Sensitivity and kinetics of signal transmission at the first visual synapse differentially impact visually-guided behavior
Figures
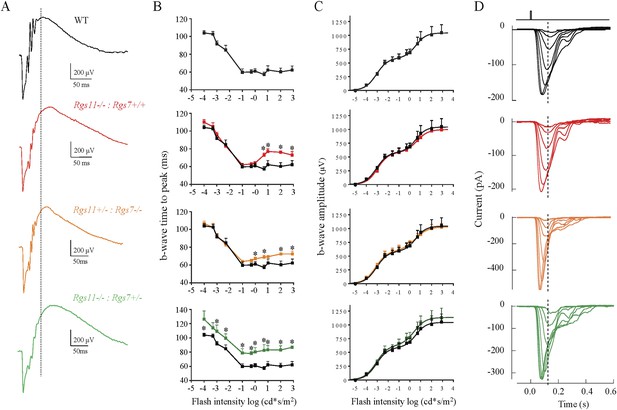
Effects of RGS haploinsufficiency on ON-BC properties.
(A) Representative electroretinography (ERG) traces of RGS11 knockout (Rgs11−/−), RGS7 knockout (Rgs7−/−) alone or in combination with haploinsufficiency of the other (Rgs11−/−, Rgs7+/− and Rgs11+/−, Rgs7−/−). Dashed line shows position of the response peak recorded at the dimmest flash. (B) Quantification of ERG b-wave onset timing across increasing light intensities. Some genotypes showed minor delay in the onset of b-wave at moderate to high light intensities (>1 cd*s/m2). (C) Dependence of ERG b-wave amplitude on flash intensity (>1 cd*s/m2 flashes [t-test, p > 0.5, n = 4–5]). Maximal amplitudes or sensitivities of b-waves were not affected in any of the genotypes. (D) Analysis of rod ON-BC light responses by single cell recordings. Responses of rod ON-BCs to flashes of light that varied in strength by factors of 2, from 0.5–23 R*/rod.
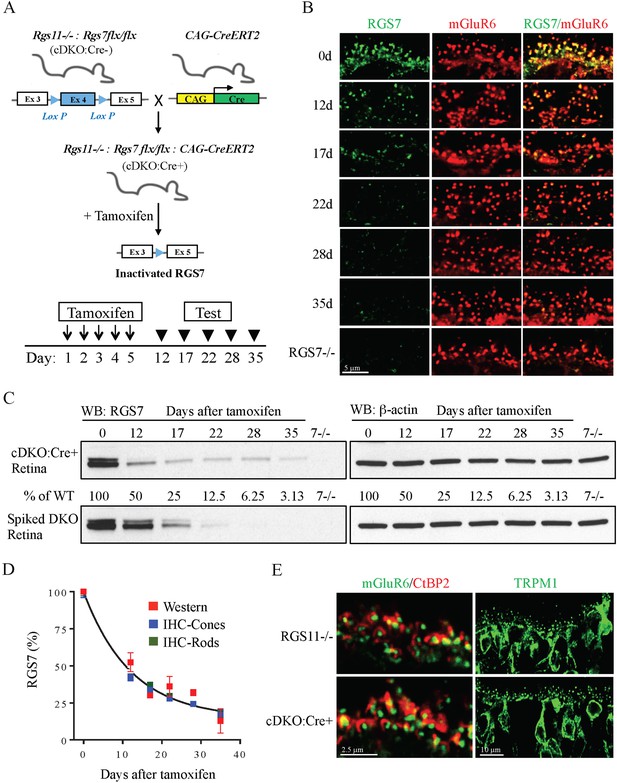
Progressive RGS7 loss in the ON-BC neurons of a conditional mouse knockout model (cDKO).
(A) Schematic representation of a breeding strategy for the conditional inactivation of RGS7 on Rgs11−/− background. To induce recombination and RGS7 loss mice were administered tamoxifen for 5 days followed by testing. (B) Immunohistochemical analysis of RGS7 expression and localization in the outer plexiform layer of mouse retinas following tamoxifen administration. Retina sections were stained with RGS7 (green) and mGluR6 (red) antibodies from cDKO:Cre+ mice at 12, 17, 22, 28, and 35 days after the start of tamoxifen administration. Retinas lacking RGS7 (Rgs7−/−) were used as a control. Two independent experiments yielding similar data were conducted. Note progressive loss of RGS7 immunoreactivity from postsynaptic puncta denoted by mGluR6. (C) Analysis of changes in RGS7 expression in total retina lysates by Western blotting. cDKO:Cre+ mice were treated with tamoxifen and retinas were collected at indicated time points. To generate retina lysates containing various RGS7 protein content, varying amounts of RGS11 knockout lysates were spiked into lysates isolated from RGS7 and RGS11 double knockout retinas (DKO). To determine the level of RGS7 reduction, a calibration curve was plotted from densities of varying amounts of RGS7 protein and used to determine the protein content in retina extracts obtained from tamoxifen-treated mice. Retinas from three separate animals were used in these experiments. (D) Quantification of RGS7 protein content at different time points following tamoxifen treatment. Values representing density of RGS7 band (red) and fluorescence intensity in mGluR6-positive puncta determined separately for rods (green) and cones (blue) were normalized to respective values observed before tamoxifen treatment (day 0). Averaged RGS7 percentages from western and IHC experiments are fitted with a single exponential decay function. Error bars are SEM values. (E) Intact synaptic morphology and retina cytoarchitecture at 28 days post-tamoxifen treatment. Retina cross-section from Rgs11−/− and cDKO:Cre+ mice were co-stained with postsynaptic marker mGluR6 and pre-synaptic marker CtBP2 and their direct apposition was noted. Immunostaining with TRPM1 (green) reveals intact morphology of ON-BC, their dendritic branching and distribution of postsynaptic puncta. Figure 2—figure supplement 1: assignment of rod vs cone synapses by immunohistochemistry. Figure 2—figure supplement 2: intact synaptic morphology and retina cytoarchitecture at 28 days post-tamoxifen treatment.
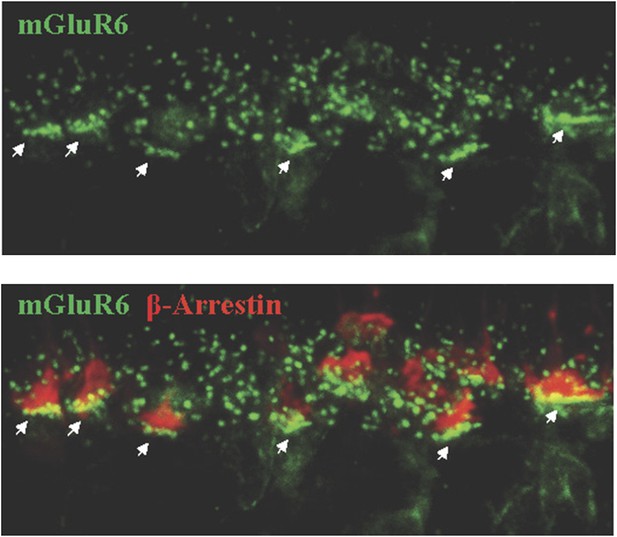
Assignment of rod vs cone synapses by immunohistochemistry.
Cone synapses (indicated by arrows) were distinguished by their position in the lower portion of the OPL where they form rows of clusters in apposition to cone pedicles (counter-stained for β-arrestin).
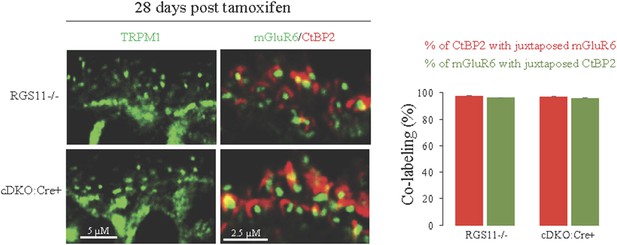
Intact synaptic morphology and retina cytoarchitecture at 28 days post-tamoxifen treatment.
Retina cross-sections from Rgs11−/− and cDKO:Cre+ mice were co-stained with postsynaptic marker mGluR6 and pre-synaptic marker CtBP2 and their direct apposition was quantified (right). Immunostaining with TRPM1 reveals intact morphology of ON-BC, their dendritic branching and distribution of postsynaptic puncta (left).
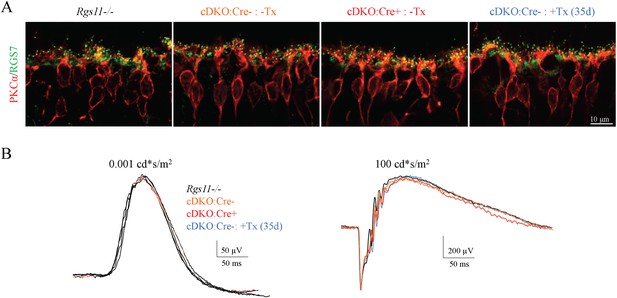
Expression of Cre recombinase without tamoxifen induction and tamoxifen administration without Cre expression do not alter RGS7 expression, localization and light response of mice.
(A) Immunohistochemical analysis of RGS7 (green) and PKCα (red) expression and localization in the outer plexiform layer of Rgs11−/−, cDKO:Cre-, and cDKO:Cre+ mouse retinas. (B) Representative ERG traces elicited from scotopic (left) and photopic (right) flashes in mice described above show unaltered retina electrophysiological responses.
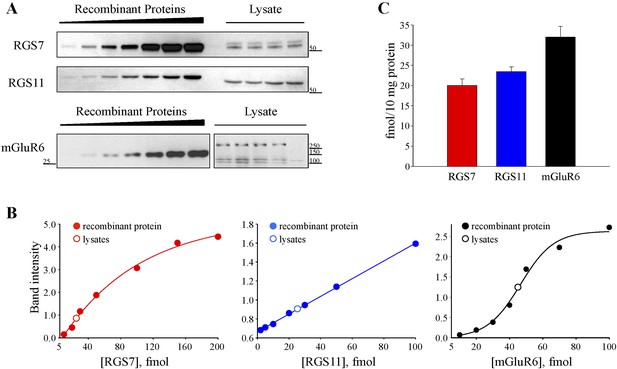
Quantification of RGS7, RGS11 and mGluR6 proteins in the retina.
(A) Representative Western blots of retina lysates analyzed alongside with recombinant standards. (B) Quantification of protein content. A calibration curve was plotted from densities of recombinant protein standards (closed circles) and used to determine the protein content (open circles) in retina extracts obtained from four separate mice. (C) Comparison of absolute RGS7, RGS11 and mGluR6 protein levels in the retinas. The experiment was performed 3 times. Error bars indicate the SEM. Figure 2—figure supplement 1: distribution of RGS7 immunofluorescence in the retina.
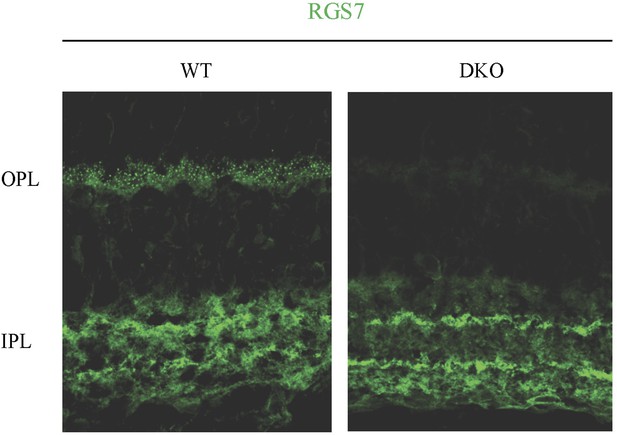
Distribution of RGS7 immunofluorescence in the retina.
Retina cross-sections were stained with anti-RGS7 antibodies as described in the ‘Materials and methods’. Staining reveals the presence of specific staining only in the outer plexiform layer.
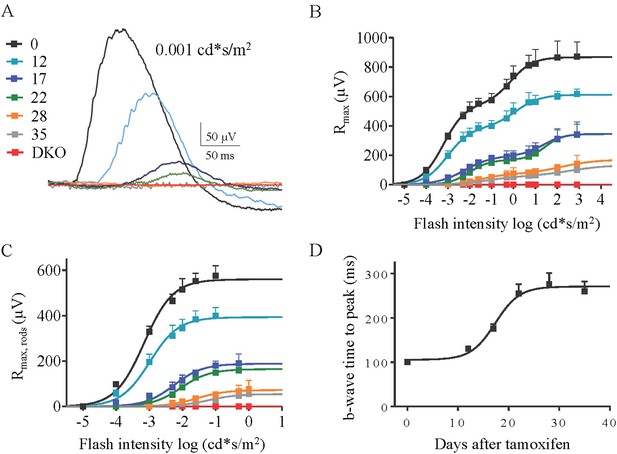
The effect of progressive RGS loss on rod ON-BC responses as revealed by scotopic ERG.
(A) Representative ERG responses elicited with dim flashes (0.001 cd*s/m2) from cDKO:Cre+ mice at 0, 12, 17, 22, 28, and 35 days post-tamoxifen administration. (B) Maximal ERG b-wave amplitudes plotted against their eliciting flash intensities. The ERG b-waves exhibit biphasic saturation pattern, with first one around 0.05 cd*s/m2, corresponding to activity of rod ON-BC, and a second one around 100 cd*s/m2, corresponding to activity of cone ON-BC. (C) Rod component of the ERG response extracted from fitting the first phase of the response in panel B. (D) Analysis of the changes in the onset of ERG b-wave elicited by half-saturating flashes. Time to peak values are plotted as a function of days after tamoxifen treatment. Errors bars are SEM.
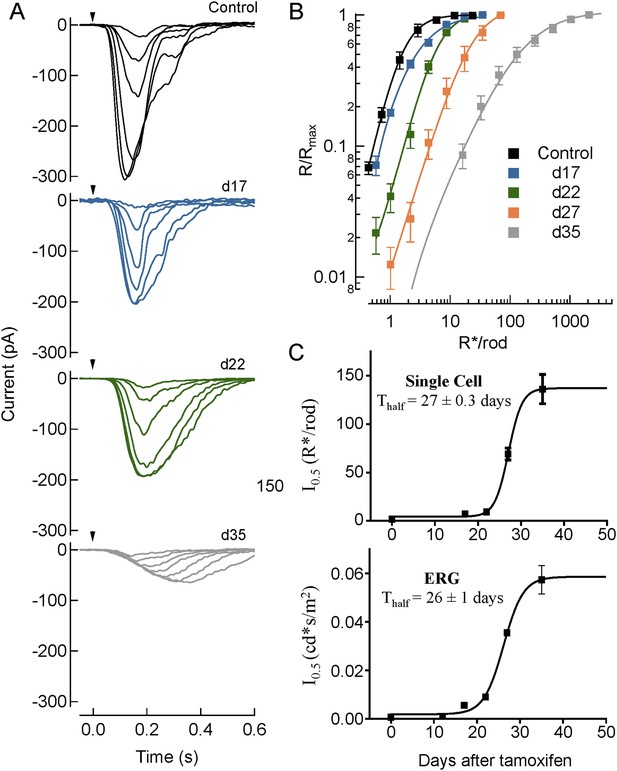
Single cell recordings from rod ON-BC recapitulate key changes in average light-evoked responses measured by ERG.
(A) Responses of rod ON-BCs to flashes of increasing strength. Shown are representative response families for 10 ms flashes (arrow) measured at an increasing number of days following tamoxifen administration. Flash strengths yielded 0.70, 1.4, 2.9, 5.7, 11, and 23 R*/rod for control (Rgs7+/+:Rgs11−/−), 0.58, 1.0, 2.2, 4.3, 8.6 and 17 R*/rod for day 17; 1.0, 2.2, 4.3, 8.7, 17, and 35 R*/rod for day 22, and 16, 32, 65, 130, 260, 520, and 1000 R*/rod for day 35. Note with time following tamoxifen administration the progressive slowing and reduction in maximum amplitude of the light-evoked response (see Table 3). (B) Average normalized response-intensity relationships for all cells recorded for control (day 0) as well as days 17, 22, 28, and 35 following tamoxifen administration. Data were sampled at 1 kHz and filtered at 50 Hz. (C) Comparison of half-saturation responses from ON-BC measured by single-cell recordings (top) (n = 4–11) and ERG (bottom) (n = 5) from cDKO:Cre+ mice at different days after tamoxifen administration. Data points plotted are half-maximal values from each tamoxifen time point fitted by sigmoidal dose-response (variable slope) equation with the least squares method. The time required for tamoxifen to reduce the sensitivity of ON-BC by half is not different when obtained by ERG (28 ± 1 days) vs single cell patch-clamp (27 ± 0.3 days). Errors bars are SEM.
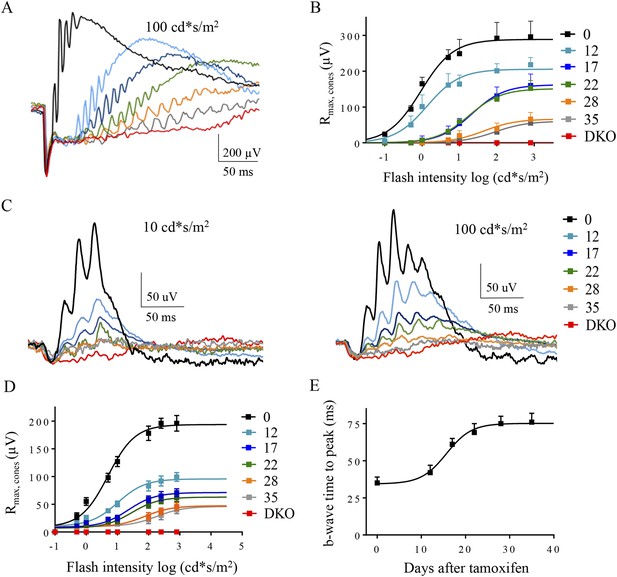
The effect of progressive RGS loss on cone ON-BC responses as revealed by photopic ERG.
(A) Representative dark-adapted ERG responses elicited with photopic (100 cd*s/m2) flashes from cDKO:Cre+ mice at 0, 12, 17, 22, 28, and 35 days post-tamoxifen administration. (B) Cone-generated component of the ERG response extracted from fitting the second phase of the response in Figure 5B. (C) Representative light-adapted ERG responses elicited with photopic flashes (10 cd*s/m2, left) and (100 cd*s/m2, right). Steady background light of 50 cd/m2 was applied to saturate rods. (D) ON-BC dose-response plot of maximal ERG b-wave amplitudes recorded with background light and plotted against their eliciting flash intensities. (E) Analysis of the changes in the ERG b-wave onset timing elicited by half-saturating flash intensities on a light background. Errors bars are SEM. Figure 2—figure supplement 1: isolation of the ERG b-wave peak.
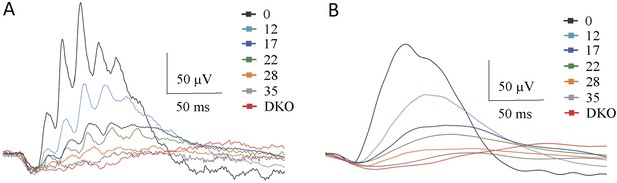
Isolation of the ERG b-wave peak.
(A) Single traces of light-adapted ERG responses elicited with photopic flashes of 100 cd*s/m2. (B) Oscillatory potentials are removed from traces by a smoothing algorithm. Steady background light of 50 cd/m2 was applied to saturate rods. See details in the ‘Materials and methods’.
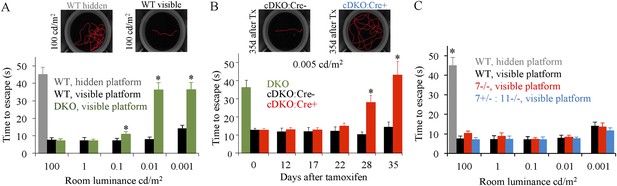
Evaluation of scotopic vision using water maze behavioral test.
(A) Development and validation of visually-guided behavioral task for the assessment of mouse vision. Mice were trained to find a randomly placed visible escape platform in a water maze. Their tracks were recorded and used to determine times to escape from water. Under bright photopic light environment, mice with constitutive elimination of RGS7 and RGS11 (DKO) find visible (7 ± 1 s), but not hidden (45 ± 4 s) platform as readily as wild-type mice (7 ± 1 s). Error bars are SEM (*p < 0.05, t-test, n = 4–5). Escape latencies thus served as a measure of their visual abilities. The room luminance was then decreased in a step-wise fashion followed by the re-assessment of mouse performance. DKO animals performed significantly worse than wild-type (WT) mice in the task at luminance levels ≤0.1 cd/m2 (*p < 0.05, t-test, n = 5 for both groups). (B) Behavioral performance of the conditional knockout mice (cDKO) mice in the visual task upon induction of RGS7 loss by tamoxifen administration. Mice were assessed under pure scotopic conditions equating to luminance of 0.005 cd/m2. Performances of cDKO:Cre+ and cDKO:Cre- littermates were compared across days after tamoxifen treatment. At days 28 and 35 cDKO:Cre+ mice performed comparably to DKO mice, taking 28 ± 4 and 42 ± 7 s (vs 36 ± 4 s for DKO) to find the platform (p > 0.05, One-Way ANOVA, n = 5 for both groups). In comparison, performance of cDKO:Cre+ mice lagged that of cDKO:Cre- littermates at day 28 (28 ± 4 vs 10 ± 2 s) and day 35 (42 ± 7 vs 14 ± 3 s) (*p < 0.05, t-test for each time point, n = 5 for each group). (C) Elimination of RGS7 or RGS11 along with RGS7 haploinsufficiency does not affect mouse performance in the visual discrimination water maze task. Behavioral performance of WT, Rgs7−/−, and Rgs7+/−:Rgs11−/− mice was assessed in the water maze based behavioral task across various luminance levels covering the photopic and scotopic ranges. Performance was compared among all genotypes at each specific luminance level.
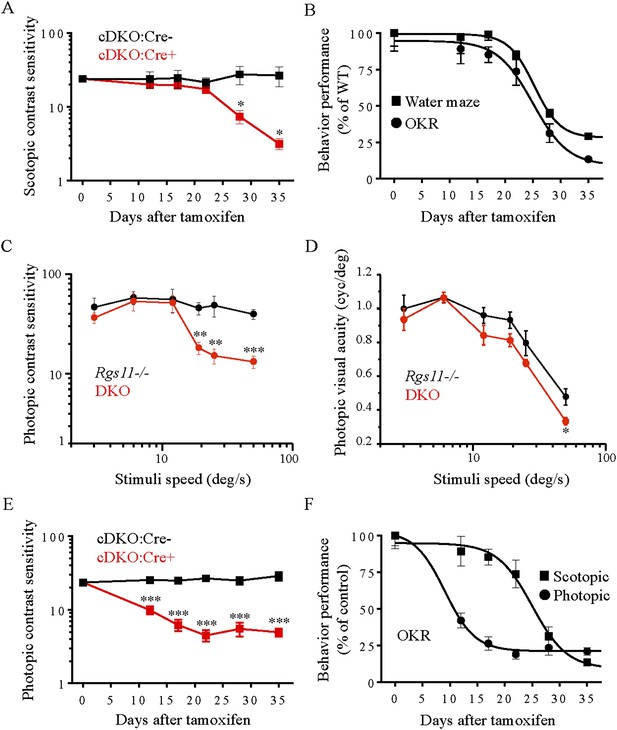
Evaluation of scotopic and photopic mouse vision by optomotor task.
(A) Scotopic (∼3 × 10−4 cd/m2) contrast sensitivity deficits in mice lacking RGS proteins (cDKO:Cre+) vs cDKO:Cre- mice as a function of time of post-tamoxifen administration (*p < 0.05, t-test, n = 4). (B) Comparison of visual performance of mice in water maze task and optomotor response tests. Mouse vision declined by half after 25 ± 1 days of tamoxifen administration in both experiments. (C) The reliance of high speed contrast sensitivity on intact ON-BC function. Deficits in photopic (70 cd/m2) contrast sensitivity of DKO mice are apparent at stimuli speeds of >12 deg/s (error bars are SEM; t-test: **p < 0.01, ***p < 0.001, n = 4–5). (D) Visual acuity is mainly unaffected by the loss of RGS11 together with RGS7 (DKO) as compared to Rgs11−/− controls. (error bars are SEM. t-test: *p < 0.05, n = 4–5 mice). (E) Photopic (70 cd/m2) contrast sensitivity deficits in mice lacking RGS proteins (cDKO:Cre+) vs cDKO:Cre- controls as a function of time of post-tamoxifen administration (error bars are SEM; t-test: ***p < 0.001, n = 7). (F) Comparison of progressive RGS elimination effects on scotopic vs photopic contrast sensitivities of mice. The half-time of contrast sensitivity decline is 25 ± 1 days for scotopic vs 9.0 ± 2 days for photopic conditions, respectively. The data for each group are normalized to their respective values upon beginning of the tamoxifen treatment (day 0) and expressed as percentage.
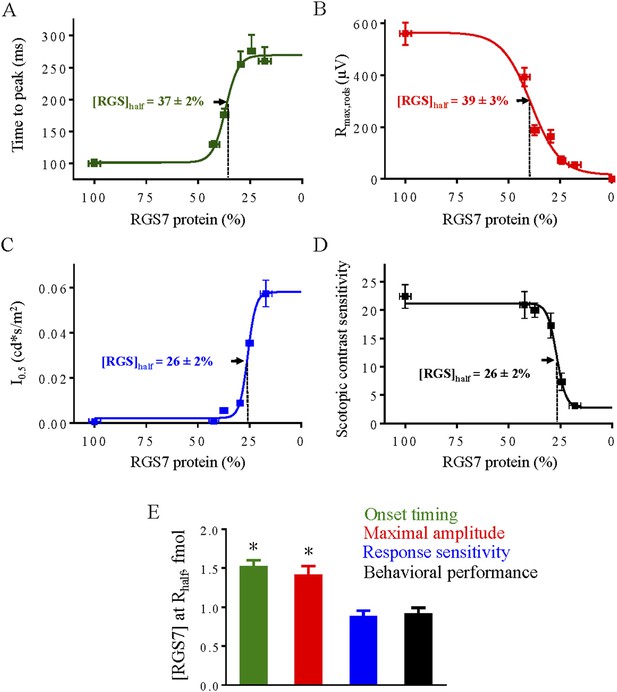
Correlation of RGS levels with key parameters of rod-rod ON-BC synaptic transmission and behavioral performance under scotopic conditions.
Dose response relationship between half-maximal values of rod ERG b-wave onset timing (A), maximal response amplitude (B), half-saturating light intensity (I0.5) (C) and performance in the behavioral task (D) were plotted as a function of RGS7 concentration quantitatively determined at each time point following tamoxifen administration. In panels A–D data points are fitted with sigmoidal dose-response (variable slope) equation with the least squares method. (E) Comparison of changes from different response parameters. RGS7% was converted to RGS7 protein levels using quantitative values determined in Figure 4. Scotopic behavioral performance is resistant to RGS reduction and tracks most closely with rod ON-BC sensitivity. One-Way ANOVA with Bonferroni's post hoc test reveals that half-maximal values for sensitivity and behavior do not significantly differ from each other (p > 0.05, n = 5), but differ from those for response kinetics and amplitude (p < 0.05, n = 5).
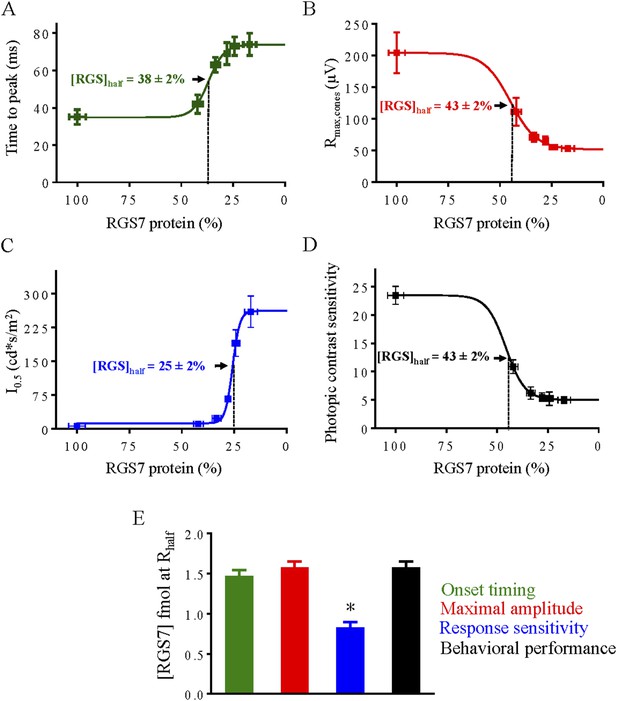
Correlation of RGS levels with key parameters of cone-cone ON-BC synaptic transmission and behavioral performance under photopic conditions.
Dose response relationship between half-maximal values of cone ERG b-wave onset timing (A), maximal response amplitude (B), half-saturating light intensity (I0.5) (C) and performance in the behavioral task (D) were plotted as a function of RGS7 concentration quantitatively determined at each time point following tamoxifen administration. In panels A–D data points are fitted with sigmoidal dose-response (variable slope) equation with the least squares method. (E) Comparison of changes from different response parameters. RGS7% was converted to RGS7 protein levels using quantitative values determined in Figure 4. Photopic behavioral performance is highly affected by RGS reduction and tracks closely with response amplitude and kinetics. One-Way ANOVA with Bonferroni's post hoc test reveals that half-maximal value for sensitivity differs from all the other parameters (p < 0.05, n = 5).
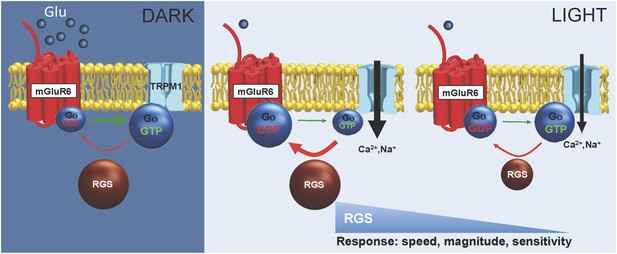
Model for RGS function in setting ON-BC responses to light.
In the dark, mGluR6 activated by high synaptic glutamate concentration produces ample amounts of GTP-bound Go, which closes TRPM1 channels. RGS proteins (RGS7 and RGS11) deactivate Go converting it back to inactive GDP-bound state but the equilibrium is dominated by excess of Go-GTP ensuring no channel activity. Upon light exposure, the activity of mGluR6 is reduced, and reduction in Go-GTP catalyzed by RGS proteins becomes dominant, allowing channels to open. When concentration of RGS proteins decline below a threshold point the speed of Go deactivation becomes rate-limiting to reduce the speed and magnitude of TRPM1 channel opening as well as its sensitivity to the reduction in glutamate concentration.
Tables
ERG b-wave parameters extracted from fitting of scotopic and photopic phases of maximal b-wave amplitudes elicited by varying flash strengths
| Genotype | I0.5, scotopic (cd*s/m2) | Rmax, (scotopic) (µV) | I0.5, photopic (cd*s/m2) | I0.5, photopic (µV) |
|---|---|---|---|---|
| WT | 0.0009 ± 0.0001 | 587 ± 17 | 3.5 ± 1.2 | 457 ± 30 |
| RGS11−/− | 0.001 ± 0.0001 | 580 ± 44 | 4.4 ± 1.4 | 419 ± 32 |
| RGS7−/− | 0.0008 ± 0.0001 | 615 ± 27 | 2.4 ± 1.1 | 510 ± 22 |
| RGS11−/− 7+/− | 0.0008 ± 0.0001 | 628 ± 51 | 2.5 ± 1.4 | 506 ± 25 |
| RGS11+/− 7−/− | 0.0007 ± 0.0001 | 593 ± 32 | 3.1 ± 1.0 | 439 ± 16 |
Effect of tamoxifen treatment on scotopic ERG b-wave parameters
| Days post tamoxifen | I0.5, scotopic (cd*s/m2) | Rmax, scotopic (µV) | Time to peak (ms) at I0.5 |
|---|---|---|---|
| 0 | 0.0007 ± 0.0001 | 560 ± 10 | 100 ± 6 |
| 12 | 0.001 ± 0.0002 | 390 ± 10 | 130 ± 5 |
| 17 | 0.005 ± 0.0001 | 190 ± 8 | 180 ± 10 |
| 22 | 0.008 ± 0.0003 | 160 ± 5 | 260 ± 21 |
| 28 | 0.03 ± 0.001 | 70 ± 3 | 280 ± 25 |
| 35 | 0.06 ± 0.006 | 50 ± 4 | 260 ± 22 |
| DKO | N.A | 0 | N.A |
-
Values are mean + SEM, n = 5 mice for all timepoints.
Parameters of rod ON-BC light responses by single cell recordings
| Days post tamoxifen | I0.5, (R*/rod)* | Rmax (pA) | Noise Variance (pA)2 | Unresponsive† (µV) |
|---|---|---|---|---|
| 0 | 1.6 ± 0.10 (4) | 340 ± 33 | 1.2 ± 0.2 | 0/4 |
| 12 | 4.8 ± 1.6 (9) | 200 ± 52 | 2.1 ± 0.4 | 4/13 |
| 22 | 6.4 ± 0.10 (8) | 200 ± 31 | 1.6 ± 0.2 | 5/13 |
| 28 | 69 ± 31 (6) | 130 ± 45 | 1.3 ± 0.1 | 5/11 |
| 35 | 160 ± 34 (13) | 68 ± 14 | 2.1 ± 0.4 | 11/24 |
-
*
Mean + SEM, number of cells is indicated in parenthesis.
-
†
Unresponsive cells were those where a flash did not evoke a response.
Effect of tamoxifen treatment on light-adapted photopic ERG b-wave parameters
| Days post tamoxifen | I0.5, photopic (cd*s/m2) | Rmax, photopic | Time to peak (ms) at I0.5 |
|---|---|---|---|
| 0 | 5 ± 1 | 204 ± 31 | 35 ± 5 |
| 12 | 10 ± 2 | 111 ± 22 | 42 ± 4 |
| 17 | 23 ± 3 | 70 ± 7 | 63 ± 6 |
| 22 | 65 ± 4 | 65 ± 6 | 69 ± 10 |
| 28 | 190 ± 29 | 55 ± 4 | 73 ± 5 |
| 35 | 260 ± 44 | 53 ± 3 | 74 ± 6 |
| DKO | N.A | 0 | N.A |
-
Values are mean + SEM, n = 5 mice for all timepoints.





