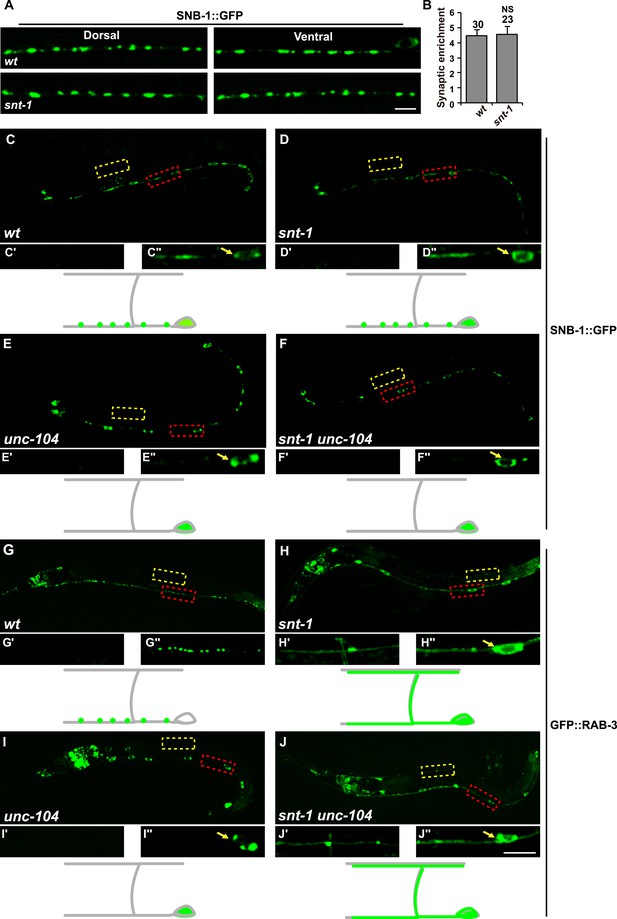
Synaptic vesicle clustering is unaffected by loss of snt-1 function.
(A) SNB-1::GFP puncta distribution in wild type and snt-1 mutants. (B) The synaptic enrichment of SNB-1::GFP puncta is indistinguishable in wild type and snt-1. Data are presented as mean ± SD; NS, not significant. In both wild type (C, C′ and C″) and snt-1 (D, D′ and D″), the SNB-1::GFP puncta are present in the synaptic area on the ventral cord, which is outside of the cell body (C″ and D″). In unc-104 (E, E′ and E″) or snt-1 unc-104 double mutants (F, F′ and F″), SNB-1::GFP accumulates in the cell bodies on the ventral cord (E″ and F″). (G) In wild type, GFP::RAB-3 is distributed in a punctate pattern in the pre-synaptic regions on the ventral cord (G“). (H) GFP::RAB-3 is diffuse throughout the whole axon including both dorsal (H') and ventral (H″) processes. (I) GFP::RAB-3 accumulates in ventral cell bodies (I″). (J) In snt-1 unc-104 double mutants, GFP::RAB-3 is diffuse throughout the whole axon in both dorsal (J') and ventral (J″) regions. Yellow boxes indicate part of the dorsal cord, which is enlarged in the lower left panels. Red boxes indicate part of the ventral cord, which is enlarged in the lower right panels (white arrows indicate DD cell bodies in the ventral cord). A schematic drawing of a DD neuron during the L1 stage is presented underneath the fluorescence images of each genotype, with the SNB-1::GFP or GFP::RAB-3 signal shown in green. Small green dots represent the pre-synaptic areas. Individual DD cell bodies are indicated as large ovals at the bottom right of each diagram. Scale bars, 5 µm.
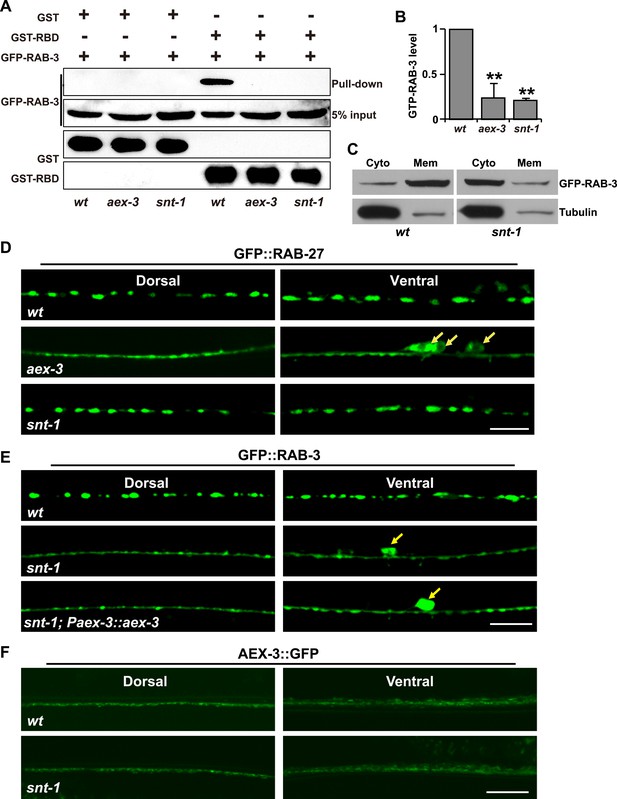
The GTP-bound form of RAB-3 is decreased in snt-1 mutants.
(A) A GST-fused RBD domain of RIM2 binds active GTP-RAB-3. The amount of GTP-RAB-3 pulled down by RBD is decreased in both aex-3 and snt-1 animals. (B) Quantification of the GTP-RAB-3 level in wild type, aex-3, and snt-1. Data are presented as mean ± SD; **p < 0.01. (C) The amount of GFP-RAB-3 in the cytosolic fraction is increased in snt-1 mutants. (D) Localization of GFP::RAB-27 puncta is affected by mutation of aex-3, but not by mutation of snt-1. The white arrows indicate the cell bodies. (E) Over-expression of aex-3 does not rescue the snt-1 mutant phenotype. Yellow arrows indicate the cell bodies. (F) The AEX-3::GFP level is unchanged in snt-1 mutants compared to wild type. Scale bars, 5 µm.
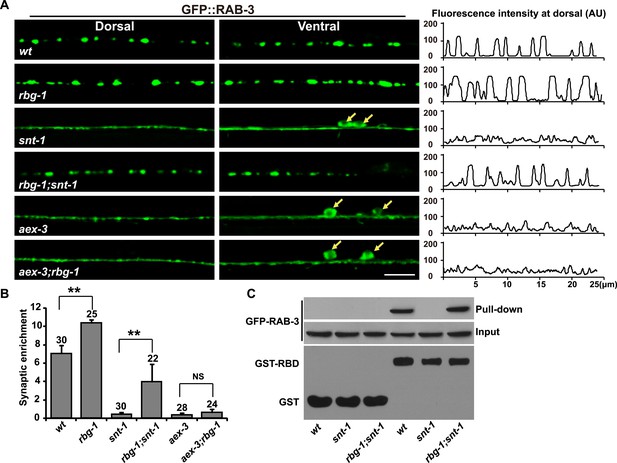
RAB-3 GAP mutations suppress the snt-1 mutant phenotype.
(A) The punctate distribution of GFP::RAB-3 is restored in rbg-1;snt-1 animals, while the aex-3 phenotype could not be suppressed by mutation of rbg-1. Yellow arrows indicate the cell bodies along the ventral cord. Scale bar, 5 µm. A representative line-scanning image for each genotype is shown in the right panel. (B) Quantification of the synaptic enrichment of GFP::RAB-3 signal in the genotypes shown in (A). Data are represented as mean ± SD. **p < 0.01; NS, not significant. (C) The amount of GTP-RAB-3 pulled down by RBD is increased in rbg-1;snt-1 animals compared to snt-1.
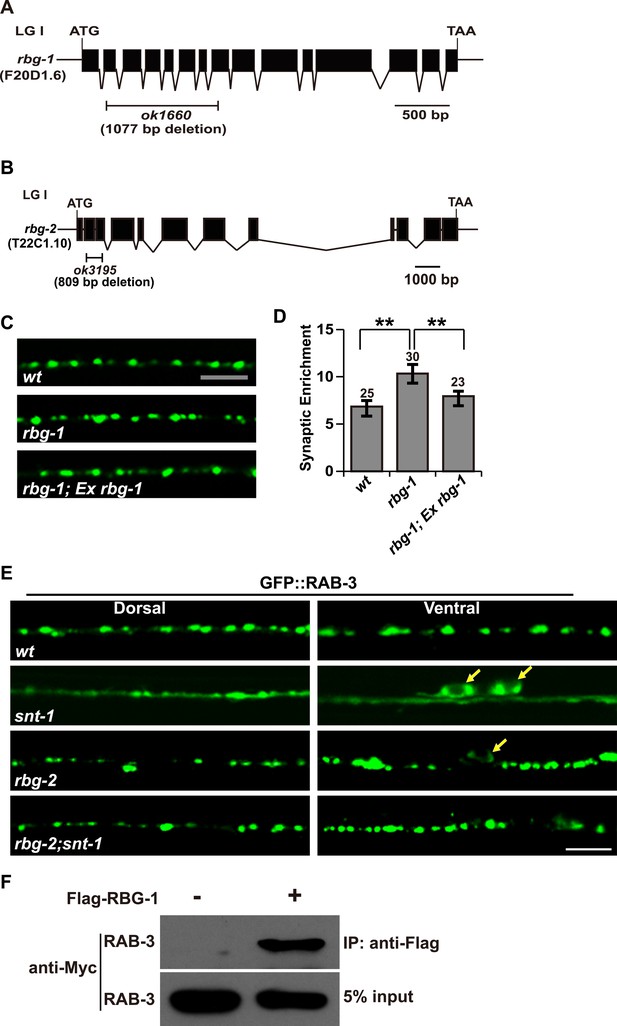
rbg-2 suppresses the snt-1 mutant phenotype.
(A) Schematic representation of the rbg-1(ok1660) deletion mutation. (B) Schematic representation of the rbg-2(ok3195) deletion mutation. Solid boxes indicate exons and thin lines indicate introns. The bar below the gene indicates the deleted region. (C) The enlarged GFP::RAB-3 puncta phenotype in rbg-1 mutants is rescued by expressing a wild-type copy of the rbg-1 gene. (D) Quantification of the synaptic enrichment of GFP::RAB-3 signal in the genotypes shown in (C). Data are represented as mean ± SD; **p < 0.01. (E) Loss of rbg-2 function suppresses the snt-1 mutant phenotype. White arrows indicate the cell bodies. (F) RAB-3 is co-precipitated with RBG-1. Scale bars, 5 µm.
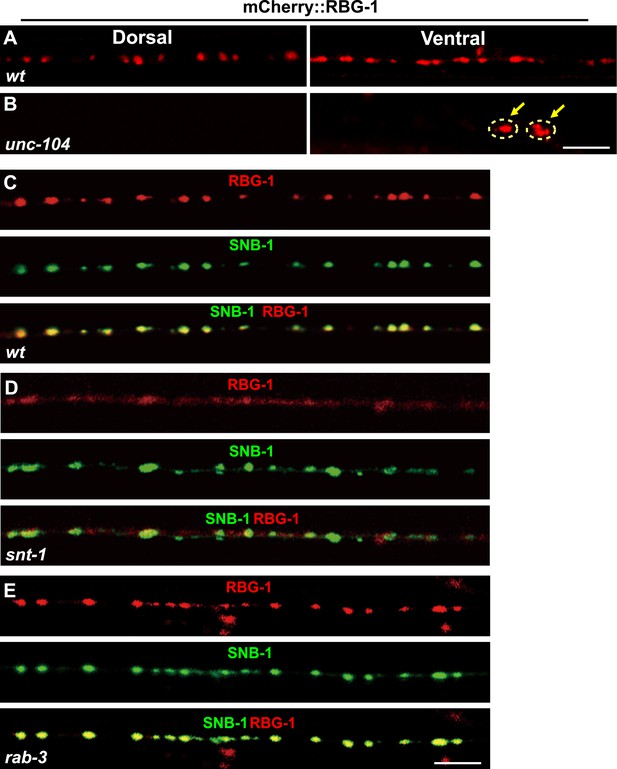
Localization of RBG-1 on synaptic vesicles requires SNT-1.
(A and B) mCherry::RBG-1 has a punctate distribution in wild type (A) but accumulates in cell bodies (yellow arrows) in unc-104 mutants (B). (C) mCherry::RBG-1 (red) is co-localized with SNB-1::GFP puncta (green). (D) In snt-1 mutants, mCherry::RBG-1 loses its punctate localization and becomes diffuse in axons, while SNB-1::GFP retains its punctate pattern. (E) mCherry::RBG-1 retains its punctate distribution and is co-localized with SNB-1::GFP in rab-3 mutants. Scale bars, 5 µm.
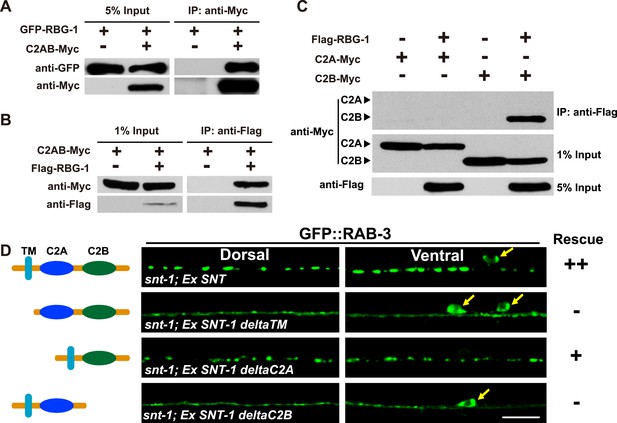
RBG-1 associates with the C2B domain of SNT-1.
(A) RBG-1 is precipitated by the intracellular domain (C2AB) of SNT-1. (B) The SNT-1 intracellular domain is precipitated by RBG-1. (C) The C2B domain of SNT-1 binds to RBG-1. (D) SNT-1 without the C2B domain fails to rescue the snt-1 mutant phenotype. Yellow arrows indicate cell bodies. The schematic diagram shows the transmembrane (TM) and intracellular calcium-binding domains (C2A and C2B) of SNT-1. Scale bar, 5 µm.
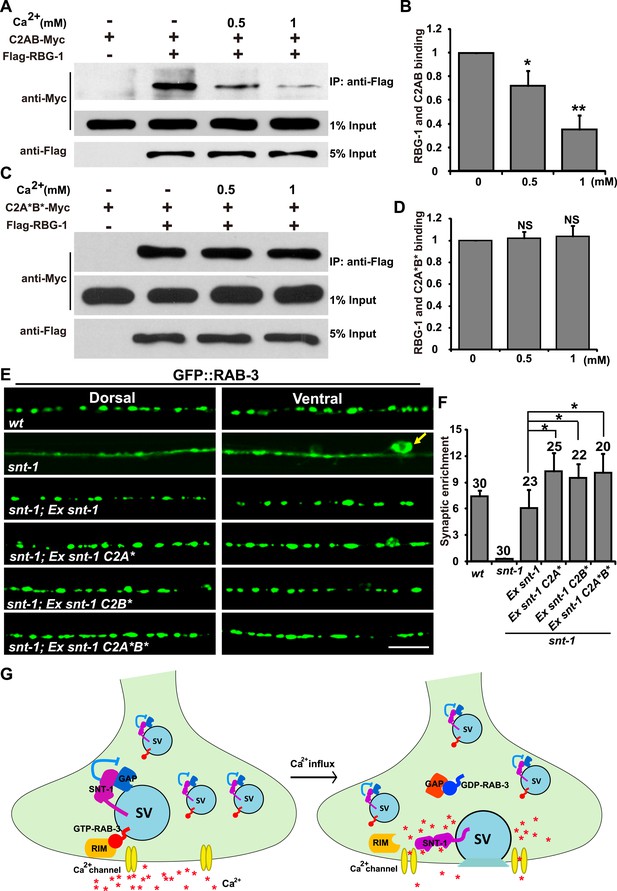
Dissociation of RAB-3 from synaptic vesicles requires the Ca2+-binding activity of SNT-1.
(A) Ca2+ treatment diminishes the binding between RBG-1 and the intracellular domain (C2AB) of SNT-1. (B) Quantification of the relative binding between RBG-1 and the C2AB domain upon Ca2+ treatment. (C) SNT-1 without Ca2+-binding sites (C2A*B*) still binds to RBG-1 in the presence of Ca2+. (D) Quantification of the relative binding between RBG-1 and the C2A*B* domain upon Ca2+ treatment. (E) Mutant SNT-1 proteins without Ca2+-binding activity stabilize RAB-3 on SVs. Ex snt-1, over-expression of SNT-1; Ex snt-1 C2A* and Ex snt-1 C2B*, over-expression of SNT-1 with mutant C2A domain or C2B domain, respectively; Ex snt-1 C2A*B*, over-expression of SNT-1 with mutant C2A and C2B domains. Scale bar, 5 µm. (F) Quantification of the synaptic enrichment in the genotypes shown in (E). (G) SNT-1 functions as a molecular switch controlling RAB-3/SV association and disassociation during SV exocytosis. Data are represented as mean ± SD. *p < 0.05; **p < 0.01; NS, not significant.
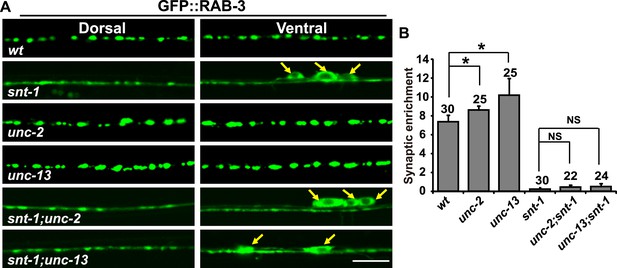
Exocytosis is uncoupled from RAB-3 synaptic vesicle dissociation in snt-1 mutants.
(A) The failure of RAB-3/SV dissociation caused by exocytosis mutants, including unc-2 and unc-13, is bypassed by mutation of snt-1. Yellow arrows indicate the cell bodies along the ventral cord. Scale bar, 5 µm. (B) Quantification of the synaptic enrichment in the genotypes shown in (A). Data are presented as mean ± SD. *p < 0.05; NS, not significant.





