The stress-responsive kinases MAPKAPK2/MAPKAPK3 activate starvation-induced autophagy through Beclin 1 phosphorylation
Figures
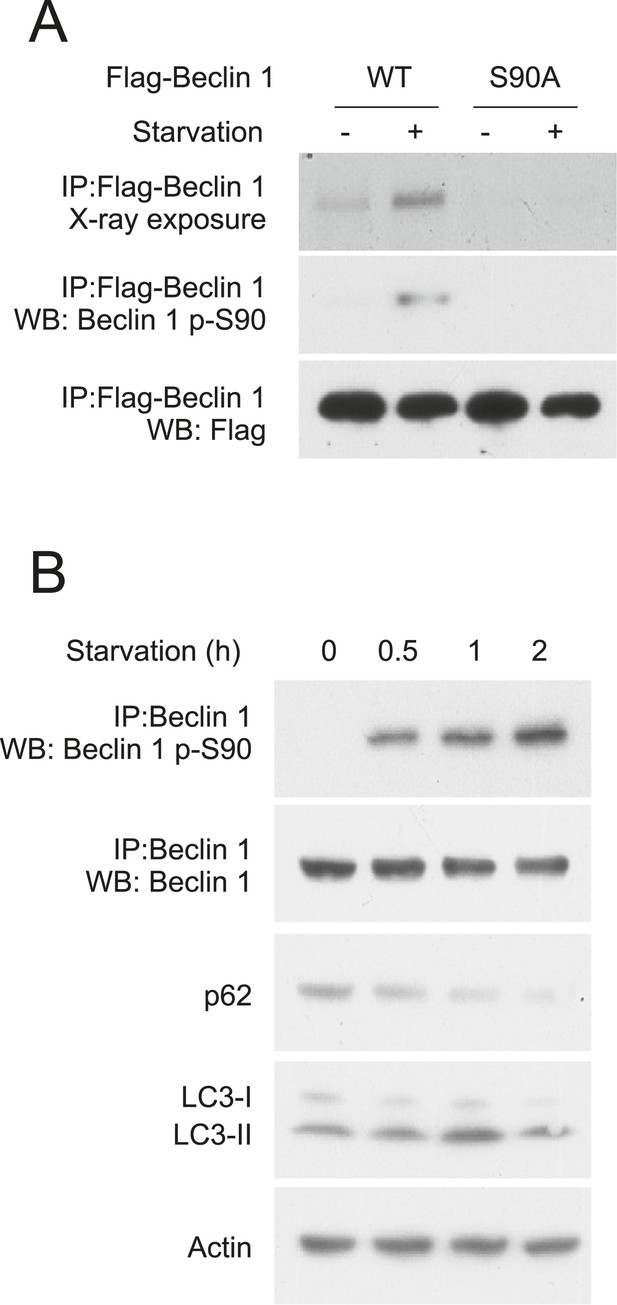
Beclin 1 S90 is phosphorylated in response to nutrient starvation.
(A) Detection of Beclin 1 S90 phosphorylation in HeLa cells transfected with indicated Flag-Beclin 1 construct by radiolabeling, immunoprecipitation with an anti-Flag antibody and autoradiography (upper gel) or by western blot analysis using an anti-Beclin 1 S90 phosphospecific antibody (middle gel). Starvation (−) refers to growth in normal medium and starvation (+) refers to growth in HBSS for 2 hr. (B) Detection of endogenous Beclin 1 S90 phosphorylation in HeLa cells by immunoprecipitation with anti-Beclin 1 followed by immunoblot analysis with anti-Beclin 1 p-S90 phosphospecific antibody and detection of autophagy by p62 and LC3 immunoblot analysis in HeLa cells at indicated time points after starvation in HBSS. Actin is shown as a loading control.
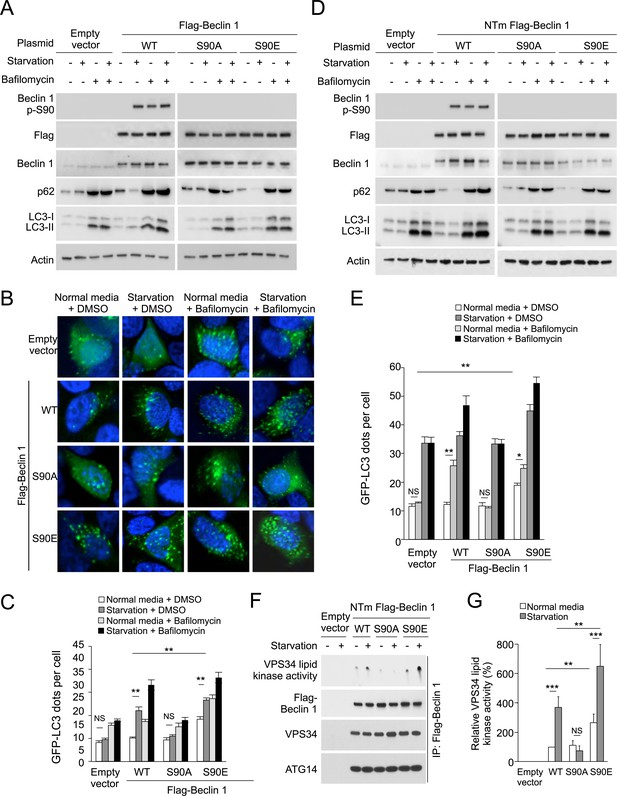
The Beclin 1 S90 phosphorylation site is required for autophagy induction in MCF7 and U2OS cells.
(A) Western blot results of MCF7 cells transiently transfected with empty vector, and Flag epitope-tagged wild-type Beclin 1, Beclin 1 S90A, or Beclin 1 S90E. The cells were grown in normal medium (starvation−) or HBSS (starvation+) for 3 hr in the presence or absence of 100 nM bafilomycin A1. (B) Representative images of GFP-LC3 puncta (autophagosomes) in MCF7 cells transiently co-transfected with indicated Flag-Beclin 1 constructs and a plasmid expressing GFP-LC3 and grown in normal medium or in HBSS for 3 hr (starvation) in the presence or absence of 100 nM bafilomycin A1. (C) Quantification of GFP-LC3 puncta in MCF7 cells in conditions shown in (B). Bars are mean + SEM of triplicate samples (>50 cells analyzed per sample). Similar results were observed in three independent experiments. ***p < 0.001, **p < 0.01, NS, not significant; one-way ANOVA. (D) Western blot detection of Beclin 1, p62 and LC3 in U2OS cells expressing doxycycline-inducible shRNA against beclin 1 (beclin 1 shRNA U2OS cells) following treatment with 1 μg/ml doxycycline for 4 days in cells transduced with retroviral constructs expressing indicated shRNA-resistant Flag-Beclin 1 (NTm, non-targetable mutant) plasmids. Cells were either grown in normal medium (starvation−) or in HBSS for 3 hr (starvation+) in the presence or absence of 100 nM bafilomycin A1. See Figure 2—figure supplement 1 for comparison of Beclin 1, p62, and LC3 western blots in the presence and absence of doxycycline. (E) Quantification of GFP-LC3 puncta (autophagosomes) in beclin 1 shRNA U2OS cells treated with 1 μg/ml doxycycline for 4 days and co-transfected with plasmids expressing GFP-LC3 and indicated shRNA-resistant Flag-Beclin 1 construct and grown in normal medium or in EBSS for 3 hr (starvation) in the presence or absence of 100 nM bafilomycin A1. Bars are mean + SEM of triplicate samples (>50 cells analyzed per sample). Similar results were observed in three independent experiments. **p < 0.01, *p < 0.05, NS, not significant; one-way ANOVA. (F) Beclin 1-associated VPS34 in vitro lipid kinase assay and amounts of VPS34 and ATG14 in anti-Beclin 1 immunoprecipitates of beclin 1 shRNA U2OS cells following treatment with 1 μg/ml doxycycline for 4 days and transfection with indicated shRNA-resistant Flag-Beclin 1 (NTm, non-targetable mutant) plasmids. Cells were either grown in normal medium (starvation−) or in HBSS for 2 hr (starvation+). Dots shown in upper panel represent the amount of PI3P generated in an in vitro VPS34 lipid kinase assay using anti-Flag-Beclin 1 immunoprecipitates as input. (G) Densometric quantitation of VPS34 in vitro lipid kinase activity in anti-Beclin 1 immunoprecipitates in conditions described in (F). Results shown represent mean + SEM of values in three independent experiments. Similar results were observed in each independent experiment. Shown are the relative values of VPS34 lipid kinase activity compared to those observed in cells expressing WT Beclin 1 in normal media (defined as 100%). To control for input in Beclin 1 anti-immunoprecipitates, values used to calculate VPS34 lipid kinase activity were normalized for levels of Beclin 1 determined by densitometric quantification of Beclin 1 western blot bands in anti-Beclin 1 immunoprecipitates. ***p < 0.001, **p < 0.01, NS, not significant; one-way ANOVA. See also Figure 2—figure supplement 1, Figure 1—figure supplement 2, Figure 2—figure supplement 3.
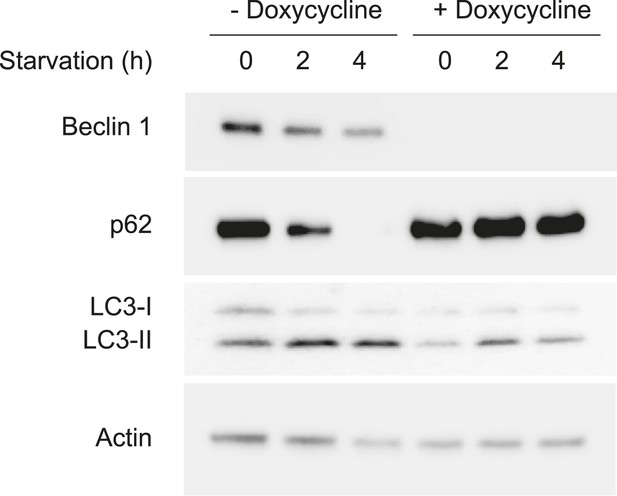
Doxycycline reduces Beclin 1 expression and starvation-induced autophagy in U2OS cells that express doxycycline-inducible beclin 1 shRNA.
Detection of Beclin 1 and autophagy levels (as measured by p62 and LC3 immunoblots) in U2OS that express doxycycline-inducible beclin 1 shRNA. Cells were cultured in the presence or absence of 1 μg/ml doxycycline for 4 days and subjected to starvation in HBSS for the indicated time period.
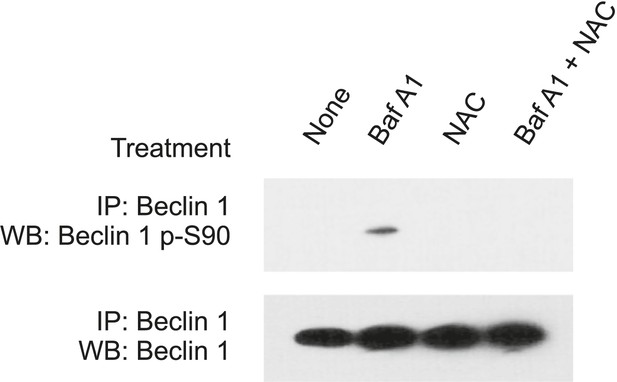
Bafilomycin A1-induced Beclin 1 S90 phosphorylation is reversed by the ROS scavenger, N-acetyl-L-cysteine.
HeLa cells were either untreated, pre-treated or not pre-treated with 5 mM N-acetyl-L-cysteine for 1 hr, followed by culture either in the presence or absence 100 nM Bafilomycin A1 for 3 hr. Baf A1, bafilomycin A1; NAC, N-acetyl-L-cysteine.

Effects of Beclin 1 S90 phosphorylation o on subcellular localization of the Beclin 1/VPS34/ATG14 complex.
Subcellular localization of Beclin 1, VPS34, and ATG14 in normal media or after starvation for 2 hr in HBSS in beclin 1 shRNA U2OS cells following treatment with 1 μg/ml doxycycline for 4 days and transfection with indicated shRNA-resistant Flag-Beclin 1 non-targetable wild-type (WT) or indicated mutant plasmids. Loading controls for the cytosol, mitochondria, and microscome fractions are GAPDH, TOM20, and PDI, respectively. Asterix denotes non-specific signal due to membrane edge artifact.
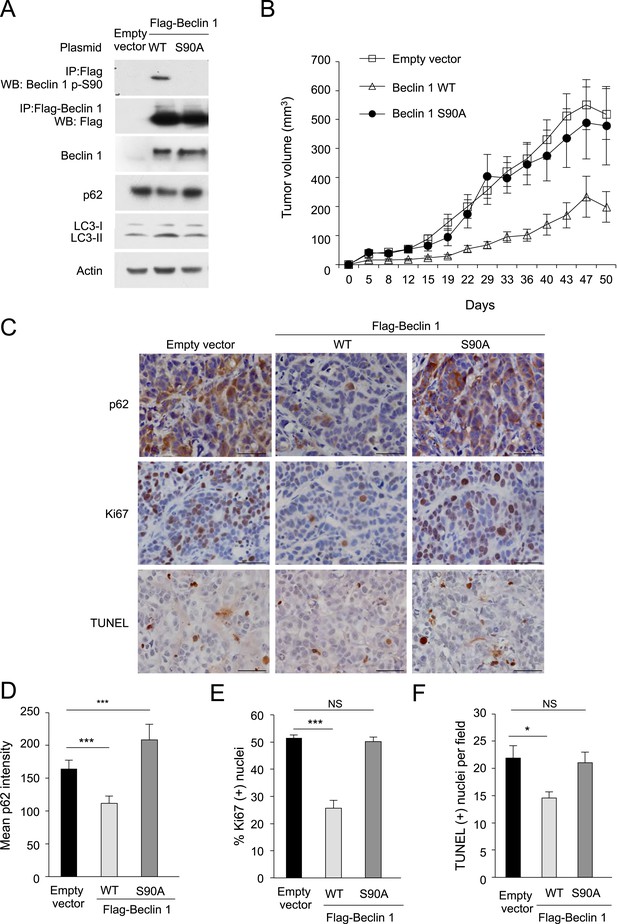
The Beclin 1 S90 phosphorylation site is required for tumor suppression function in MCF7 cells.
(A) Western blot detection of Beclin 1 p-S90, Flag-Beclin 1, p62, and LC3 in MCF7 cells stably transduced with retroviruses expressing indicated Beclin 1 construct. (B) Xenograft growth of cells in (A) in nu/nu mice. Tumor volumes shown represent mean + SEM for at least 11 mice per group. Differences in tumor growth among the different groups were analyzed using a linear mixed effect model; P = NS for Beclin 1 S90A vs empty vector; p < 0.001 for Beclin 1 WT vs empty vector. (C) Representative images of p62 staining, Ki67 staining, and TUNEL-labeling of indicated MCF7 xenograft tumor genotype. (D–F) Quantification of relative reciprocal p62 intensity per high-power field (D), percentage of Ki67-positive nuclei per high-power field (E) and number of TUNEL-positive nuclei per high power field (F). At least 10 randomly selected fields per xenograft section were analyzed by an observer blinded to genotype. Bars are mean + SEM for 5–8 xenografts per MCF7 xenograft tumor genotype. ***p < 0.001, **p < 0.01, *p < 0.05, NS, not significant; one-way ANOVA with Dunnett's test.
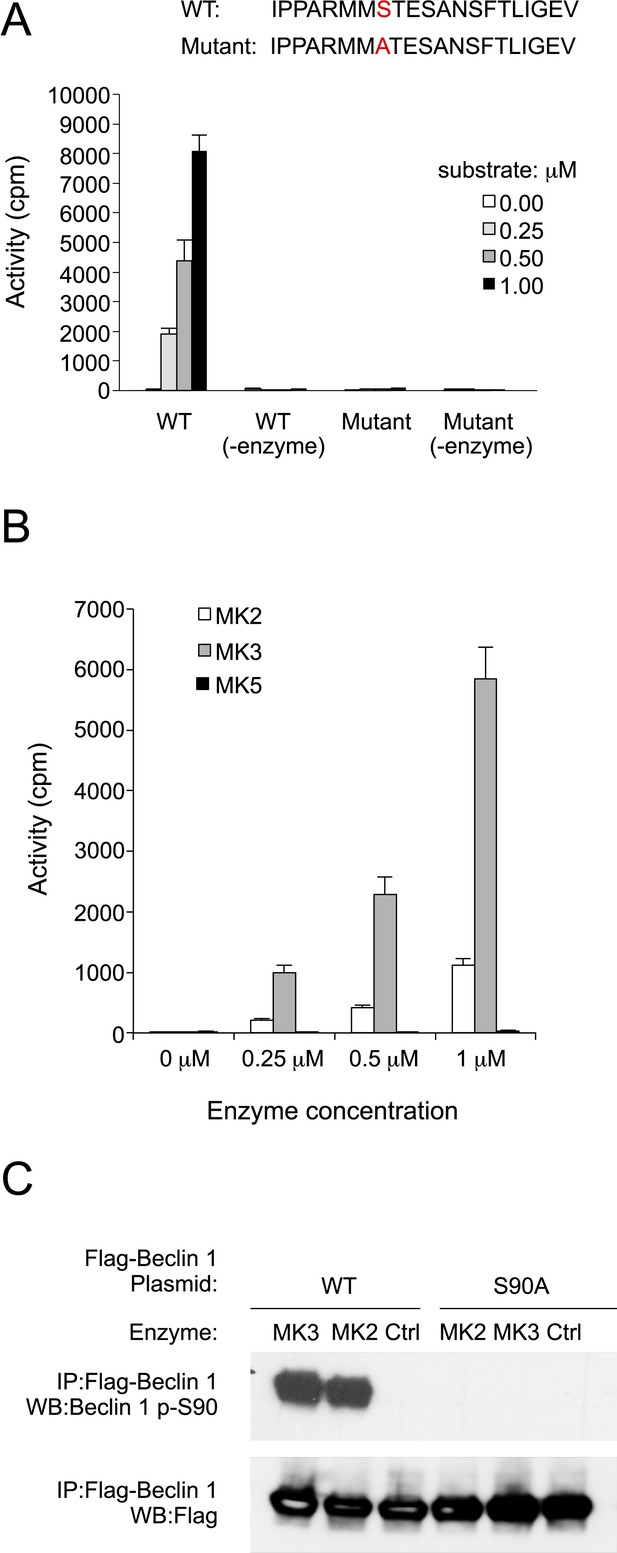
MK2 and MK3 mediate phosphorylation of Beclin 1 S90.
(A) In vitro kinase activity of MK3 using indicated Beclin 1 peptides as a substrate. (B) In vitro kinase activity of MK2, MK3, and MK5 using the wild-type (WT) Beclin 1 peptide shown in A as a substrate. (C) In vitro kinase activity of MK2 and MK3 using Flag epitope-tagged wild-type Beclin 1 or Beclin 1 S90 purified from HEK293T cells as a substrate. See also Figure 4—figure supplement 1 and Supplementary file 1.
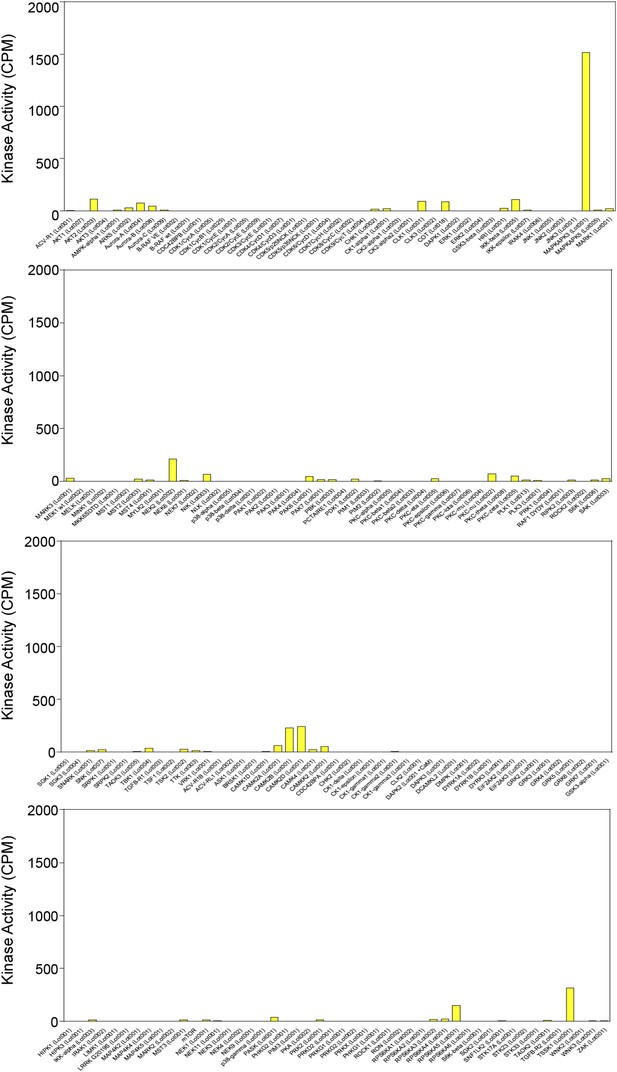
Results of in vitro kinase screen using the Beclin 1 83–97 peptide as a substrate.
Kinases included in screen are listed in alphabetical order on the x-axis. See Supplementary file 1 for details.
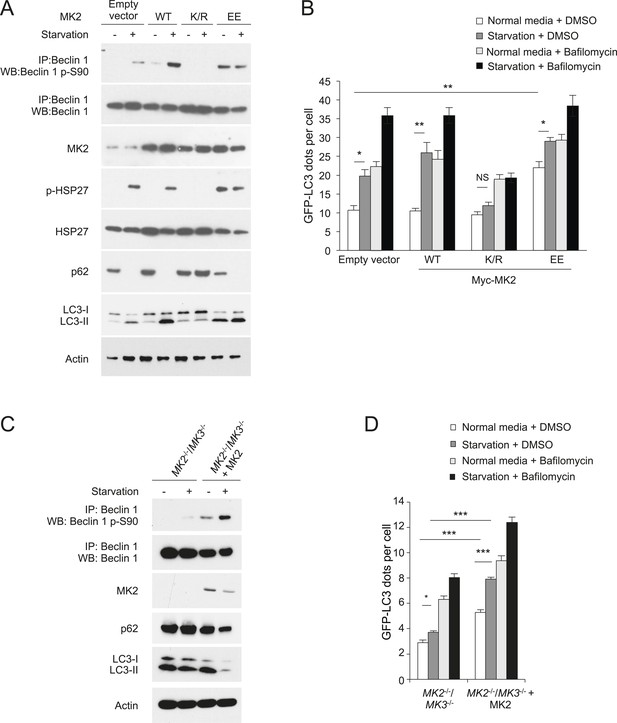
MK2 positively regulates autophagy.
(A) Effects of wild-type, dominant negative (K/R) and constitutively active (EE) MK2 on endogenous Beclin 1 S90 phosphorylation, MK2/MK3 activation (levels of p-HSP27) and autophagy (levels of p62 degradation and LC3-II conversion) in HeLa cells grown in normal medium (starvation−) or HBSS for 2 hr (starvation+). Actin is shown as a loading control. (B) Effects of wild-type, dominative negative (K/R) and constitutively active (EE) MK2 on autophagy (GFP-LC3 puncta numbers in the presence or absence of 100 nM bafilomycin A1) in HeLa cells grown in normal medium (starvation−) or HBSS for 3 hr (starvation+). Bars are mean + SEM of triplicate samples (>50 cells analyzed per sample). Similar results were observed in three independent experiments. **p < 0.01, *p < 0.05, NS, not significant; one-way ANOVA for indicated comparisons. (C) Beclin 1 S90 phosphorylation and autophagy (levels of p62 degradation and LC3-II conversion) in MK2−/−/MK3−/− MEFs and MK2−/−/MK3−/− MEFs stably transformed with wild-type MK2. Cells were grown in normal medium (starvation−) or HBSS for 2 hr (starvation+). (D) Quantitation of GFP-LC3 puncta (autophagosomes) in MK2−/−/MK3−/− MEFs and MK2−/−/MK3−/− MEFs stably transformed with wild-type MK2 during growth in normal media or HBSS (starvation) for 3 hr in the presence or absence of 100 nM bafilomycin A1. Bars are mean + SEM of triplicate samples (>50 cells analyzed per sample). Similar results were observed in three independent experiments. ***p < 0.001, NS, not significant; one-way ANOVA. p < 0.001 for the magnitude of change between normal and starvation conditions in MK2−/−/MK3−/− MEFs vs that in in MK2−/−/MK3−/− + MK2 MEFs; two-way ANOVA. See also Figure 5—figure supplement 1.
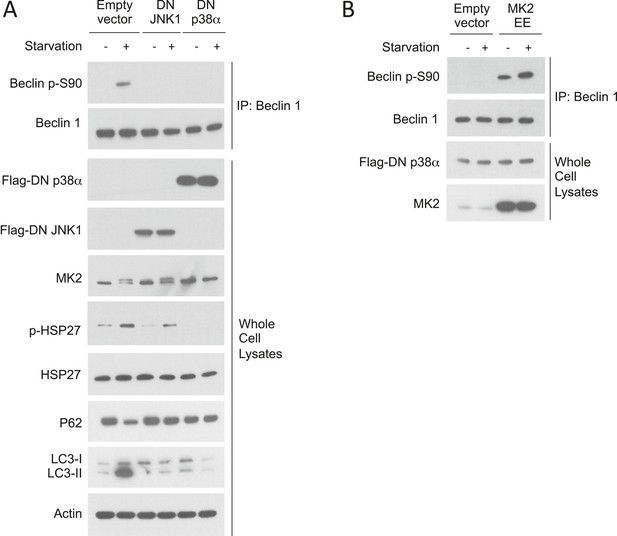
Dominant p38α inhibits starvation-induced MK2 activation and Beclin 1 S90 phosphorylation.
(A) Effects of dominant-negative (DN) JNK1 and DN p38α on Beclin 1 S90 phosphorylation, MK2 activation (MK2 band shift and p-HSP27) and autophagy (p62 degradation and LC3-II conversion) in HeLa cells grown in normal media (starvation−) or HBSS for 2 hr (starvation+). (B) Constitutively active MK2 (MK2 EE) blocks DN p38α suppression of starvation-induced Beclin 1 S90 phosphorylation. HeLa cells were co-transfected with plasmids expressing Flag-DN p38α and either empty vector or MK2 EE and grown in normal media (starvation−) or HBSS for 2 hr (starvation+).
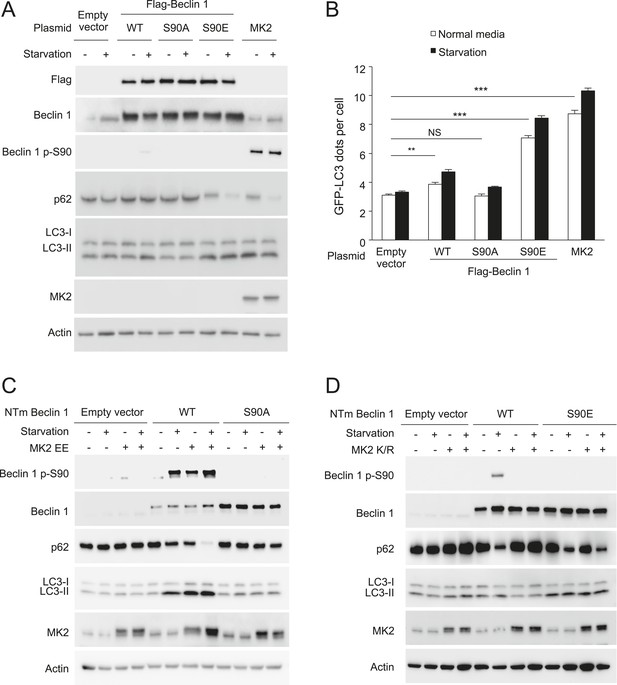
MK2 positively regulates autophagy through the Beclin 1 S90 phosphorylation site.
(A) Western blot detection of Beclin 1 S90 phosphorylation and autophagy (p62 degradation and LC3-II conversion) in MK2−/−/MK3−/− MEFs retrovirally transduced with indicated expression constructs and grown in normal media or HBSS (starvation) for 4 hr. (B) Quantification of GFP-LC3 puncta (autophagosomes) in MK2−/−/MK3−/− MEFs retrovirally transduced with indicated expression constructs and grown in normal media or HBSS (starvation) for 4 hr. Bars are mean + SEM of triplicate samples (>50 cells analyzed per sample). Similar results were obtained in two independent experiments. ***p < 0.01, NS, not-significant; one-way ANOVA with Dunnett's test. (C) Effects of constitutively active MK2 (MK2 EE) on Beclin 1 S90 phosphorylation and autophagy (as measured by p62 and LC3 western blot analysis) in U2OS doxycycline-inducible beclin 1 shRNA knockdown cells expressing either shRNA-resistant wild-type Flag-Beclin 1 or mutant Flag-Beclin 1 S90A. Cells were treated with 1 μg/ml doxycycline for 4 days prior to western blot analyses with indicated antibodies and transfected with indicated shRNA-resistant plasmids after 2 days of doxycycline treatment. Cells were grown in normal media or HBSS (starvation) for 2 hr. NTm, non-targeting mutant. (D) Effects of dominant-negative MK2 (MK2 K/R) on Beclin 1 S90 phosphorylation and autophagy (as measured by p62 and LC3 western blot analysis) in U2OS doxycycline-inducible beclin 1 shRNA knockdown cells expressing either shRNA-resistant wild-type Flag-Beclin 1 or mutant Flag-Beclin 1 S90A. Cells were treated with 1 μg/ml doxycycline for 4 days prior to western blot analyses with indicated antibodies, and transfected with indicated shRNA-resistant plasmids after 2 days of doxycycline treatment. Cells were grown in normal media or HBSS (starvation) for 2 hr. NTm, non-targeting mutant.
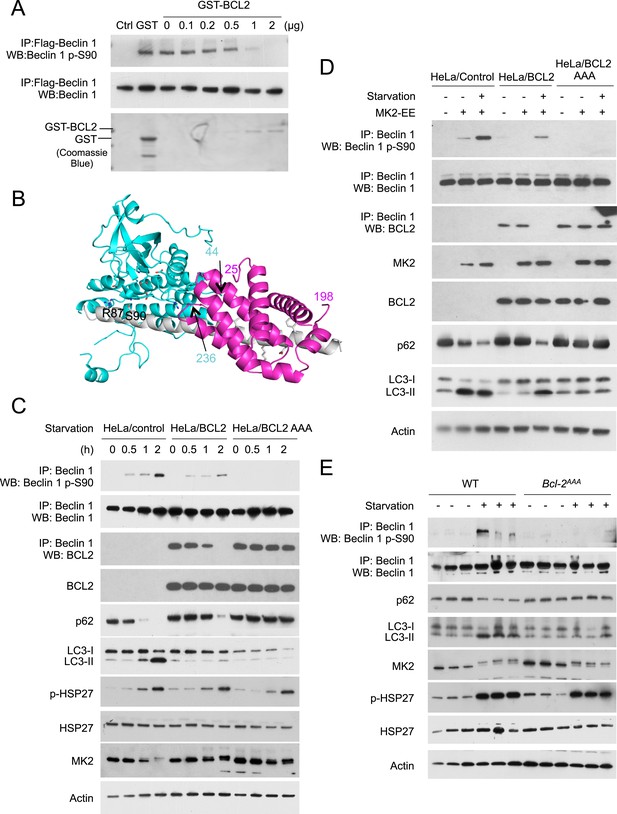
BCL2 inhibits MK2-dependent Beclin 1 S90 phosphorylation.
(A) In vitro kinase activity of MK2 using Flag-Beclin 1 purified from HEK293 cells as a substrate in the presence of the indicated amount of GST-BCL2. (B) Structural model showing that steric hindrance may prevent simultaneous binding of BCL2/BCL2L1 and MK2 to Beclin 1. MK2 (residues 44–216, 236–284 and 288–345), BCL2L1 (residues 2–25 and 83–198) and Beclin 1 (residues 79–181) are shown in cyan, pink and grey ribbon, respectively. MK2 residues important for catalysis and Beclin 1 residues important for binding to BCL2L1, or for phosphorylation (S90 and R87) are shown in stick, with atoms colored by element type: oxygen, red; nitrogen, blue; sulfur, green; and carbon, cyan or grey for MK2 or Beclin 1 respectively. Numbers indicate the last residue preceding or following regions missing from this model, that might cause further steric conflicts. (C) Detection of Beclin 1 S90 phosphorylation and autophagy levels (as measured by p62 and LC3 immunoblots) in HeLa cells stably transfected with empty vector (HeLa/control), wild-type BCL2 (HeLa/BCL2), or a BCL2 T69A/S70A/S87A mutant (HeLa/BCL2 AAA) and subjected to starvation in HBSS for the indicated time period. p-Hsp27 protein levels are shown as an indicator of MK2 activation. (D) Detection of Beclin 1 S90 phosphorylation and autophagy levels (as measured by p62 and LC3 immunoblots) in HeLa/Control, HeLa/BCL2, and HeLa/BCL2 AAA cells transiently transfected with a control empty vector or constitutively active MK2 (MK2 EE) and grown in normal medium (starvation−) or HBSS for 2 hr (starvation+). (E) Starvation-induced Beclin 1 S90 phosphorylation and autophagy induction in vastus lateralis muscle of Bcl2AAA mice and control wild-type littermates. Mice were subjected to starvation for 48 hr prior to tissue collection and western blot analysis of muscle lysates with indicated antibodies. Each lane represents a muscle sample from an independent mouse.
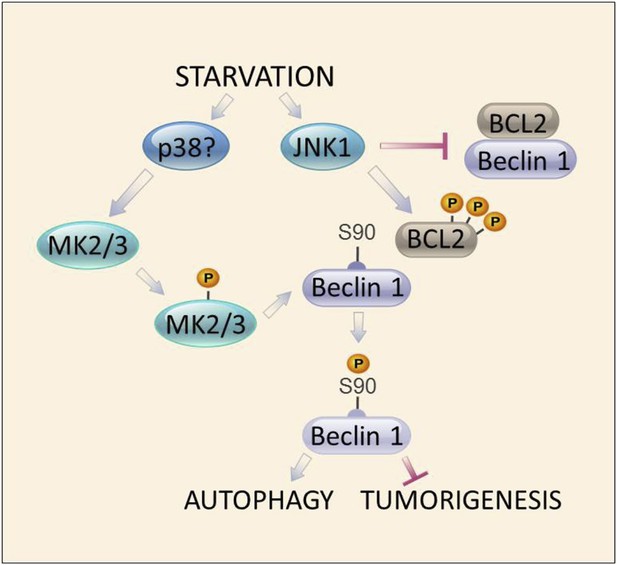
Model of convergent functions of MAPK signaling molecules in amino acid starvation-induced autophagy and regulation of Beclin 1 S90 phosphorylation.
In response to nutrient starvation, JNK1 results in multi-site phosphorylation of BCL2 and disruption of BCL2/Beclin 1 binding (Wei et al., 2008). This permits active MK2/3 to phosphorylate Beclin 1 at residue serine 90 (Figures 4–5), which is essential for the autophagy and tumor suppressor function of Beclin 1 (Figures 2–3). Mutations in BCL2 that block multi-site phosphorylation by JNK1 (and block starvation-induced disruption of BCL2/Beclin 1 binding [He et al., 2012]) block MK2-mediated Beclin 1 S90 phosphorylation both in vitro and in vivo (Figure 7). The MAPK signaling molecule, p38, may function upstream to activate MK2/MK3 during amino acid starvation (Figure 5—figure supplement 1); however, a definitive role for other kinases has not been excluded. Together, our data suggest a model in which autophagy activation during amino acid starvation requires two simultaneous functions of MAPK signaling molecules: (1) MK2/MK3-mediated Beclin 1 S90 phosphoryation; and (2) JNK1-mediated disruption of BCL2 binding to Beclin 1 (which functions to block MK2/MK3-mediated Beclin 1 S90 phosphorylation).
Additional files
-
Supplementary file 1
Raw data of in vitro kinase screen with 190 kinases using the Beclin 1 peptide spanning from amino acids 83–97 as a substrate.
- https://doi.org/10.7554/eLife.05289.016





