A cortical disinhibitory circuit for enhancing adult plasticity
Figures
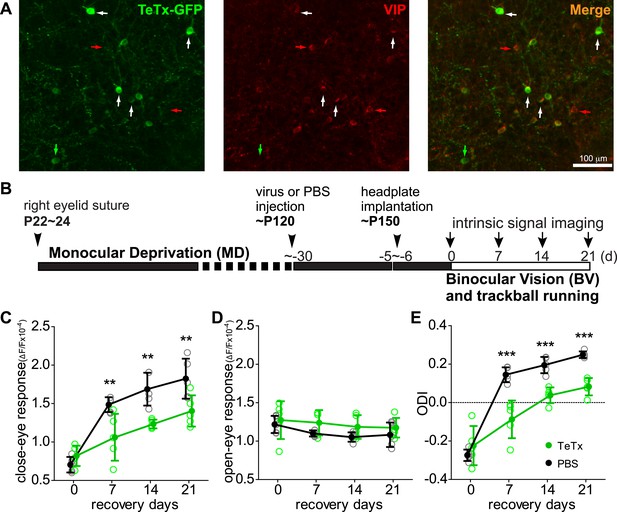
Synaptic transmission from VIP neurons is necessary for the enhancement of recovery of the amblyopic eye by running.
(A) Representative fluorescent images of binocular V1 area from a VIP-Cre mouse injected with AAV-DIO-TeTx-GFP. Slices were immunostained for GFP to indicate viral infected neurons and VIP for VIP-positive neurons. White arrows indicate the cells positive for both GFP and VIP staining. Red arrows indicate the cells stained positive for VIP only; green arrow indicates a cell positive only for GFP. (B) Experimental schedule. (C and D) Changes in intrinsic signal responses evoked by the visual stimulation through the closed eye (C) and the open eye (D) in AAV-DIO-TeTx-injected (VIP-TeTx, n = 5) experimental and PBS-injected control mice (PBS, n = 6). (E) Ocular dominance index (ODI) computed from responses to contralateral (closed) and ipsilateral (open) eyes shown in (C and D). ODI represents normalized difference in response magnitude between the two eyes; higher ODI indicates more domination of the contralateral eye. Open circles represent measurements in individual animals, solid circles indicate mean of the open circles. (Data are plotted as mean ± S.D., ***p < 0.001, **p < 0.01 between groups at given time point; Two-way ANOVA followed by multiple comparisons with Bonferroni correction).
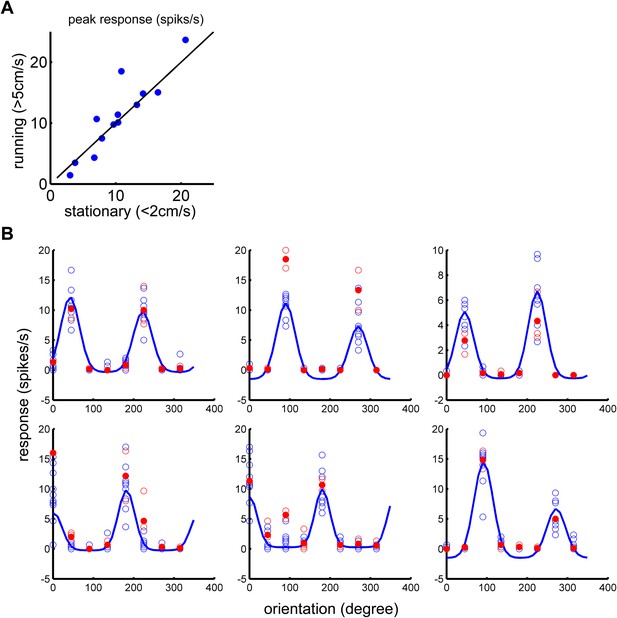
Silencing the synaptic transmission of VIP neurons abolished the effect of running on visually responsive neurons.
(A) AAV-DIO-TeTx-GFP was injected into the V1 of VIP-Cre mice. The visual response to the drifting gratings was examined by silicon tetrode recording 4 weeks after viral injection. Peak responses of the preferred orientation were compared between running and stationary states. Each dot represents visual responses of one cell (n = 13 from two animals). (B) Data showing representative visual responses of six cells. Orientation tuning curves (blue traces) were plotted using the average visual responses during stationary state (blue open circles, each circle represent one repetition of visual stimulation at indicated orientation). Open red circles represent visual responses during running state, and the solid red circles represent the average of open red circles at indicated orientation.
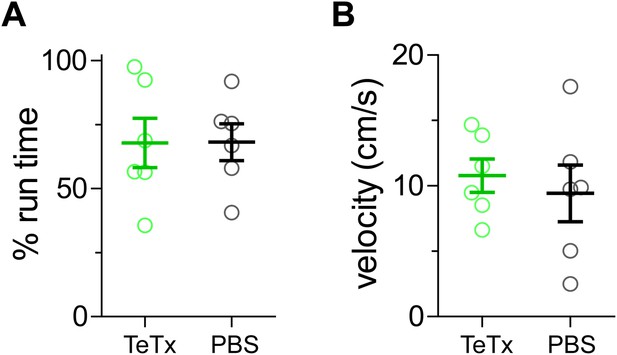
VIP-Cre mice injected with AAV-DIO-TeTx-GFP (TeTx) ran similarly to PBS-injected control mice (PBS).
(A) The percentage of running time during the 4-hr visual stimulation on day 14 of recovery from amblyopia. (B) The average running velocity during the running state. Each circle represents one mouse. (Data are plotted as mean ± S.D., p > 0.05 between TeTx and PBS groups).
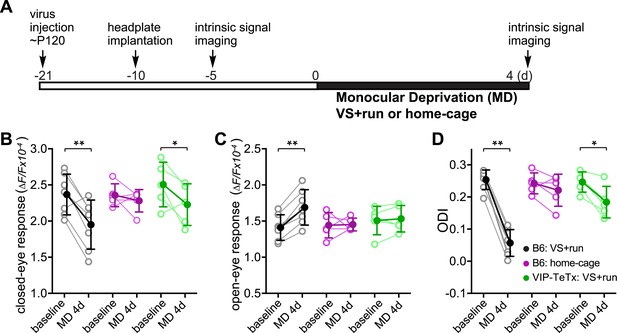
Synaptic transmission from VIP neurons facilitates the enhancement of ocular dominance plasticity in adult mice by running.
(A) Experimental schedule. (B and C) Amplitudes of intrinsic signal responses evoked by the visual stimulation through the closed eye (B) and the open eye (C) before (baseline) and after 4-day monocular deprivation of the contralateral eye (MD 4d). (D) Ocular dominance index (ODI) computed from response amplitude to contralateral (closed) and ipsilateral (open) eyes shown in (B and C). Open circles represent measurements in individual animals. B6: C57BL/6J wild type mice (VS + run: n = 7; home-cage: n = 5); VIP-TeTx: VIP-Cre mice that received a cortical injection of AAV-DIO-TeTx and treated with VS + run during MD (n = 5). Solid circles represent the average of the corresponding open circles or open triangles (±S.D.). *p < 0.05, **p < 0.01 between baseline and after MD 4d; two-way ANOVA followed by multiple comparisons with Bonferroni correction.
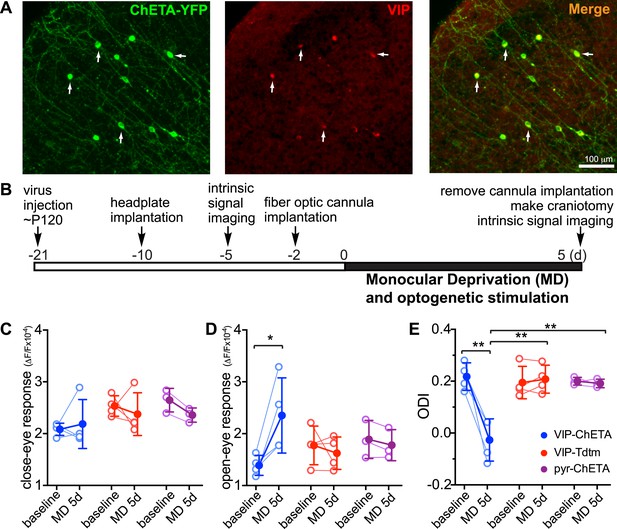
Activation of VIP neurons is sufficient to enhance visual cortical plasticity in adult mice.
(A) Representative fluorescent images of binocular V1 area from a VIP-Cre mouse injected with AAV-DIO-ChETA-YFP. The slices were immunostained for YFP to indicate viral infected neurons and VIP for VIP-positive neurons. White arrows indicate the cells positive for both YFP and VIP staining. (B) Experimental schedule. (C and D) Changes in intrinsic signal responses evoked by the visual stimulation through the closed eye (C) and the open eye (D). VIP-Cre mice were injected with AAV-DIO-ChETA-YFP (VIP-ChETA, n = 4), AAV-DIO-TdTomato (VIP-Tdtm, n = 4), or AAV-ChETA (pyr-ChETA, n = 3). (E) Ocular dominance index (ODI) computed from response amplitude to contralateral (closed) and ipsilateral (open) eyes shown in (C and D). Open circles represent measurements in individual animals, and solid circles indicate mean of the open circles. (Data are plotted as mean ± S.D., *p < 0.05, **p < 0.01; paired t-test for comparing baseline and MD 5d of the VIP-ChETA group; other comparisons were analyzed with the two-way ANOVA followed by multiple comparisons with Bonferroni correction).
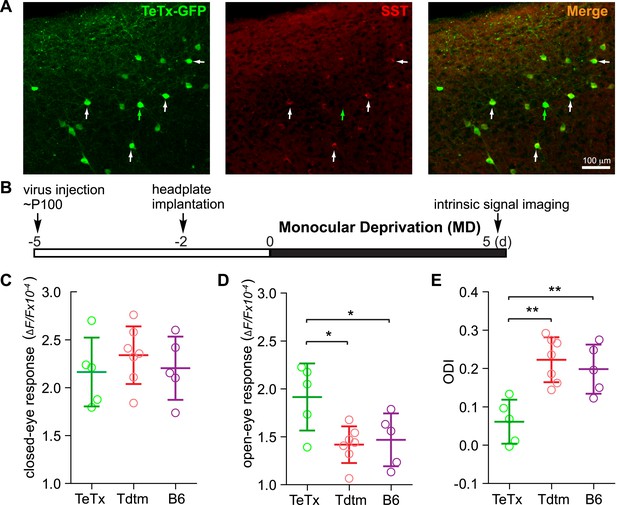
Short-term silencing of SST neurons is sufficient to enhance visual cortical plasticity in adult mice.
(A) Representative fluorescent images of the binocular area in V1 from SST-Cre mouse injected with AAV-DIO-TeTx-GFP. The slices were immunostained for GFP to indicate viral infected neurons and SST for SST-positive neurons. White arrows indicate the cells positive for both GFP and SST staining, and green arrow indicates a cell positive only for GFP. (B) Experimental schedule. (C and D) Amplitudes of intrinsic signal responses evoked by visual stimulation through the closed eye (C) and the open eye (D), in SST-Cre mice treated with AAV-DIO-TeTx-GFP (TeTx, n = 5) or with AAV-DIO-TdTomato (Tdtm, n = 7) and in C57BL/6J mice (B6, n = 5). (E) Ocular dominance index (ODI) computed from response amplitude to contralateral (closed) and ipsilateral (open) eyes shown in (C and D). Open circles represent measurements in individual animals. (Data are plotted as mean ± S.D., *p < 0.05, **p < 0.01; One-way ANOVA followed by multiple comparisons with Bonferroni correction).
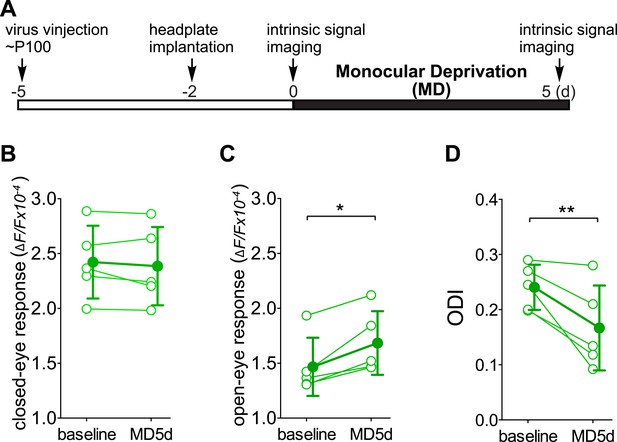
Chronic intrinsic signal imaging confirms that the short-term silencing of SST neurons is sufficient to enhance visual cortical plasticity in adult mice by specifically potentiating open-eye response.
(A) Experimental schedule. (B and C) Amplitudes of intrinsic signal responses evoked by visual stimulation through the closed eye (B) and the open eye (C) in SST-Cre mice treated with AAV-DIO-TeTx-GFP (n = 5) before (baseline) and after 5 days of monocular deprivation of the contralateral eye (MD 5d). (D) Ocular dominance index (ODI) computed from response amplitude to contralateral (closed) and ipsilateral (open) eyes shown in (B and C). Open circles represent measurements in individual animals. Error bars represent mean ± S.D., *p < 0.05, **p < 0.01; paired t-test.





