G-protein-coupled receptors regulate autophagy by ZBTB16-mediated ubiquitination and proteasomal degradation of Atg14L
Figures
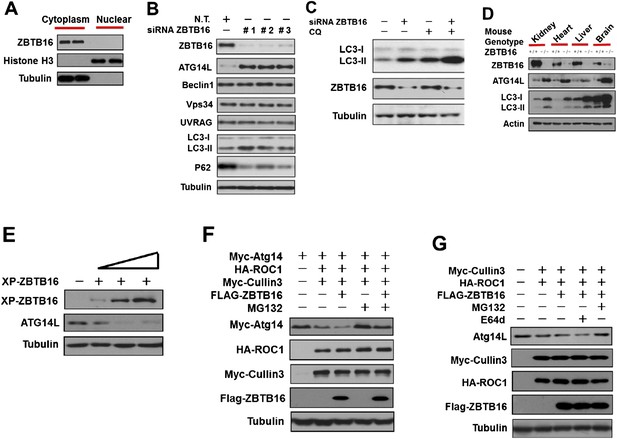
ZBTB16 mediates the proteasomal degradation of Atg14L.
(A) The cytoplasmic and nuclear fractions of HeLa cells cultured in normal media were separated by using Paris Kit (Ambion) and analyzed by western blotting using indicated antibodies. (B) HeLa cells were transfected with control siRNA (N.T.) or siRNA targeting ZBTB16 and cultured for 72 hr. The cell lysates were analyzed by western blotting with indicated antibodies. (C) HeLa cells were transfected with control siRNA (N.T.) or siRNA targeting ZBTB16 and cultured for 72 hr. Before harvesting, the cells were treated with or without 10 μM CQ (chloroquine) for 4 hr. The cell lysates were analyzed by western blotting with indicated antibodies. (D) The levels of ZBTB16, Atg14L, LC3, and actin (control) in the lysates isolated from wt and zbtb16−/− littermates were analyzed by western blotting using indicated antibodies. (E) HeLa cells were transfected with the Xpress-tagged ZBTB16 expression vector and cultured for 36 hr. The cell lysate was analyzed by western blotting using anti-Xpress and anti-ATG14L antibodies. Anti-tubulin was used as a loading control. (F) 293T cells were transfected with Myc-Atg14, Myc-Cul3, HA-ROC1, and FLAG-ZBTB16 expression vectors and cultured for 24 hr. MG132 (25 µM) was added in the last 8 hr as indicated. The cell lysates were then harvested and analyzed by western blotting using indicated antibodies. (G) 293T cells were transfected with Myc-Cul3, HA-ROC1, and FLAG-ZBTB16 expression vectors and cultured for 24 hr. MG132 (25 µM) or E64D (10 µM) was added in the last 8 hr as indicated. The cell lysates were then harvested and analyzed by western blotting using indicated antibodies.
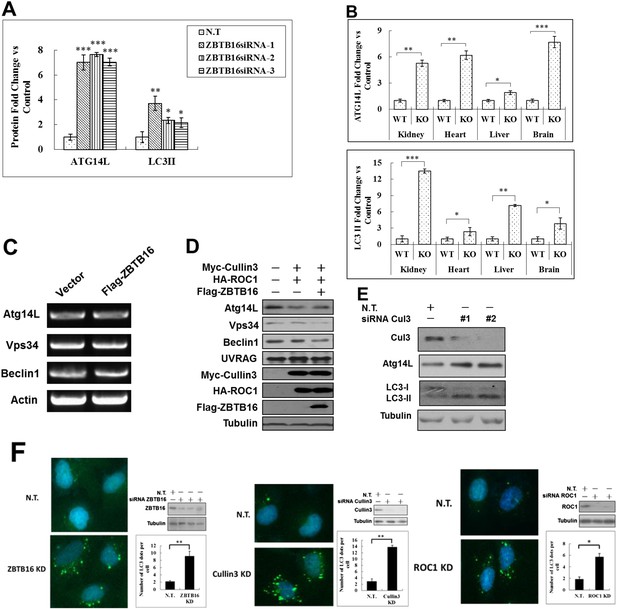
ZBTB16 mediates the proteasomal degradation of Atg14L.
(A) Quantification for Figure 1B. HeLa cells were transfected with control siRNA (N.T.) or siRNA targeting ZBTB16 and cultured for 72 hr. The cell lysates were analyzed by western blotting with indicated antibodies. The data are expressed as the mean of biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**); p < 0.001 (***). (B) Quantification for Figure 1D. The tissues of wt and zbtb16−/− littermates were isolated and homogenized in lysis buffer. The levels of ZBTB16, Atg14L, LC3, and actin (as a loading control) were analyzed by western blotting using indicated antibodies. The data are expressed as the mean of biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**); p < 0.001 (***). (C) HeLa cells were transfected with control FLAG vector and FLAG-ZBTB16 expression vector and cultured for 48 hr. Transfection efficiency was monitored by co-transfection of GFP vector. The mRNA was extracted and analyzed by RT-PCR. (D) 293T cells were transfected with expression vectors of Myc-Cullin3, HA-ROC1, and FLAG-ZBTB16 and cultured for 24 hr. The cell lysates were analyzed by western blotting using indicated antibodies. Anti-tubulin was used as a loading control. (E) HeLa cells were transfected with control siRNA (N.T.) or siRNA targeting Cul3 and cultured for 72 hr. The cell lysates were analyzed by western blotting with indicated antibodies. (F) Left: representative images of GFP-LC3 puncta (autophagosomes) in H4-GFP-LC3 expressing control non-targeting (N.T.) or ZBTB16 siRNA cultured in normal conditions and the quantitation. Bars are mean ± SEM of triplicate samples (500 cells analyzed per sample). Similar results were observed in three independent experiments. p < 0.01 (**). Middle: representative images of GFP-LC3 puncta (autophagosomes) in H4-GFP-LC3 expressing control non-targeting (N.T.) or Cullin3 siRNA cultured in normal conditions and the quantitation. Bars are mean ± SEM of triplicate samples (500 cells analyzed per sample). Similar results were observed in three independent experiments. p < 0.01 (**). Right: representative images of GFP-LC3 puncta (autophagosomes) in H4-GFP-LC3 expressing control non-targeting (N.T.) or ROC1 siRNA cultured in normal conditions and the quantitation. Bars are mean ± SEM of triplicate samples (500 cells analyzed per sample). Similar results were observed in three independent experiments. p < 0.05 (*).
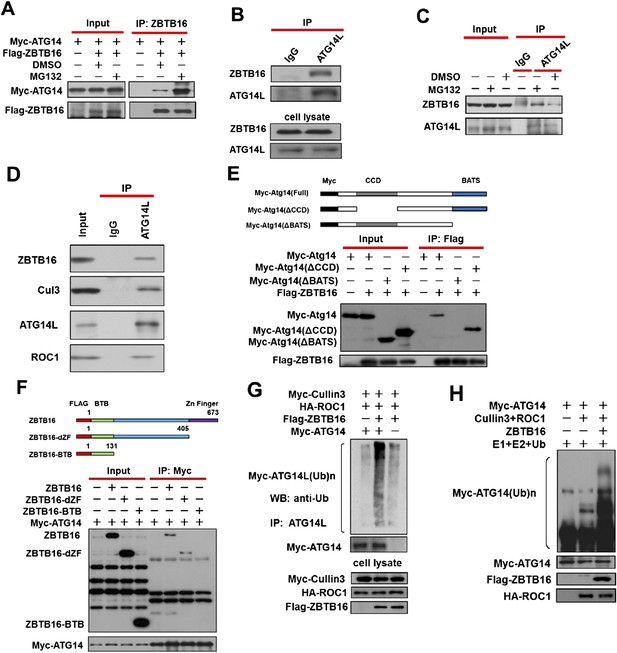
Ubiquitination of ATG14L by ZBTB16.
(A) 293T cells were transfected with expression vectors of FLAG-ZBTB16 and Myc-Atg14 and cultured for 24 hr. The cells were treated with MG132(10 µM) for the last 4 hr before harvesting. The cell lysates were immunoprecipitated with anti-ZBTB16 antibody, and the immunocomplexes were analyzed by western blotting using anti-Myc antibody. (B) 293T cells were cultured for 24 hr and then harvested and lysed in Buffer II. The lysates were immunoprecipitated with anti-ATG14L antibody, and the immunocomplexes were analyzed by western blotting using anti-ZBTB16 antibody. (C) HeLa cells were treated with MG132(10 µM) for 4 hr and then harvested and lysed in Buffer II. The lysates were immunoprecipitated with anti-ATG14L antibody, and the immunocomplexes were analyzed by western blotting using anti-ZBTB16 antibody. (D) HeLa cell lysates were immunoprecipitated with anti-ATG14L antibody, or a control IgG, and the immunocomplexes were analyzed by western blotting using indicated antibodies. (E) 293T cells were transfected with the expression vectors of FLAG-ZBTB16, Myc-ATG14L, truncated Myc-Atg14L(ΔBATS), Myc-Atg14L(ΔCCD) as indicated and cultured for 24 hr. The cells were then harvested and lysed in NP-40 buffer. The lysates were immunoprecipitated with anti-Flag antibody, and the immunocomplexes were analyzed by western blotting using anti-Myc antibody. (F) 293T cells were transfected with the expression vectors of Myc-ATG14L, FLAG-ZBTB16, truncated FLAG-ZBTB16-dZF, ZBTB16-BTB as indicated and cultured for 24 hr. The cells were then harvested and lysed in NP-40 buffer. The lysates were immunoprecipitated with anti-Myc antibody, and the immunocomplexes were analyzed by western blotting using anti-Flag antibody. (G) 293T cells were transfected with expression vectors of Myc-Atg14, HA-ROC1, FLAG-ZBTB16, and Myc-Cul3 expression vectors as indicated and cultured for 24 hr. MG132 (25 µM) was added in the last 6 hr as indicated. The cell lysates were harvested and immunoprecipitated with anti-ATG14L. The immunocomplexes were analyzed by western blotting using anti-Ub antibody for ubiquitin. (H) Myc-Cullin3, FLAG-ZBTB16, ROC1, and ATG14L proteins were individually purified from 293T cells transfected with indicated expression vectors by immunoprecipitation. The eluted proteins were incubated with recombinant E1, E2, and Ub. The reactions were terminated by boiling for 5 min in SDS sample buffer. The sample was analyzed by western blotting using anti-Myc.
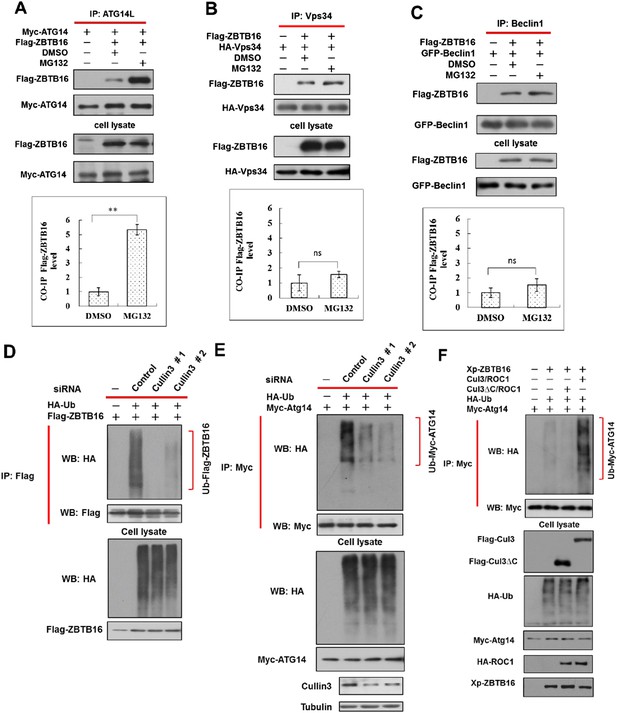
Ubiquitination of ATG14L by ZBTB16 and Cullin3.
(A) 293T cells were transfected with expression vectors of FLAG-ZBTB16 and Myc-Atg14L and cultured for 24 hr. The cells were treated with MG132 (10 µM) for the last 4 hr before harvesting and then lysed in NP-40 buffer. The lysates were immunoprecipitated with anti-ATG14L antibody, and the immunocomplexes were analyzed by western blotting using anti-Flag antibody. The data are expressed as the mean of 3 biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**). (B) 293T cells were transfected with expression vectors of FLAG-ZBTB16 and HA-Vps34 and cultured for 24 hr and then treated with MG132 (10 µM) for an additional 4 hr. The cells were lysed in NP-40 buffer. The lysates were immunoprecipitated with anti-Vps34 antibody, and the immunocomplexes were analyzed by western blotting using anti-Flag. The data are expressed as the mean of 3 biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. ns, no significance. (C) 293T cells were transfected with expression vectors of FLAG-ZBTB16 and GFP-Beclin1 for 24 hr and then treated with MG132 (10 µM) for 4 hr and then lysed in NP-40 buffer. The lysates were immunoprecipitated with anti-Beclin1 antibody, and the immunocomplexes were analyzed by western blotting using anti-Flag antibody. Statistical analysis was performed on biological repeats of three-independent sets of experiments using ImageJ. The data are expressed as the mean of 3 biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. ns, no significance. (D–E) HeLa cells were first transfected with control or Cul3 siRNA for 48 hr and then transfected with the indicated constructs and cultured for another 24 hr. The cells were then treated with MG132 for 4 hr before harvesting. Fully denatured lysates were diluted with 0.5% NP-40 lysis buffer and IP with anti-Flag antibody or anti-Myc antibody as indicated. The lysates were WB with indicated antibodies. (F) Expression vectors for FLAG-tagged Cul3 or Cul3∆C deleted C-terminal E2 binding domain were cotransfected with that of Myc-tagged ATG14, ROC1, Xpress-tagged ZBTB16, and HA-tagged ubiquitin into 293T cells as indicated for 24 hr. The cells were then treated with MG132 for 2 hr before harvesting. Fully denatured lysates were diluted with 0.5% NP-40 lysis buffer and IP with anti-Myc antibody. The lysates were WB with indicated antibodies.
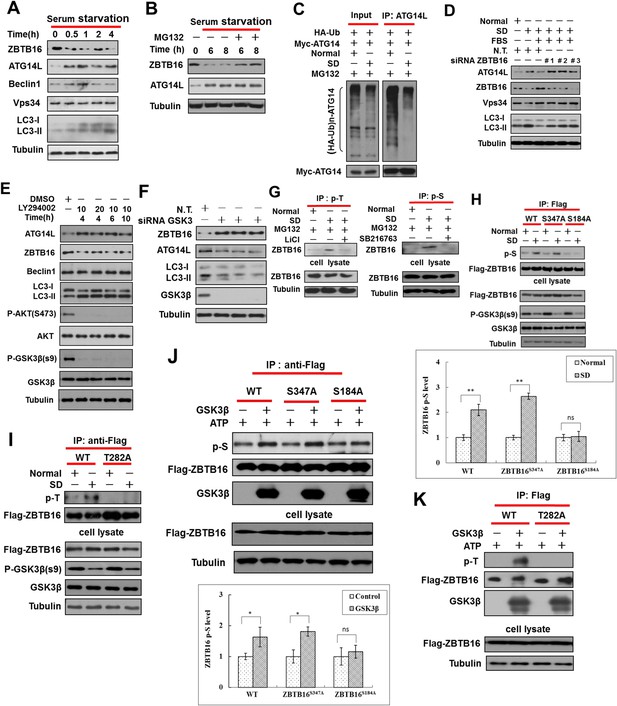
Serum starvation regulates ZBTB16 activity through GSK3β.
(A) HeLa cells were cultured in serum-free condition for indicated periods of time, and then the cell lysates were harvested and analyzed by western blotting using indicated antibodies. (B) HeLa cells were serum starved for indicated periods of time with or without MG132 (10 µM), and then the cell lysates were harvested and analyzed by western blotting using indicated antibodies. (C) HeLa cells were transfected with the expression vectors of HA-Ub and Myc-ATG14L and cultured for 36 hr and then cultured either in the presence or absence of serum (SD) for an additional 4 hr. The cell lysates were harvested and immunoprecipitated with anti-ATG14L. The immunocomplexes were analyzed by western blotting using anti-HA antibody for ubiquitin or anti-Myc as indicated. (D) HeLa cells were transfected with control non-targeting or ZBTB16 targeting siRNA. The cells were cultured in the presence of serum for 46 hr after transfection, subject to serum deprivation (SD) for 24 hr, and then re-stimulated by 10% FBS as indicated for another 2 hr. The total cell lysates were analyzed by western blotting with indicated antibodies. (E) HeLa cells were treated with PI3 kinase inhibitor LY294002 at indicated concentrations (μM) and time periods, and the cell lysates were harvested and analyzed by western blotting using indicated antibodies. (F) HeLa cells were transfected with control siRNA or siRNA targeting GSK3β and cultured for 72 hr. The total cell lysates were analyzed by western blotting with indicated antibodies. (G) HeLa cells were treated with or without SD for 4 hr and then treated with or without MG132 (10 µM), LiCl (10 mM), or SB216763 (20 µM) for an additional 4 hr. The cells were lysed in NP-40 buffer with phosphatase inhibitors and immunoprecipitated with pan-phospho-Thr antibody or pan phospho-Ser antibody. The immunocomplexes were analyzed by western blotting with rabbit anti-ZBTB16. (H) The expression vectors of Flag-tagged wild type or ZBTB16 point mutants were transfected into HeLa cells and cultured for 24 hr. Then, the cells were subject to normal or SD conditions as indicated with MG132 (10 µM) for 8 hr. The cells were lysed in NP-40 lysis buffer with phosphatase inhibitors and immunoprecipitated with anti-Flag antibody. The immunocomplexes were analyzed by western blotting with pan phospho-Ser antibody. (I) The expression vectors of Flag-tagged wild type or mutants of ZBTB16 were transfected into HeLa cells and cultured for 24 hr. The cells were subject to serum deprived or normal condition with MG132 for 8 hr. Then, the cells were lysed in NP-40 lysis buffer with phosphatase inhibitors and immunoprecipitated with anti-Flag antibody. The immunocomplexes were analyzed by western blotting with pan-phospho-Thr antibody. (J–K) The expression vectors of Flag-tagged wild type or mutant ZBTB16 were transfected into 293T cells and cultured for 24 hr. The cell lysates were collected and analyzed by immunoprecipitation with anti-Flag antibody. The immunoprecipitated ZBTB16 was incubated with or without recombinant GSK3β kinase in vitro at 30°C for 1 hr and then analyzed by western blotting analysis with anti-phosphor-Ser (J) or anti-phosphor-Thr (K). Quantification data are expressed as mean of 3 biological replicates ± SD.
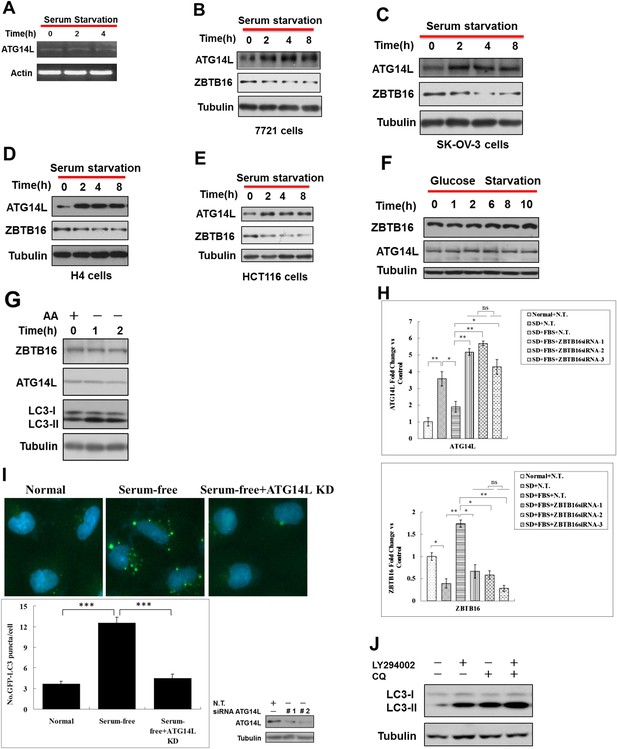
Serum starvation regulates ZBTB16 activity through GSK3β.
(A) HeLa cells were serum starvation for the indicated periods of time, and then the mRNA was extracted and subjected to RT-PCR. (B–E) 7721 cells (B), SK-OV-3 cells (C), H4 cells (D) and HCT116 cells (E) were cultured in serum-free condition for indicated periods of time, and then the cell lysates were harvested and analyzed by western blotting using indicated antibodies. (F) HeLa cells were treated without glucose for the indicated periods of time, and the cell lysates were analyzed by western blotting with indicated antibodies. (G) HeLa cells were cultured in the medium with or without amino acids for the indicated periods of time. The cell lysates were analyzed by western blotting with indicated antibodies. (H) HeLa cells were transfected with control non-targeting or ZBTB16 targeting siRNA. The cells were cultured in the presence of serum for 46 hr after transfection, subject to serum deprivation (SD) for 24 hr, and then re-stimulated by 10% FBS as indicated for another 2 hr. The total cell lysates were analyzed by western blotting with indicated antibodies. The data are expressed as the mean of biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**), ns, no significance. (I) Top: representative images of GFP-LC3 puncta (autophagosomes) in H4-GFP-LC3 cells expressing control or ATG14L siRNA and cultured under serum starvation condition for 8 hr. Bottom: quantitation of GFP-LC3 puncta in serum starvation conditions is shown as above. Bars are mean ± SEM of triplicate samples (500 cells analyzed per sample). Similar results were observed in three independent experiments. p < 0.001 (***). (J) HeLa cells were treated with 10 μM LY294002 or 10 μM CQ for 4 hr. The cell lysates were analyzed by western blotting with indicated antibodies.
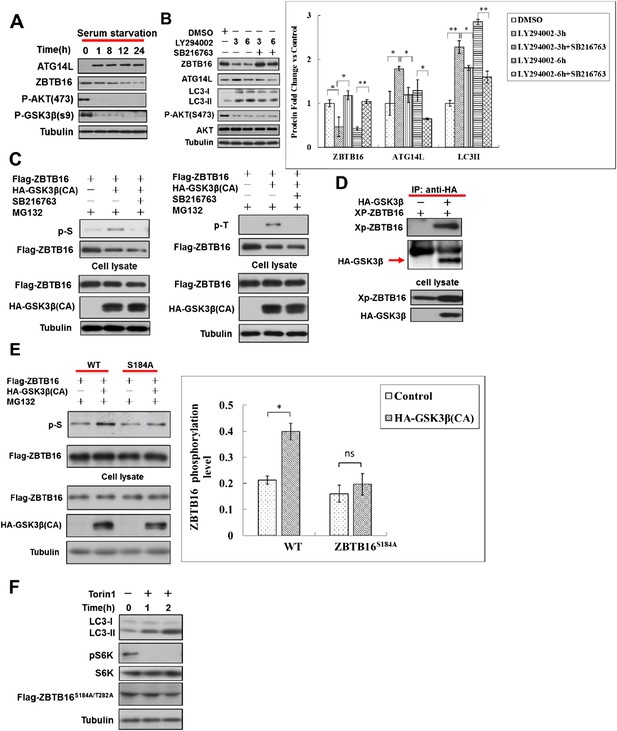
Serum starvation regulates ZBTB16 activity through GSK3β.
(A) HeLa cells were cultured without serum for the indicated periods of time, and then the cell lysates were harvested and analyzed by western blotting using indicated antibodies. (B) HeLa cells were treated with LY294002 (10 µM) or GSK3 inhibitor SB216763 (10 µM) for indicated periods of time in the presence of FBS. The cell lysates were harvested and analyzed by western blotting using indicated antibodies. The data are expressed as the mean of biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**). (C) HeLa cells were transfected with the expression vectors of Flag-ZBTB16, constitutively active HA-GSK3β(CA) for 36 hr and then treated with or without SB216763(10 µM), MG132(10 µM) for an additional 4 hr. The cells were lysed in NP-40 buffer with phosphatase inhibitors and immunoprecipitated with anti-Flag antibody. The immunocomplexes were analyzed by western blotting with pan phospho-Ser antibody or phospho-Thr antibody. (D) 293T cells were transfected with expression vectors for HA-GSK3β and XP-ZBTB16 for 24 hr. The cell lysates were harvested and immunoprecipitated with anti-HA. The immunocomplexes were analyzed by western blotting using indicated antibodies. (E) HeLa cells were transfected with the expression vectors of Flag-ZBTB16, mutant Flag-ZBTB16, constitutively active HA-GSK3β(CA) for 36 hr and then treated with MG132 (10 µM) for an additional 4 hr. The cells were lysed in NP-40 buffer with phosphatase inhibitors and immunoprecipitated with anti-Flag antibody. The immunocomplexes were analyzed by western blotting with pan phospho-Ser antibody. The lysates were WB with indicated antibodies. The data are expressed as the mean of biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*). (F) HeLa cells were transfected with expression vectors of Flag-ZBTB16S184A/T282A and cultured for 24 hr. Before harvesting the sample, the cells were treated with mTOR inhibitor Torin1 (500 nM) in nutrient-rich conditions for the indicated periods of time. The cell lysates were analyzed by western blotting with indicated antibodies.
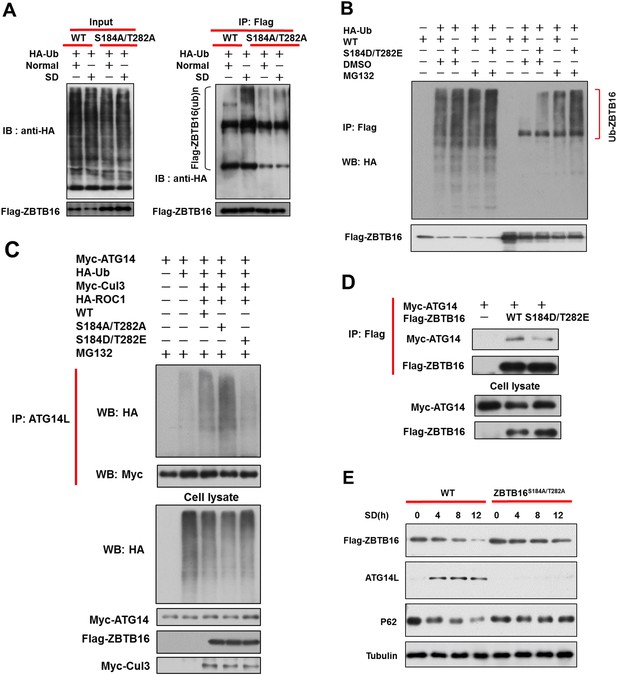
Phosphorylation of S184/T282 of ZBTB16 by GSK3b is functionally important for regulating Atg14L under serum starvation condition to promote autophagy.
(A) The expression vectors of Flag-tagged wild type or mutants ZBTB16 and HA-Ub were transfected into HeLa cells and cultured for 24 hr. HeLa cells were treated with or without serum-deprivation (SD) for 4 hr. The cell lysates were collected and subjected to immunoprecipitation with anti-Flag antibody. The immunocomplexes were analyzed by western blotting using anti-HA for ubiquitin or anti-Flag as indicated. (B) 293T cells were transfected with the expression vectors of wide-type FLAG-ZBTB16, mutant FLAG-ZBTB16, HA-Ub as indicated and cultured for 20 hr. The cells were then treated with or without MG132 for 4 hr. Fully denatured lysates were diluted with 0.5% NP-40 lysis buffer and IP with anti-Flag antibody. The lysates were WB with indicated antibodies. (C) 293T cells were transfected with the expression vectors of wide-type FLAG-ZBTB16, mutant FLAG-ZBTB16, HA-Ub, Myc-ATG14, HA-ROC1, Myc-Cul3 as indicated and cultured for 36 hr. The cells were then treated with MG132 for 2 hr. Fully denatured lysates were diluted with 0.5% NP-40 lysis buffer and IP with anti-ATG14L antibody. The lysates were WB with indicated antibodies. (D) HeLa cells were transfected with expression vectors of wide-type FLAG-ZBTB16, mutant FLAG-ZBTB16, Myc-ATG14 as indicated and then lysed in NP-40 buffer. The lysates were immunoprecipitated with anti-Flag antibody, and the immunocomplexes were analyzed by western blotting using anti-Myc antibody. (E) The expression vectors of Flag-tagged wild type or ZBTB16 point mutants were transfected into HeLa cells and cultured for 12 hr. The cells were cultured in serum-free condition for indicated periods of time. The cell lysates were then harvested and analyzed by western blotting using indicated antibodies.
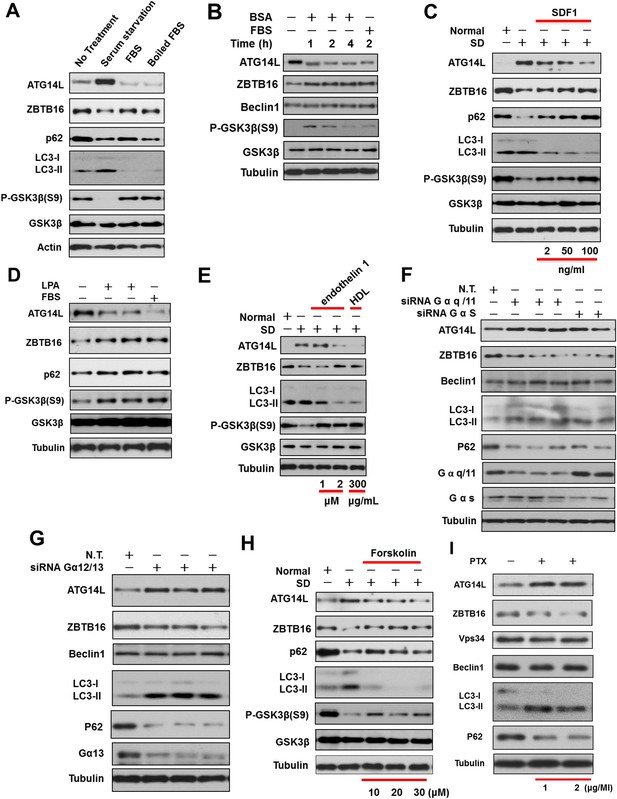
Regulation of Atg14L by GPCR mediated signaling pathways.
(A) HeLa cells were cultured in the presence (no treatment) or absence of serum (serum starvation) for 24 hr and then re-stimulated by 10% FBS or boiled FBS (boiled for 30 min at 95°C) for 1 hr. The cell lysates were analyzed by western blotting with the indicated antibodies. (B) Serum-starved HeLa cells were stimulated with 10% FBS or 10 mg/ml BSA (A2058, Sigma) for indicated periods of time and then harvested and analyzed by western blotting using indicated antibodies. (C) H4 cells were cultured in the presence or absence of serum for 24 hr. SDF1 was added to serum-starved cells at indicated concentrations for 1 hr. The cells were lysed, and the lysates were analyzed by western blotting with indicated antibodies. (D) HeLa cells were serum starved for 24 hr and then stimulated with 20 µM LPA or 10% FBS for 1 hr. The cells were lysed, and the lysates were analyzed by western blotting with indicated antibodies. (E) HeLa cells were serum deprived for 24 hr and then stimulated with endothelin 1 or HDL at indicated concentrations for 4 hr. The cells were lysed, and the lysates were analyzed by western blotting with indicated antibodies. (F) HeLa cells were transfected with siRNAs for control, Gαq/11, or GαS and cultured for 72 hr. The cell lysates were analyzed by western blotting with indicated antibodies. (G) HeLa cells were transfected with siRNAs for control or Gα12/13 and cultured for 72 hr. The cell lysates were analyzed by western blotting with indicated antibodies. (H) HeLa cells were serum-deprived (SD) for 24 hr and then stimulated with forskolin for 2 hr. The cell lysates were harvested and analyzed by western blotting using indicated antibodies. (I) HeLa cells were treated with Gαi/o inhibitor Pertussis toxin (PTX) at 1 or 2 μg/ml for 6 hr, and the cell lysates were harvested and analyzed by western blotting using indicated antibodies.
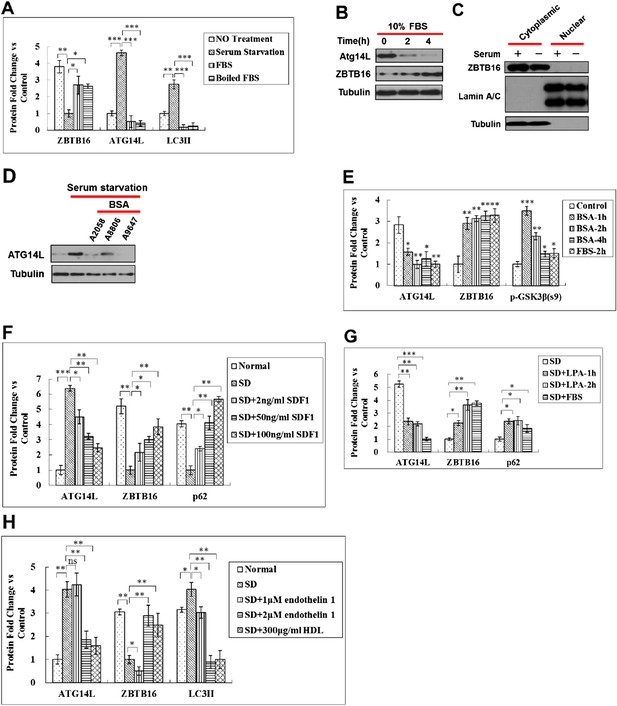
Regulation of Atg14L by ligands and agonists of GPCR.
(A) Quantification for Figure 5A. HeLa cells were cultured in the presence (no treatment) or absence of serum (serum starvation) for 24 hr, and then re-stimulated by 10% FBS or boiled FBS (boiled for 30 min at 95°C) for 1 hr. The cell lysates were analyzed by western blotting with the indicated antibodies. The data are expressed as the mean of biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**). p < 0.001 (***). (B) HeLa cells were serum starved for 30 hr and then stimulated with serum for the indicated periods of time. The cell lysates were analyzed by using western blotting with the indicated antibodies. (C) HeLa cells were cultured in the presence or absence of serum (SD) for 6 hr and then the cell lysate was harvested and analyzed by western blotting using indicated antibodies. Antibodies for lamin A/C and tubulin were used to determine purity of nuclear and cytoplasmic samples, respectively. (D) HeLa cells were serum starved for 24 hr, and then stimulated with for different bovine serum albumin (BSA) batches for 1 hr, and the cell lysates were harvested and analyzed by western blotting using indicated antibodies. (E) Quantification for Figure 5B. Serum-starved HeLa cells were stimulated with 10% FBS or 10 mg/ml BSA (A2058, Sigma) for indicated periods of time and then harvested and analyzed by western blotting using indicated antibodies. The data are expressed the mean of biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**); p < 0.001 (***). (F) Quantification for Figure 5C. H4 cells were cultured in the presence or absence of serum for 24 hr. SDF1 was added to serum-starved cells at indicated concentrations for 1 hr. The cells were lysed, and the lysates were analyzed by western blotting with indicated antibodies. The data are expressed as the mean of biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**); p < 0.001 (***). (G) Quantification for Figure 5D. HeLa cells were serum-starved for 24 hr, and then stimulated with 20 µM LPA or 10% FBS for 1 hr. The cells were lysed, and the lysates were analyzed by western blotting with indicated antibodies. The data are expressed as the mean of biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**), p < 0.001 (***). (H) Quantification for Figure 5E. HeLa cells were serum-deprived for 24 hr, and then stimulated with endothelin 1 or HDL at indicated concentrations for 4 hr. The cells were lysed, and the lysates were analyzed by western blotting with indicated antibodies. The data are expressed as the mean of biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*), p < 0.01 (**). ns, no significance.
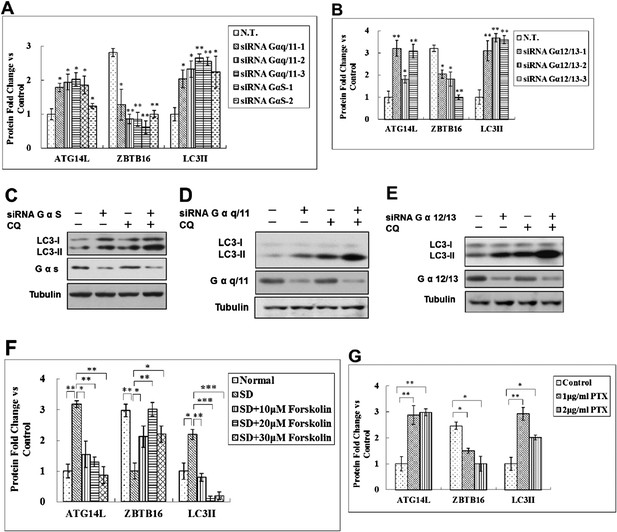
Regulation of Atg14L by GPCR mediated signaling pathways.
(A) Quantification for Figure 5F. HeLa cells were transfected with control siRNA, Gαq/11, GαS and cultured for 72 hr. The cell lysates were analyzed by western blotting with indicated antibodies. The data are expressed as the mean of 3 biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**). (B) Quantification for Figure 5G. HeLa cells were transfected with control siRNA, Gα12/13 and cultured for 72 hr. The cell lysates were analyzed by western blotting with indicated antibodies. The data are expressed as the mean of 3 biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**). (C) HeLa cells were transfected with control siRNA (N.T.) or siRNA targeting GαS and cultured for 72 hr. Before harvesting the sample, the cells were treated with or without 10 μM CQ for 4 hr. The cell lysates were analyzed by western blotting with indicated antibodies. (D) HeLa cells were transfected with control siRNA (N.T.) or siRNA targeting Gαq/11 and cultured for 72 hr. Before harvesting the sample, the cells were treated with or without 10 μM CQ for 4 hr. The cell lysates were analyzed by western blotting with indicated antibodies. (E) HeLa cells were transfected with control siRNA (N.T.) or siRNA targeting Gα12/13 and cultured for 72 hr. Before harvesting the sample, the cells were treated with or without 10 μM CQ for 4 hr. The cell lysates were analyzed by western blotting with indicated antibodies. (F) Quantification for Figure 5H. HeLa cells were serum-deprived (SD) for 24 hr, and then stimulated with forskolin for 2 hr. The cell lysates were harvested and analyzed by western blotting using indicated antibodies. The data are expressed as the mean of 3 biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**), p < 0.001 (***). (G) Quantification for Figure 5I. HeLa cells were treated with Gαi/o inhibitor PTX at 1 or 2 μg/ml for 6 hr, and the cell lysates were harvested and analyzed by western blotting using indicated antibodies. The data are expressed as the mean of 3 biological replicates (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student's t-test. p < 0.05 (*); p < 0.01 (**).
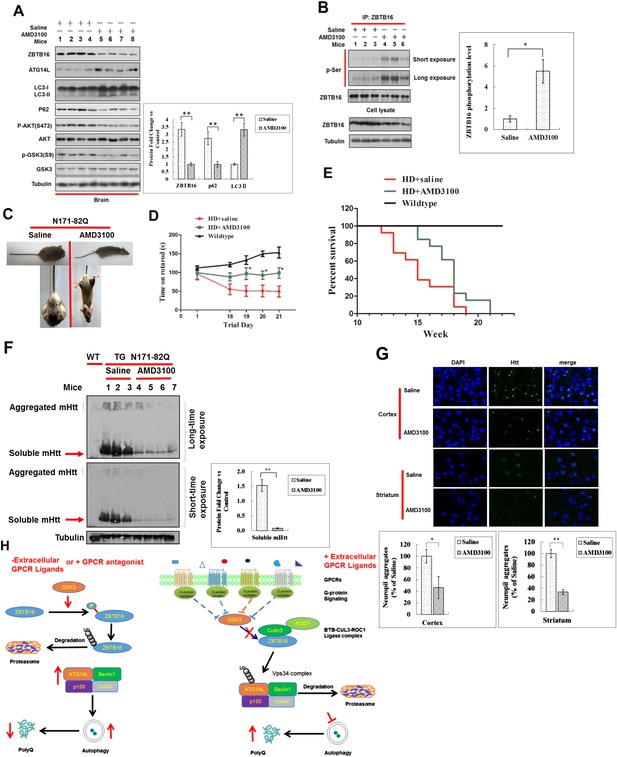
Up-regulation of Atg14L and autophagy by GPCR antagonist AMD3100 in vivo ameliorated the neural dysfunction of HD transgenic mice.
(A) WT mice were dosed once intraperitoneally with AMD3100 at 10 mg/kg body weight and 24 hr later, the brain tissues were collected and analyzed by western blotting using indicated antibodies. Anti-Tubulin was used as a loading control. The levels of ZBTB16, p62, and LC3-II were quantified as graphs on the right side. (B) WT mice were dosed once intraperitoneally with AMD3100 at 10 mg/kg body weight and 24 hr later, the brain tissues were lysed in NP-40 buffer with phosphatase inhibitors and immunoprecipitated ZBTB16 antibody. The immunocomplexes were analyzed by western blotting with phospho-Ser antibody and anti-ZBTB16 antibody. The levels of ZBTB16 phosphorylation were quantified shown as a graph on the right. (C) Dosing of AMD3100, but not saline alone, for two weeks reduced the clasping of the hindlimbs of N171-82Q mice at 13 weeks of age. (D) AMD3100 improved motor function of N171-82Q mice as determined by rotarod testing. The mice received training on rotarod for 10 min each day for 3 days before being tested on the first day of 11 week of age before dosing of AMD3100 started (Day 1). The mice received rotarod training again on day 15–17 during AMD3100 or saline dosing course and were tested again on day 18–21 as shown. For testing, the speed of the rod was set to 5 rpm and increased by 0.5 rpm/s. The data were collected as an average of three trials for each mouse everyday. Mice were allowed to rest for 20 min between trials. N = 13 for each group. The data are presented as mean values ± s.e.m.*. p < 0.05 by t-test for significance. AMD3100 (10 mg/kg body weight) or saline treatment was delivered by intraperitoneal injection from 11 weeks of age daily (every 24 hr). The experiments were carried out in double-blind manner (the person who conducted rotarod testing was unaware if the mouse had received saline or AMD3100, which was provided by another person). (E) Survival of wild-type (WT), N171-82Q injected with saline (HD + saline), N171-82Q mice injected with AMD3100 (HD + AMD3100). N = 13 for each group. p < 0.05 by log-rank test for significance. The experiments were carried out in double-blind manner (the person who monitored mouse survival was unaware if the mouse had received saline or AMD3100, which was provided by another person). (F) The brain tissues of WT mice or N171-82Q mice dosed for 4 weeks by saline alone or AMD3110 at the age of 15 weeks were isolated and analyzed by western blotting using indicated antibodies. Anti-tubulin was used as a loading control. AMD3100 (10 mg/kg body weight/day) or saline treatment was delivered by intraperitoneal injection from 11 weeks of age every 24 hr. The levels of soluble mHtt were quantified as graphs on the right side. (G) Immunostaining of tissue sections from the cortex and striatum of N171-82Q mice treated with saline or AMD3100 daily for 4 weeks using rabbit EM48. DAPI staining is for nucleus. Quantitative assessment of neuropil aggregate density is as graphs below. (H) A model for regulation of autophagy under normal nutritional conditions: inhibiting GPCR signaling leads to the activation of GSK3β, which mediates the phosphorylation of ZBTB16 to promote its auto-ubiquitination and inhibit the ubiquitination and proteasomal degradation of Atg14L that in turn leads to increased activity of class III PI3 kinase and production of PI3P, and activation of autophagy.
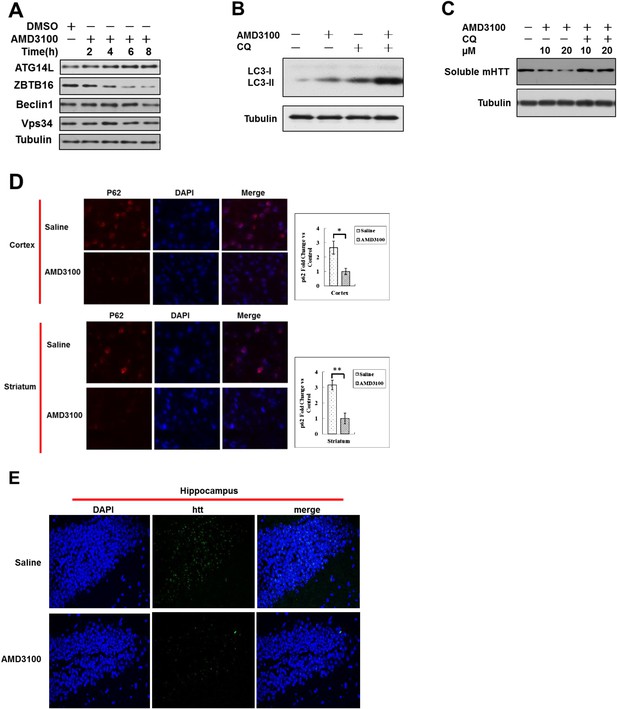
Up-regulation of Atg14L and autophagy by GPCR antagonist AMD3100 in vivo ameliorated the neural dysfunction of HD transgenic mice.
(A) HeLa cells were treated with AMD3100 at 10 µM for indicated periods of time, and then the cell lysates were harvested and analyzed by western blotting using indicated antibodies. (B) HeLa cells were treated with 10 µM AMD3100 for 4 hr. Before harvesting, the cells were treated with or without 10 µM CQ for 4 hr. The cell lysates were analyzed by western blotting with indicated antibodies. (C) HeLa cells were transfected with the expression vectors of GFP-140Q and cultured for 12 hr, and then treated with different doses AMD3100 for another 12 hr. The cells were treated with or without 20 μM CQ for 4 hr before harvesting. The lysates were WB with anti-GFP antibodies to detect soluble mHTT. (D) Anti-p62 immunostaining of tissue sections from the cortex and striatum of N171-82Q mice treated with vehicle or AMD3100 daily for 4 weeks. DAPI staining is for nucleus. (E) EM48 immunostaining of hippocampal sections from Q171-N82 mice at the age of 15 weeks after 1 month of treatment with vehicle or AMD3100 at 10 mg/kg body weight daily by intraperitoneal injection.
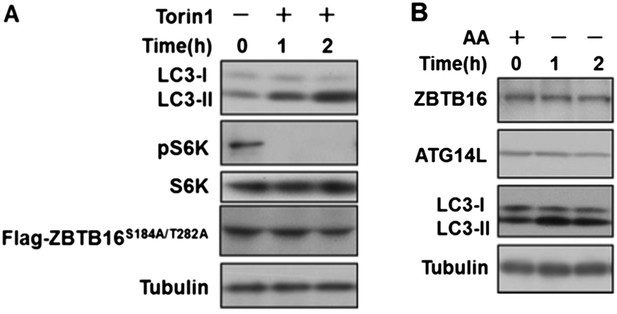
A. HeLa cells were transfected with expression vectors of Flag-ZBTB16S184A/T282A and cultured for 24h. Before harvesting the sample, the cells were treated with mTOR inhibitor Torin1(500nM) in nutrient-rich conditions for the indicated periods of time. The cell lysates were analyzed by western blotting with indicated antibodies. B. HeLa cells were cultured in the medium with or without amino acids for the indicated periods of time. The cell lysates were analyzed by western blotting with indicated antibodies.
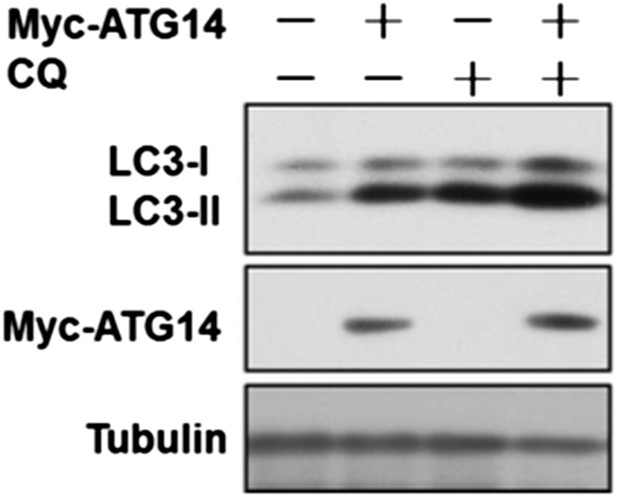
HeLa cells were transfected with expression vectors of Myc-Atg14 and cultured for 24h. Before harvesting the sample, the cells were treated with or without 10 µM CQ for 4h. The cell lysates were analyzed by western blotting with indicated antibodies.
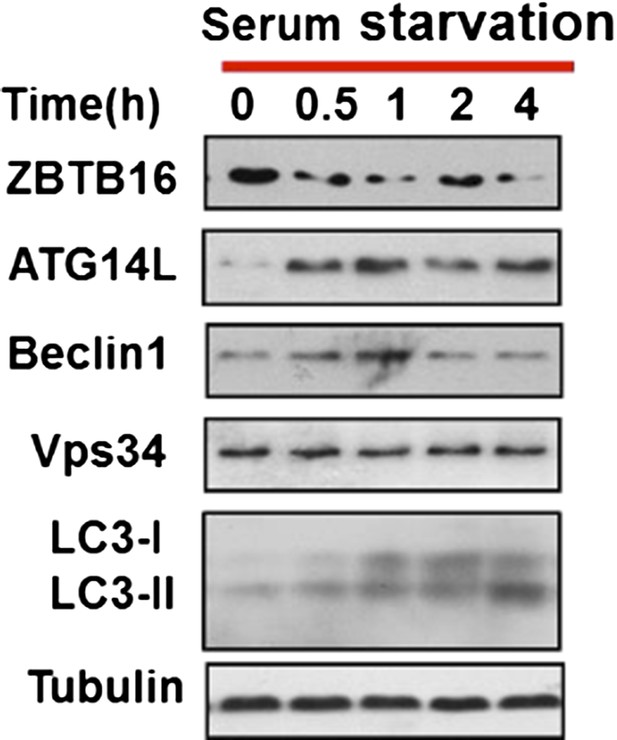
HeLa cells were cultured in serum-free condition for indicated periods of time and then the cell lysates were harvested and analyzed by western blotting using indicated antibodies.





