Blood-stage immunity to Plasmodium chabaudi malaria following chemoprophylaxis and sporozoite immunization
Figures
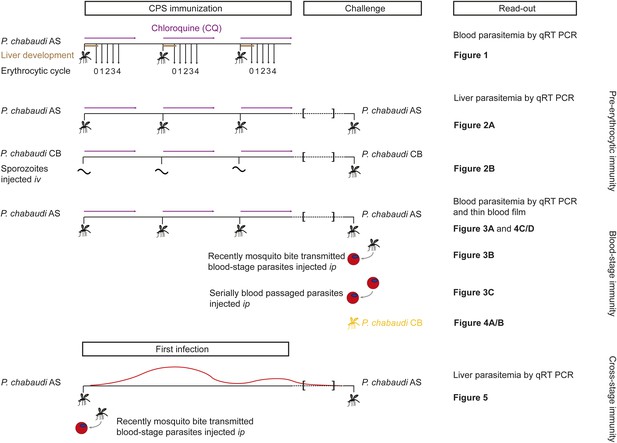
Overview of experimental procedures.
To quantify transient blood-stage exposure during chemoprophylaxis and sporozoite (CPS) immunization female C57BL/6 were immunized three times at 2-week intervals with P. chabaudi AS-infected mosquito bites (typically 9.15 [median, range 6.9–13.6] [Spence et al., 2012]). Following each immunization mice received 100 mg per kg chloroquine (CQ) per os daily for 10 days, starting from the day of infection. A small blood sample was taken 48 hr after each mosquito transmission (before merozoite egress from the liver, P. chabaudi develops in the liver for 52 hr [Stephens et al., 2012]; erythrocytic cycle 0), and then every 24 hr until erythrocytic replication cycle 4. Blood parasitemia was analyzed by sensitive quantitative RealTime (qRT) PCR (Figure 2). Pre-erythrocytic immunity was evaluated in mice immunized three times with either P. chabaudi AS-infected mosquito bites (Figure 3A) or by intravenous (iv) injection of defined numbers of P. chabaudi CB sporozoites (Figure 3B). P. chabaudi CB was used since mosquitoes infected with this parasite harbor an increased number of sporozoites in their salivary glands (Spence et al., 2012), which made injections of high numbers of sporozoites technically feasible. Mice were challenged 100 days after the last immunization by mosquitoes infected with the respective homologous strain. Liver parasitemia was examined 42 hr after challenge by qRT PCR. Blood-stage immunity was assessed in mice immunized with P. chabaudi AS-infected mosquito bites by qRT PCR and thin blood film following homologous challenge with either infected mosquito bites (Figures 4A, 5C/D) or intraperitoneal (ip) injection of parasitized erythrocytes, which were either derived from a donor mouse infected by mosquito bite (recently mosquito transmitted, Figure 4B) or after 26–32 serial blood-passages (Figure 4C). Heterologous protection was assessed using P. chabaudi CB-infected mosquito bites (Figure 5A/B). To evaluate cross-stage protection mice received a first infection with P. chabaudi AS either by mosquito bite or by ip injection of recently mosquito transmitted parasitized erythrocytes. The resulting blood-stage infection was eventually self-cured without intervention. Mice were re-challenged 100 days after their first infection with P. chabaudi AS-infected mosquito bites and liver parasitemia was evaluated by qRT PCR (Figure 6).
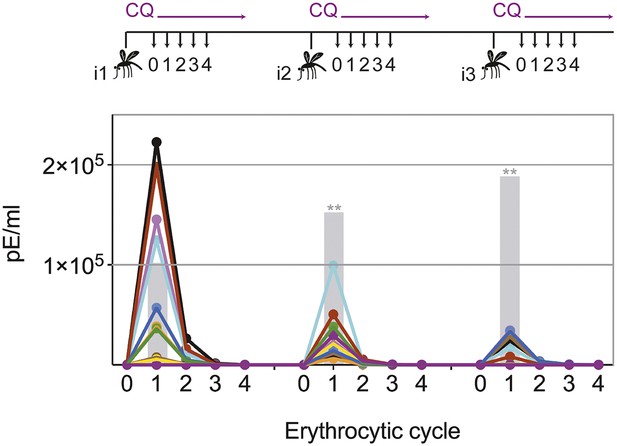
Chloroquine permits transient blood-stage parasitemia during each immunization.
The number of parasitized erythrocytes (pE) per ml of whole blood was enumerated by quantitative RealTime PCR after each CPS immunization (i1, i2, i3) with P. chabaudi AS-infected mosquito bites under chloroquine (CQ) cover. The number of pE (at the late trophozoite stage) was quantified immediately before merozoite egress from the liver, at 48 hr post mosquito transmission (erythrocytic cycle 0), and then every 24 hr until erythrocytic replication cycle 4. Daily parasitemia of 10 CPS immunized mice (each color represents an individual mouse) are shown. Blood-stage parasites were detected within the first erythrocytic cycle after every immunization in all but one mouse after the final immunization. Gray bars represent the mean parasitemia in the first erythrocytic cycle of naive mice infected as controls for mosquito transmission efficiency separate with each immunization (n = 3–5). Significant differences in the number of blood-stage parasites in the first erythrocytic cycle between naive and CPS immunized mice are indicated (Mann Whitney test, **p ≤ 0.01).
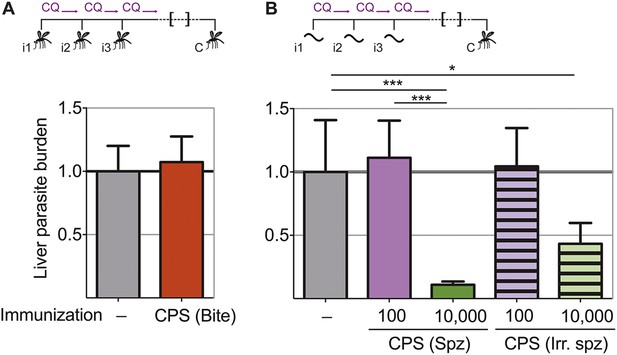
Pre-erythrocytic immunity following CPS immunization requires high doses of sporozoites.
Liver parasite burden was determined 42 hr after mosquito bite challenge as copy number of P. chabaudi-specific 18S rRNA. (A) Mice were CPS immunized three times (i1, i2, i3) with P. chabaudi AS-infected mosquito bites under chloroquine (CQ) cover (CPS (Bite)) and challenged (C) 96–104 days after immunization by bites of P. chabaudi AS-infected mosquitoes (pooled data from three independent experiments; naive infection controls (−) n = 25, CPS (Bite) n = 35). (B) 100 or 10,000 untreated or irradiated (Irr.) P. chabaudi CB sporozoites (spz) were injected iv three times under CQ cover. Mice were challenged 96 days after immunization by bites of P. chabaudi CB-infected mosquitoes (naive infection controls (−) n = 12, all other groups n = 20). All data are displayed relative to the mean of corresponding liver parasite burden of naïve infection controls and presented as mean ± SEM, (A) Mann–Whitney test: no significant difference between the groups; (B) Kruskal Wallis with Dunn's multiple comparisons test *p ≤ 0.05, ***p ≤ 0.001.
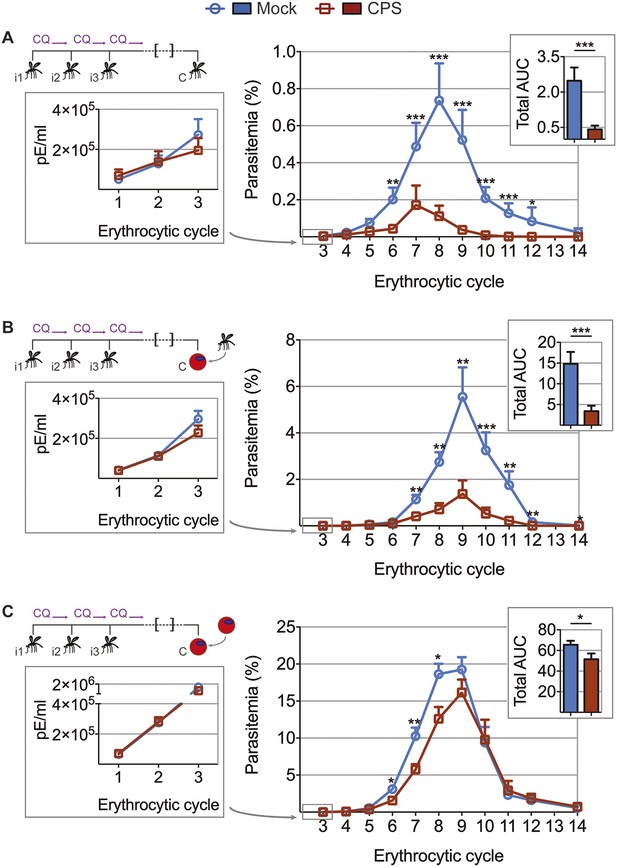
CPS immunization elicits blood-stage immunity.
Mice were CPS immunized three times (i1, i2, i3) using chloroquine (CQ) and P. chabaudi AS-infected or uninfected mosquito bites (mock immunized). Approximately 100 days after the final CPS immunization, mice were challenged (C) with P. chabaudi AS. Erythrocytic parasitemia was evaluated daily by quantitative RealTime PCR (cycle 1–3, displayed as parasitized erythrocytes (pE) per ml whole blood; left) and from cycle 3–14 by thin blood-film (expressed as % parasitized erythrocytes [parasitemia] 0.01% parasitemia corresponds to 1,000,000 pE per ml; middle). The total area under the curve (AUC) was calculated for each mouse between erythrocytic cycle 3 and 14 (right). (A) Mosquito bite challenge: parasitemia from 1st to 3rd (n = 10) and between 3rd and 14th erythrocytic cycle (representative of three independent experiments, n = 12–19), total AUC between cycle 3 and 14 (n = 19). (B) Direct blood challenge using 10,000 erythrocytic parasites obtained from a donor mouse infected by mosquito bite; injected intraperitoneal (ip): parasitemia between 1st and 3rd (n = 10) and 3rd and 14th erythrocytic cycle (representative of three independent experiments, n = 10), total AUC between cycle 3 and 14 (n = 10). (C) Blood challenge using 10,000 serially blood passaged parasites; injected ip: parasitemia from 1st to 3rd (n = 10) and between 3rd and 14th erythrocytic cycle (representative of two independent experiments, n = 8–10), total AUC between cycle 3 and 14 (n = 10). All data are presented as mean ± SEM, Mann–Whitney test per time point *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
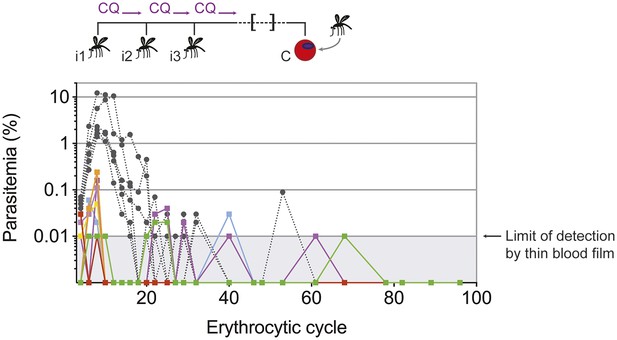
Chronic blood-stage infection in CPS immunized mice.
Naive infection controls or mice that were CPS immunized three times (i1, i2, i3) using chloroquine (CQ) and P. chabaudi AS-infected mosquito bites were challenged (C) by intraperitoneal (ip) injection of 100,000 parasitized erythrocytes derived from a mosquito bite initiated infection 100 days later. Parasitemia was evaluated by thin blood film for 96 erythrocytic cycles. Different colors represent individual CPS immunized mice (n = 8), naive infection controls are presented in grey with dashed lines (n = 6). The limit of detection by thin blood film is 0.01% parasitemia (1 parasite in 10,000 erythrocytes or 1,000,000 parasites per ml blood).
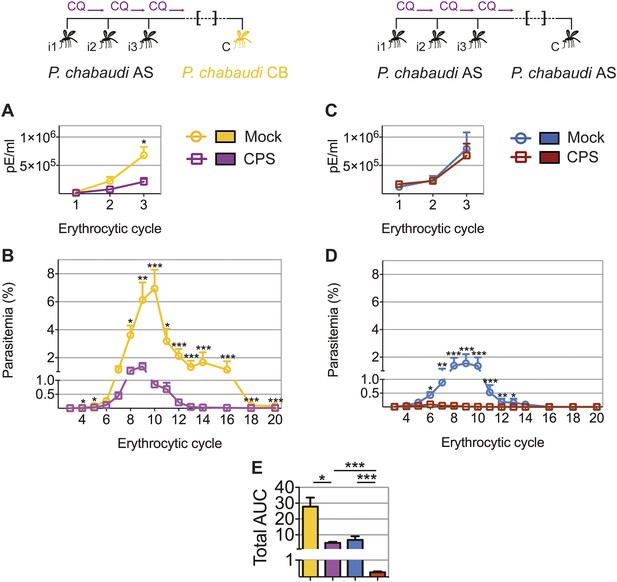
CPS immunization elicits heterologous blood-stage immunity.
Mice were CPS immunized three times (i1, i2, i3) under chloroquine (CQ) cover by P. chabaudi AS-infected mosquito bites or mock immunized with uninfected mosquito bites, and challenged (C) 96–107 days later by mosquito bite. Erythrocytic parasitemia was evaluated daily by quantitative RealTime PCR for blood-stage parasites (cycle 1–3, displayed as parasitized erythrocytes (pE) per ml whole blood) and from cycle 3–20 by thin blood-film (expressed as % parasitized erythrocytes [parasitemia], 0.01% parasitemia corresponds to 1,000,000 pE per ml). (A/B) Heterologous challenge with P. chabaudi CB infected mosquitoes (A) Parasitemia between 1st and 3rd (n = 10) and (B) from 3rd to 20th erythrocytic cycle (n = 20). (C/D) Homologous challenge with P. chabaudi AS-infected mosquitoes (C) Parasitemia between 1st and 3rd (n = 10) and (D) from 3rd to 20th erythrocytic cycle (CPS immunized n = 20, mock immunized n = 19). (E) Total AUC comparing mock and CPS immunized mice receiving heterologous or homologous mosquito bite challenge. Data are presented as mean ± SEM, (A–D) Mann–Whitney test per time point (E) Kruskal Wallis with Dunn's multiple comparisons test *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
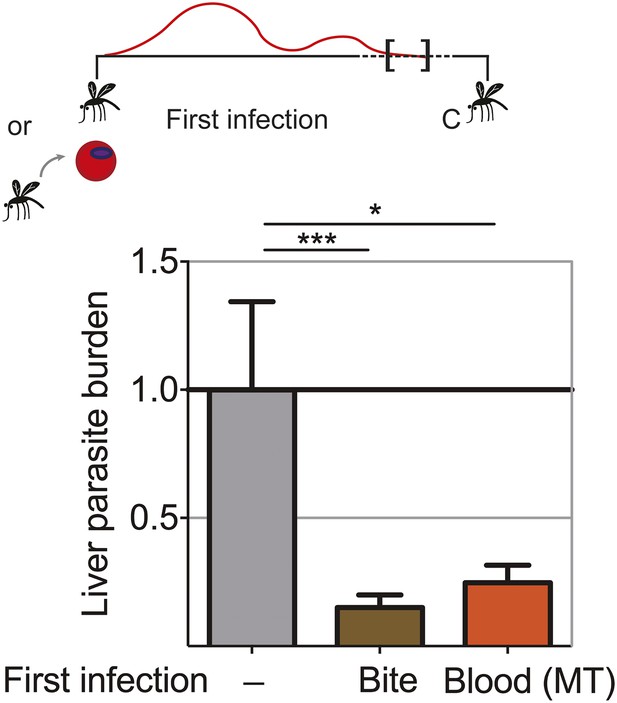
Extended blood-stage parasitemia elicits pre-erythrocytic immunity.
Mice received a first infection with P. chabaudi AS either by mosquito bite (Bite) or intraperitoneal (ip) injection of 10,000 parasitized erythrocytes obtained from a donor mouse infected by mosquito bite (Blood (MT)). Blood-stage parasitemia was self-cured before challenge (C; 98 days post-infection) using P. chabaudi AS-infected mosquito bites. Liver parasite burden was determined 42 hr after mosquito bite challenge as copy number of P. chabaudi-specific 18S rRNA. Data are displayed relative to the mean of corresponding liver parasite burden of naive infection controls (−). Pooled data from two independent experiments (Naive (−) and Bite n = 30, Blood (MT) n = 20) are presented as mean ± SEM, Kruskal Wallis with Dunn's multiple comparisons test *p ≤ 0.05, ***p ≤ 0.001.
Tables
Relationship between the dose of immunizing pre-erythrocytic and blood-stage parasites and the acquisition of immunity.
| Low dose | High dose | |
|---|---|---|
| Sporozoites (liver-stage parasites) | Mosquito bite or 100 sporozoites iv: no pre-erythrocytic immunity (Figure 3A/B) | 10,000 sporozoites iv: pre-erythrocytic immunity (Figure 3B) |
| Blood-stage parasites | Blood-stage infection curtailed by chloroquine: partial blood-stage immunity (Figure 4) | Fulminant, self-cured blood-stage infection: blood-stage immunity (Spence et al., 2013); pre-erythrocytic immunity (Figure 6) |





