The vacuole/lysosome is required for cell-cycle progression
Figures
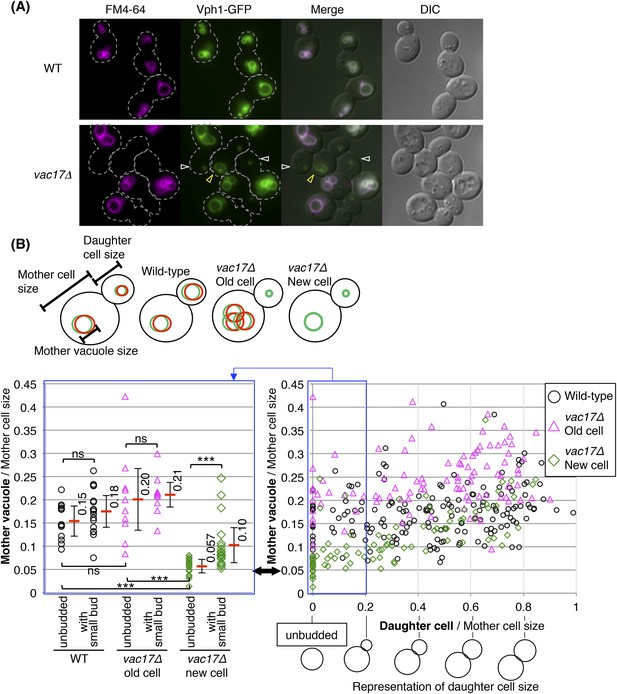
Yeast require vacuoles of a specific size prior to formation of a bud.
(A) Wild-type and vac17Δcells which express Vph1-GFP from its endogenous locus, were pulse labeled with the vacuole specific dye FM4-64. Wild-type cells have both FM4-64 and Vph1-GFP signals in both mother and daughter cells. vac17Δ cells have both Vph1-GFP and FM4-64 on the vacuole in old mother cells, however the daughter cells solely have a Vph1-GFP labeled vacuole. White arrowheads; new vacuoles in daughter cells. Yellow arrowheads; new vacuoles in new mother cells. Dashed line; outline of cells. (B) (Left panel) A new cell does not form a bud until its vacuole reaches a specific size. Graph indicates the vacuole diameter of wild-type, vac17Δ old cell and vac17Δ new cells with no bud and mother cells with a small bud (less than 20% of diameter of the mother cell). Cell/vacuole diameter was measured by ImageJ. Each cell/vacuole diameter was normalized to its mother cell diameter. Black arrow; minimum size of mother vacuoles in cells with a bud. Average in each category (red bar). Error bar; standard deviation (SD). Not a significant difference; ns, p-value > 0.10. A statistically significant difference; *** (p-value < 1 × 10−3). (Right panel) The vacuoles of the new mother cells of vac17Δ grow faster than vacuoles in either a wild-type or vac17Δ old mother cell. Scatter plots of bud sizes and mother vacuole sizes.
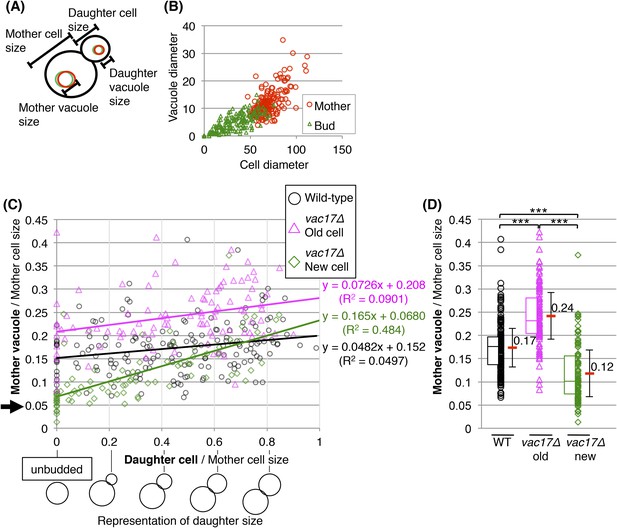
Yeast require vacuoles of a specific size prior to formation of a bud.
(A) Schematic of diameters measured. (B) Vacuole diameter is proportional with the cell diameter. Scatter plot of cell diameter and vacuole diameter in mother and daughter cells. (C) When viewed across the cell-cycle, the increase in vacuole size of vac17Δ new cells is greater than either wild-type or vac17Δ old cells. Trend lines from linear equations of data from wild-type (black line); vac17Δ old mother cells (pink line), and vac17Δ new mother cells (green line) are shown. (D) Vacuole inheritance is important for regulating vacuole size in the mother cell. The vacuole size of vac17Δ old mother cells is larger than that of wild-type. Average in each category (red bar). Error bar; SD. *** (p-value < 1 × 10−4). Middle line in box plot indicates the median of the data set. The upper edge of the box indicates the 75th percentile of the data set, and the lower edge indicates the 25th percentile.
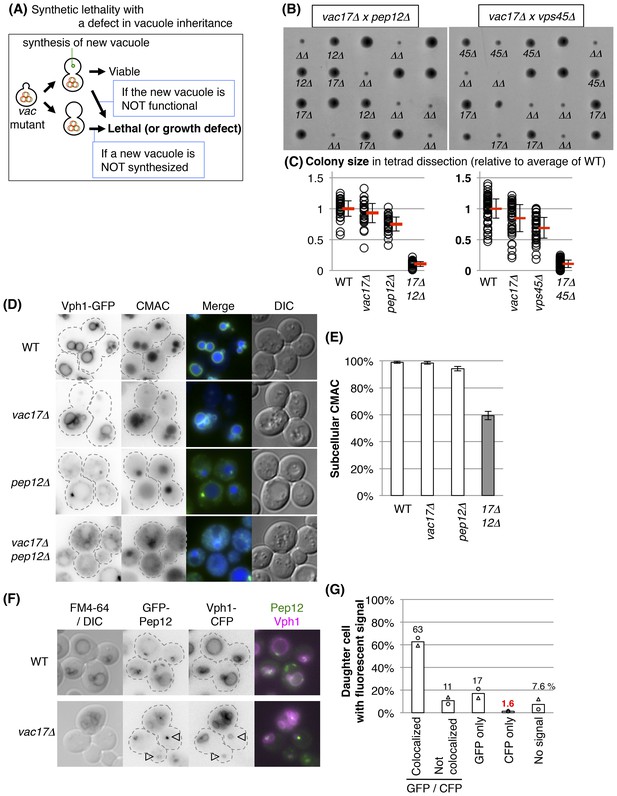
Pep12 and Vps45 are required for the synthesis of a new vacuole.
(A) Schematic of pathways predicted to exhibit synthetic lethality with mutations in vacuole inheritance. When vacuole inheritance is defective, the bud generates a new vacuole that is independent of the mother vacuole. If vacuoles play an essential role, then cells with no mechanism to generate a vacuole will not be viable. Furthermore if the new vacuole is defective in its essential function(s), the cell will not be viable. (B) The pep12Δ and vps45Δ mutants exhibit a synthetic growth defect with vac17Δ. Results of tetrad dissection. Haploid colonies from tetrads derived from heterozygous diploids of VAC17/vac17Δ PEP12/pep12Δ and VAC17/vac17Δ VPS45/vps45Δ were arrayed vertically on YPD (rich medium) plates incubated at 24°C for 3 days. vac17Δ = 17Δ; pep12Δ = 12Δ; vps45Δ = 45Δ; vac17Δ pep12Δ or vac17Δ vps45Δ double mutant = ΔΔ are indicated. (C) Quantification of colony size, relative to the average of wild-type colonies. A total of 28 full tetrads and 48 full tetrads were analyzed for vac17Δ pep12Δ and vac17Δ vps45Δ, respectively. Average size in each genotype (red bar). Error bar; SD. (D) Both vacuole inheritance and new synthesis are important to maintain functional vacuoles. In the vac17Δ pep12Δ mutant several cells appear to lack a vacuole. Wild-type cells incubated with 10 μM CMAC for 30 min exhibited a blue fluorescent signal in the vacuole lumen. The limiting membrane of the vacuole is indicated by Vph1-GFP expressed from its endogenous locus. Wild-type and vac17Δ cells show normal localization of Vph1-GFP and CMAC. Single pep12Δ cells show abnormal distribution in Vph1-GFP, but not CMAC. The vac17Δ pep12Δ double mutant cells show defects in the localization of Vph1-GFP and CMAC. (E) Quantification of cells with a CMAC positive subcellular structure. Any CMAC containing structure with or without Vph1-GFP was scored as a structure. Error bars; SD calculated from four independent experiments with at least 100 cells counted in each strain/experiment. (F) New vacuoles are generated from Pep12-positive endosomes. GFP-Pep12/Vph1-CFP expressed in wild-type and vac17Δcells were pulse labeled with FM4-64. GFP-Pep12 and Vph1-CFP were expressed from the endogenous loci in both strains. Open arrowheads; new vacuoles. (G) Quantification of percent daughter cells with Vph1-CFP and/or GFP-Pep12 in vac17Δ cells. Averages from two independent experiments; at least 100 cells counted per experiment. Open circles and triangles indicate each experiment.
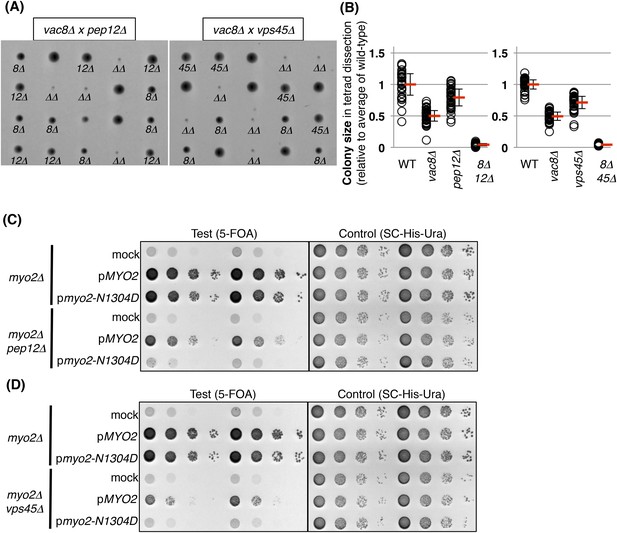
Pep12 and Vps45 are required for the viability of vacuole inheritance mutants.
(A) A vac8Δ mutant exhibits a synthetic growth defect with either pep12Δ and vps45Δ. Results of tetrad dissections of heterozygous diploids, VAC8/vac8Δ PEP12/pep12Δ and VAC8/vac8Δ VPS45/vps45Δ. vac8Δ = 8Δ; pep12Δ = 12Δ; vps45Δ = 45Δ; vac8Δ pep12Δ or vac8Δ vps45Δ double mutant = ΔΔ are indicated. (B) Quantification of colony size, relative to average of wild-type colonies. A total 35 tetrads and 37 tetrads were analyzed for vac8Δ pep12Δ and vac8Δ vps45Δ, respectively. (C) The pep12Δ mutant exhibits a synthetic growth defect with myo2-N1304D, which is a vacuole inheritance mutant due to a defect in binding to Vac17. Survival occurs when wild-type MYO2 is expressed in myo2Δ cells, and in the myo2Δ pep12Δ mutant. In addition, the myo2-N1304D mutant expressed in myo2Δ cells is sufficient for survival. In contrast, the myo2-N1304D mutant expressed in myo2Δ pep12Δ cells is lethal. Plasmids were transformed into a myo2Δ strain and a myo2Δ pep12Δ strain containing YCp50 [URA3] MYO2. Plasmids tested were pRS413 [HIS3] (mock), pRS413 MYO2 (pMYO2), or pRS413 myo2-N1304D (pmyo2-N1304D). Transformed colonies were cultured in SC-His-Ura liquid media and serial dilutions spotted onto SC+5-FOA plates to counter select against YCp50 [URA3] MYO2 (middle panel) and the same culture was also tested on SC-His-Ura plate (right panel). (D) A vps45Δ mutant exhibits a synthetic growth defect with the myo2-N1304D mutant. Wild-type MYO2 expressed in myo2Δ cells, or in the myo2Δ vps45Δ mutant, is sufficient for cell viability. In addition, the myo2-N1304D mutant expressed in myo2Δ cells is sufficient for cell viability. In contrast, the myo2-N1304D mutant expressed in myo2Δ vps45Δ cells is lethal.
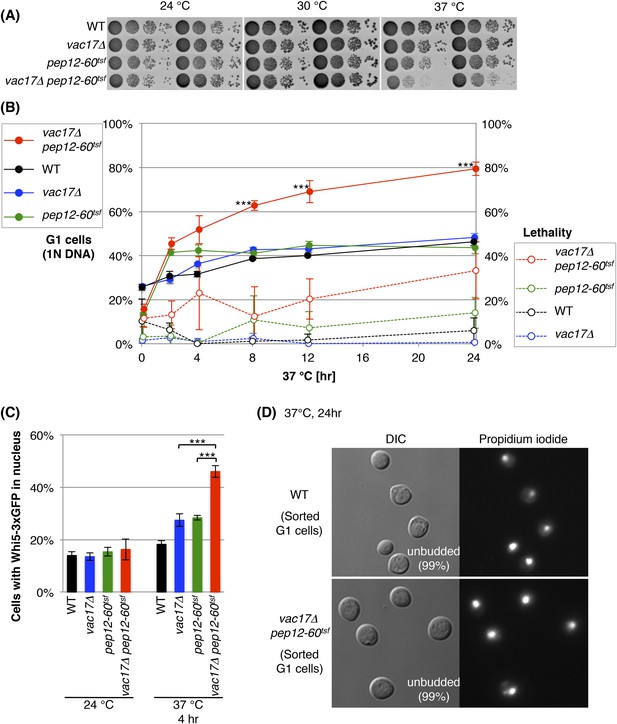
The vacuole is required for cell-cycle progression from early G1.
(A) The vac17Δ pep12-60tsf double mutant shows synthetic growth defects at the restrictive temperature, 37°C. Wild-type, vac17Δ, pep12-60tsf and vac17Δ pep12-60tsf strains were cultured in liquid media and serial dilutions were spotted onto YPD plates. The plates were incubated at 24°C, 30°C and 37°C for 2 days. (B) The vac17Δ pep12-60tsf double mutant arrests in G1 phase at the restrictive temperature 37°C. Percent cells in G1 phase (solid lines). Yeast strains tested; wild-type, vac17Δ, pep12-60tsf, and vac17Δ pep12-60tsf. Cultures were incubated at 24°C overnight, and then sifted to 37°C for 0, 2, 4, 8, 12, or 24 hr. The percentage of G1 cells (1N DNA) was measured using propidium iodide (PI) staining and assessed by flow cytometry. The same cultures were analyzed for lethality (percent dead cells) (dashed lines). After incubation at 37°C, the number of yeast cells were assessed with a hemocytometer, and their ability to form colonies at 24°C on YPD plates was tested. Lethality was inferred from the number of cells that survived the treatment. Error bars; SD calculated from four independent experiments. *** (p-value < 1 × 10−3). (C) The vac17Δ pep12-60tsf double mutant arrests in early G1 phase at the restrictive temperature 37°C. Cells were scored for the presence of Whi5-3xGFP in the nucleus. Wild-type, vac17Δ, pep12-60tsf, and vac17Δ pep12-60tsf cells, which express Whi5-3xGFP from its endogenous locus, were incubated at 24°C overnight, and then sifted to 37°C for 0 or 4 hr. Error bars; SD calculated from three independent experiments with at least 100 cells counted in each strain/experiment. *** (p-value < 1 × 10−3). (D) Arrested cells that have 1N DNA content are unbudded. Wild-type and vac17Δ pep12-60tsf cells were incubated at 24°C overnight, and then sifted to 37°C for 24 hr. After fixation, yeast were stained with PI, and cells with 1N DNA were sorted by flow cytometry. The sorted cells were observed by microscopy. For both wild-type and the vac17Δ pep12-60tsf double mutant 99% of the cells with 1N DNA were unbudded. Sorted cells from three individual experiments were counted. At least 400 cells were counted for each experiment.
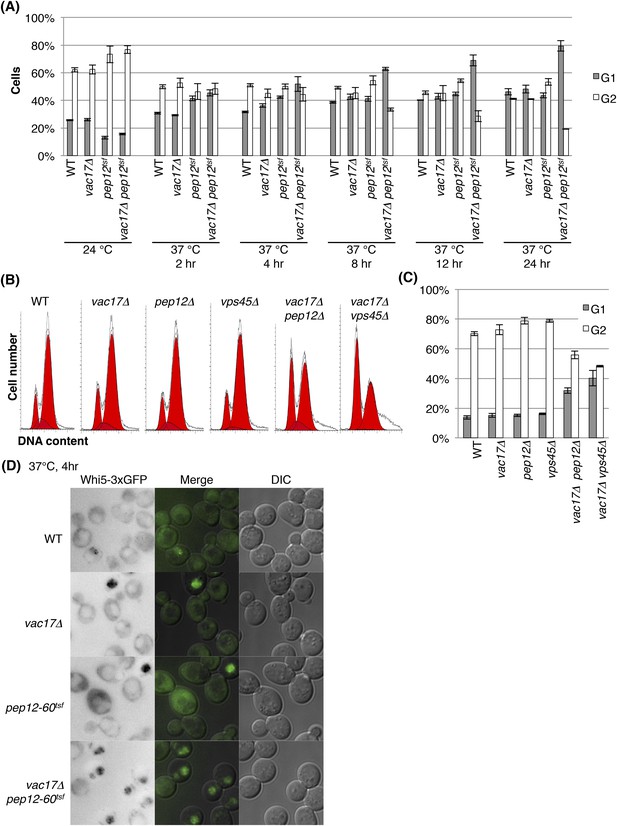
The vacuole is required for cell-cycle progression from early G1 phase.
(A) The vac17Δ pep12-60tsf double mutant is arrested in G1 phase at the restrictive temperature, 37°C. Percent cells in G1 (1N DNA) and G2 (2N DNA) phase after incubation at 37°C for 0, 2, 4, 8, 12, or 24 hr. Percent cells arrested in G1 are also shown in Figure 3B. Error bars; SD calculated from four independent experiments. (B) vac17Δ pep12Δ and vac17Δ vps45Δ double mutants exhibit an accumulation of cells arrested in G1 phase. Flow cytometry analysis of PI staining of yeast strains; wild-type, vac17Δ, pep12Δ, vps45Δ, vac17Δ pep12Δ, and vac17Δ vps45Δ. (C) Quantification of percent cells in G1 and G2 phase. Error bars; SD calculated from four independent experiments. (D) The vac17Δ pep12-60tsf double mutant arrests in early G1 phase at the restrictive temperature, 37°C. Wild-type, vac17Δ, pep12-60tsf, and vac17Δ pep12-60tsf cells which express Whi5-3xGFP from its endogenous locus, were incubated at 24°C overnight, and then sifted to 37°C for 0 or 4 hr. Images of Whi5-3xGFP localization after cells were incubated at 37°C for 4 hr. Quantification is shown in Figure 3C.
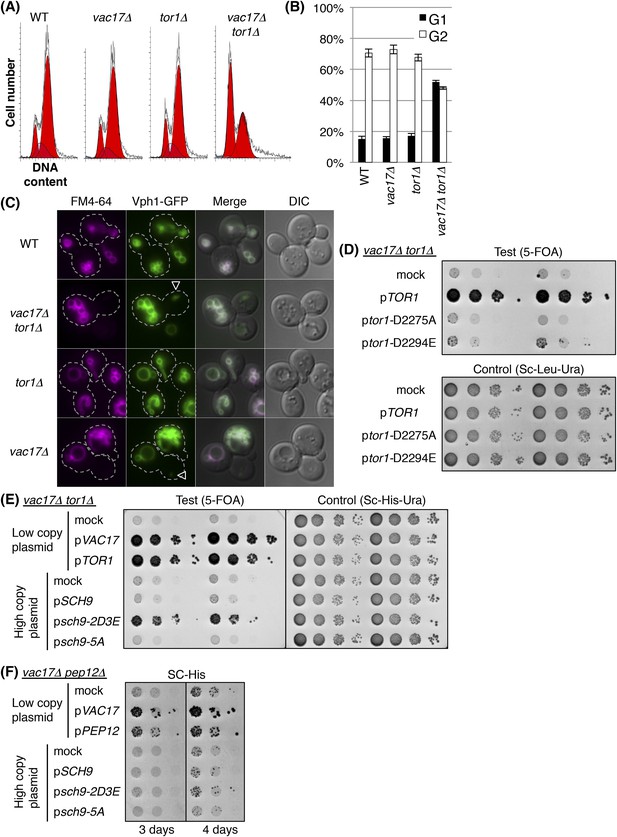
TORC1-SCH9 signaling from the new vacuole is required for cell-cycle progression.
(A) The vac17Δ tor1Δ double mutant exhibits an accumulation of G1 phase cells. Flow cytometry analysis with PI staining of yeast strains; wild-type, vac17Δ, tor1Δ, and vac17Δ tor1Δ. (B) Quantification of percent cells in G1 and G2 phase. Error bars; SD calculated from four independent experiments. (C) A new vacuole is synthesized in the new daughter cells of the vac17Δ tor1Δ double mutant. Wild-type, vac17Δ, tor1Δ, and vac17Δ tor1Δ cells which express Vph1-GFP from its endogenous locus, were pulse labeled with FM4-64. Arrowheads; new vacuole in daughter cells. (D) The kinase activity of target of rapamycin 1 (Tor1) is required for growth of the vacuole inheritance mutant, vac17Δ. Plasmids were transformed into a vac17Δ tor1Δ mutant containing pRS416 [URA3] TOR1. Plasmids tested were pRS315 [LEU2] (mock), pRS315 HA-TOR1, pRS315 HA-tor1-D2275A, or pRS315 HA-tor1-D2294E. Transformed colonies were cultured in liquid media and serial dilutions spotted onto SC+5-FOA or SC-Leu-Ura plates. Plates were incubated at 24°C for 4 days. (E) TORC1 signals from the new vacuole via Sch9. The phospho-mimetic sch9-2D3E mutant partially rescues the growth defect of the vac17Δ tor1Δ mutant. pRS413 (mock), pRS413 VAC17, pRS413 TOR1, pVT102-H (mock), pVT102-H SCH9, pVT102-H sch9-2D3E, or pVT102-H sch9-5A expressed in vac17Δ tor1Δ with pRS416 TOR1. Transformed colonies were cultured in liquid media and serial dilutions were spotted onto SC-His+5-FOA or SC-His-Ura plates, and incubated at 24°C for 4 days. (F) Sch9 signaling requires a functional vacuole. The phospho-mimetic sch9-2D3E mutant does not rescue the growth defect of the vac17Δ pep12Δ mutant. pRS413 (mock), pRS413 VAC17, pRS413 TOR1, pVT102-H (mock), pVT102-H SCH9, pVT102-H sch9-2D3E, or pVT102-H sch9-5A plasmids were expressed in a vac17Δ pep12Δ strain. Transformed colonies were cultured in liquid media and serial dilutions spotted onto an SC-His plate, and incubated at 24°C for 3 to 4 days.
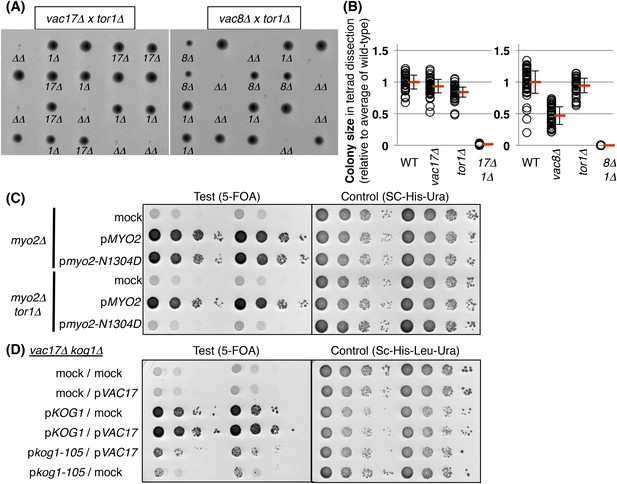
TORC1-SCH9 is required for the viability of vacuole inheritance mutants.
(A) The tor1Δ mutant exhibits a synthetic growth defect with vac17Δ and vac8Δ. Results of tetrad dissection of heterozygous diploids, VAC17/vac17Δ TOR1/tor1Δ and VAC8/vac8Δ TOR1/tor1Δ. vac17Δ = 17Δ; vac8Δ = 8Δ; tor1Δ = 1Δ; vac17Δ tor1Δ or vac8Δ tor1Δ double mutants = ΔΔ are indicated. (B) Quantification of colony size, relative to average of wild-type colonies. A total of 34 tetrads and 39 tetrads were analyzed for vac17Δ tor1Δ and vac8Δ tor1Δ, respectively. (C) The tor1Δ mutant exhibits a synthetic growth defect with the myo2-N1304D mutant. Plasmids were transformed into a myo2Δ and myo2Δ tor1Δ strain containing YCp50 MYO2. Plasmids tested were pRS413 (mock), pRS413 MYO2, or pRS413 myo2-N1304D. Transformed colonies were cultured in liquid media and serial dilutions were spotted onto SC+5-FOA or SC-His-Ura plate, and incubated at 24°C for 4 days. (D) The vac17Δ kog1-105 mutant showed synthetic growth defects. pRS313 (mock) and pRS415 (mock), pRS313 (mock) and pRS415 VAC17, pRS313 KOG1 and pRS415 (mock), pRS313 KOG1 and pRS415 VAC17, pRS313 kog1-105 and pRS415 VAC17, or pRS313 kog1-105 and pRS415 (mock) expressed in vac17Δ kog1Δ with pRS316 KOG1. Transformed colonies were cultured in liquid media and serial dilutions were spotted on SC-His-Leu+5-FOA or SC-His-Leu-Ura plates, and incubated at 24°C for 5 days.
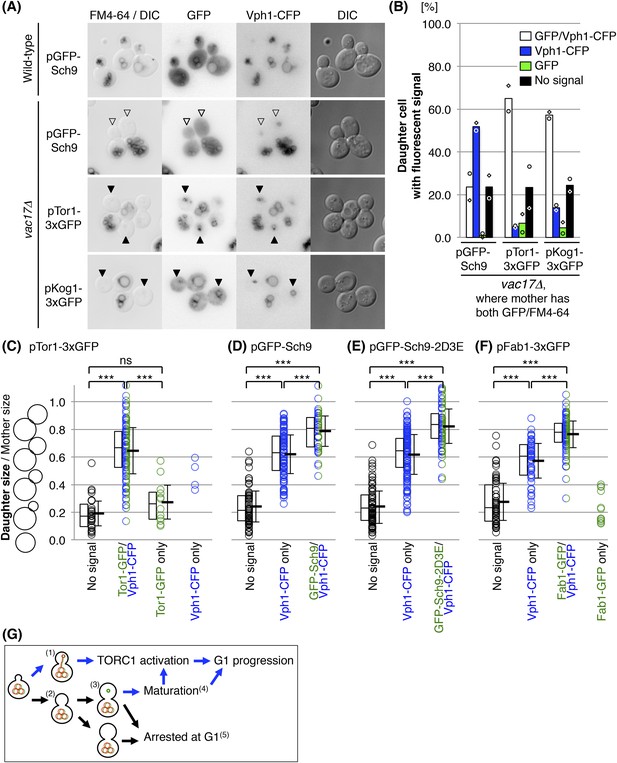
The newly synthesized vacuoles initially lack Sch9 and Fab1.
(A) Sch9 does not localize to the newly formed vacuole. Indicated plasmids were transformed into wild-type and vac17Δ strains, which express Vph1-CFP from its endogenous locus: pRS416 GFP-SCH9, pRS416 TOR1-3xGFP(D330), or pRS416 KOG1-3xGFP. Transformed cells were pulse labeled with FM4-64. Open arrowheads indicate a newly formed vacuole (Vph1-CFP) that was not inherited (lack of FM4-64), and is lacking GFP-Sch9. Closed arrowheads indicate a newly formed vacuole (Vph1-CFP) that was not inherited (lack of FM4-64), and with the GFP fusion protein, either Tor1-3xGFP(D330) or Kog1-3xGFP. (B) Quantification of cells with fluorescence (Vph1-CFP and/or GFP) in daughter cells, where the mother has both GFP and FM4-64 signals. Averages from two independent experiments, with n = 69 and n = 104 for GFP-Sch9, n = 166 and n = 110 for Tor1-3xGFP(D330), and n = 106 and n = 126 cells for Kog1-3xGFP, respectively. Open circles and squares indicate results of each experiment. (C) Tor1 is immediately recruited to the newly formed vacuoles. FM4-64 labeled vac17Δ cells that express Vph1-CFP from its endogenous locus, and Tor1-3xGFP expressed from a CEN plasmid with its endogenous promoter were used. Most small budded cells do not have fluorescent signals for any of the proteins, indicating that these small buds do not have a vacuole. In most cases, as the bud increases in size, Vph1-CFP and Tor1-3xGFP appear simultaneously. The middle line in the box plot indicates the median of the data set. The upper edge of the box indicates the 75th percentile of the data set, and the lower edge indicates the 25th percentile. ns; not a significant difference (p-value > 0.10); *** (p-value < 1 × 10−6). (D) Sch9 recruitment is delayed compared to Tor1, but eventually occurs. FM4-64 labeled vac17Δ cells expresses Vph1-CFP from its endogenous locus, and GFP-Sch9 expressed from a CEN plasmid with its endogenous promoter were used. In medium sized buds (0.62(±0.14) daughter size/mother size) only Vph1-CFP is present. The average bud size where both Vph1 and Sch9 are present is 0.79(±0.11). (E) Recruitment of Sch9-2D3E to the new vacuole is similar to the recruitment of wild-type Sch9. FM4-64 labeled vac17Δ cells which express Vph1-CFP from its endogenous locus, and GFP-Sch9-2D3E expressed from a CEN plasmid with its endogenous promoter were used. (F) The timing of the recruitment of Fab1 was similar to that observed for Sch9. FM4-64 labeled vac17Δ cells which express Vph1-CFP from its endogenous locus, and Fab1-3xGFP expressed from a CEN plasmid with its endogenous promoter were used. In medium sized buds (0.57(±0.13) daughter size/mother size) only Vph1-CFP is present. The average bud size where both Vph1 and Fab1 are present is 0.76(±0.10). (G) Model: The vacuole is essential for cell-cycle progression and functions in part through the TORC1 pathway. When the daughter cell receives vacuoles from the mother cell(1), the daughter can progress from G1. If the cell fails to inherit a vacuole(2), the cell generates a new vacuole(3), which is followed by maturation of the new vacuole prior to G1 progression(4). Without a functional vacuole, the daughter cell arrests at G1 phase(5).
Tables
Yeast strains used in this study
| Strain | Genotype | Source | Figure |
|---|---|---|---|
| LWY7235 | MATa, ura3-52, leu2-3,-112, his3-Δ200, trp1-Δ901, lys2-801, suc2-Δ9 | (Bonangelino et al., 1997) | – |
| LWY11678 | MATa, VPH1-GFP::KanMX | This study | Figures 1, 2, 4, Figure 1—figure supplement 1 |
| LWY12144 | MATa, VPH1-GFP::KanMX, vac17Δ::TRP1 | This study | Figures 1, 2, 4, Figure 1—figure supplement 1 |
| LWY15258 | MATa/α, VAC17/vac17Δ::TRP1, PEP12/pep12Δ::KanMX | This study | Figure 2 |
| LWY15612 | MATa/α, VAC17/vac17Δ::TRP1, VPS45/vps45Δ::KanMX | This study | Figure 2 |
| LWY14490 | MATa, VPH1-GFP::KanMX, pep12Δ::KanMX | This study | Figure 2 |
| LWY14493 | MATa, VPH1-GFP::KanMX, vac17Δ::TRP1, pep12Δ::KanMX | This study | Figure 2 |
| LWY15515 | MATa, GFP-PEP12::natNT2, VPH1-CFP::KanMX | This study | Figure 2 |
| LWY15506 | MATa, GFP-PEP12::natNT2, VPH1-CFP::KanMX, vac17Δ::TRP1 | This study | Figure 2 |
| LWY15263, LWY14462, LWY12369 | MATa | This study | Figures 3, 4, Figure 3—figure supplement 1 |
| LWY5798 | MATa, vac17Δ::TRP1 | (Tang et al., 2003) | – |
| LWY15244, LWY14468, LWY12366 | MATa, vac17Δ::TRP1 | This study | Figures 3, 4, Figure 3—figure supplement 1 |
| CBY9 | MATα, pep12-60tsf, leu2-3,112::pBHY11 CPY-Inv LEU2 | (Burd et al., 1997) | – |
| LWY15250 | MATa, pep12-60tsf | This study | Figure 3, Figure 3—figure supplement 1 |
| LWY15249 | MATa, vac17Δ::TRP1, pep12-60tsf | This study | Figure 3, Figure 3—figure supplement 1 |
| LWY15799 | MATa, WHI5-3xGFP::His3MX | This study | Figure 3, Figure 3—figure supplement 1 |
| LWY15791 | MATa, WHI5-3xGFP::His3MX, vac17Δ::TRP1 | This study | Figure 3, Figure 3—figure supplement 1 |
| LWY15789 | MATa, WHI5-3xGFP::His3MX, pep12-60tsf | This study | Figure 3, Figure 3—figure supplement 1 |
| LWY15814 | MATα, WHI5-3xGFP::His3MX, vac17Δ::TRP1, pep12-60tsf | This study | Figure 3, Figure 3—figure supplement 1 |
| LWY12364 | MATa, tor1Δ::KanMX | This study | Figure 4 |
| LWY12367 | MATα, vac17Δ::TRP1, tor1Δ::KanMX | This study | Figure 4 |
| LWY12168 | MATa, VPH1-GFP::KanMX, tor1Δ::KanMX | This study | Figure 4 |
| LWY12193 | MATa, VPH1-GFP::KanMX, vac17Δ::TRP1, tor1Δ::KanMX | This study | Figure 4 |
| LWY14142 | MATa, vac17Δ::TRP1, tor1Δ::KanMX, pRS416 TOR1 | This study | Figure 4 |
| LWY12358, LWY14487 | MATa, vac17Δ::TRP1, pep12Δ::KanMX | This study | Figure 4, Figure 3—figure supplement 1 |
| LWY11657 | MATa, VPH1-CFP::KanMX | This study | Figure 5 |
| LWY13781 | MATa, VPH1-CFP::KanMX, vac17Δ::TRP1 | This study | Figure 5 |
| LWY15610 | MATa/α, VAC8/vac8Δ::HIS3, PEP12/pep12Δ::KanMX | This study | Figure 2—figure supplement 1 |
| LWY15614 | MATa/α, VAC8/vac8Δ::HIS3, VPS45/vps45Δ::KanMX | This study | Figure 2—figure supplement 1 |
| LWY2947 | MATα, myo2Δ::TRP1, YCp50-MYO2 | (Catlett and Weisman, 1998) | Figure 2—figure supplement 1, Figure 4—figure supplement 1 |
| LWY12443 | MATα, pep12Δ::KanMX, myo2Δ::TRP1, YCp50-MYO2 | This study | Figure 2—figure supplement 1 |
| LWY15581 | MATα, vps45Δ::KanMX, myo2Δ::TRP1, YCp50-MYO2 | This study | Figure 2—figure supplement 1 |
| LWY14497 | MATa, pep12Δ::KanMX | This study | Figure 3—figure supplement 1 |
| LWY14475 | MATa, vps45Δ::KanMX | This study | Figure 3—figure supplement 1 |
| LWY14463 | MATa, vac17Δ::TRP1, vps45Δ::KanMX | This study | Figure 3—figure supplement 1 |
| LWY1 | MATa/α, TOR1/tor1Δ::KanMX | This study | Figure 4—figure supplement 1 |
| LWY15616 | MATa/α, VAC8/vac8Δ::HIS3, TOR1/tor1Δ::KanMX | This study | Figure 4—figure supplement 1 |
| LWY12001 | MATa, tor1Δ::KanMX, myo2Δ::TRP1, YCp50-MYO2 | This study | Figure 4—figure supplement 1 |
| LWY13595 | MATa, vac17Δ::TRP1, kog1Δ::KanMX, pRS316 KOG1 | This study | Figure 4—figure supplement 1 |
-
Each above haploid strain is ura3-52, leu2-3,-112, his3-Δ200, trp1-Δ901, lys2-801, suc2-Δ9, and diploid strain is ura3-52/ura3-52, leu2-3,-112/leu2-3,-112, his3-Δ200/his3-Δ200, trp1-Δ901/trp1-Δ901, lys2-801/lys2-801, suc2-Δ9/suc2-Δ9.
Plasmids used in this study
| Plasmid name | Description | Source | Figure |
|---|---|---|---|
| pBlueScript SK+ GFP-PEP12::natNT2 | Amp | This study | Figure 2 |
| pRS416 TOR1 | CEN, URA3 | This study | Figure 4 |
| pRS413 | CEN, HIS3 | (Sikorski and Hieter, 1989) | Figure 4 |
| pRS315 HA-TOR1 | CEN, HIS3 | Gift from Dr Robbie Loewith | Figure 4 |
| pRS315 HA-tor1-D2275A | CEN, HIS3 | This study | Figure 4 |
| pRS315 HA-tor1-D2294E | CEN, HIS3 | This study | Figure 4 |
| pRS413 VAC17 | CEN, HIS3 | This study | Figure 4 |
| pRS413 TOR1 | CEN, HIS3 | This study | Figure 4 |
| pVT102-H | 2μ, HIS3 | (Vernet et al., 1987) | Figure 4 |
| pVT102-H SCH9 | 2μ, HIS3 | This study | Figure 4 |
| pVT102-H sch9-2D3E | 2μ, HIS3 | This study | Figure 4 |
| pVT102-H sch9-5A | 2μ, HIS3 | This study | Figure 4 |
| pRS416 GFP-SCH9 | CEN, URA3 | (Urban et al., 2007) | Figure 5 |
| pRS416 GFP-sch9-2D3E | CEN, URA3 | This study | Figure 5 |
| pRS416 TOR1-3xGFP(D330) | CEN, URA3 | This study | Figure 5 |
| pRS416 KOG1-3xGFP | CEN, URA3 | This study | Figure 5 |
| pRS416 FAB1-3xGFP | CEN, URA3 | (Jin et al., 2008) | Figure 5 |
| pRS413 | CEN, HIS3 | (Sikorski and Hieter, 1989) | Figure 2—figure supplement 1, Figure 4—figure supplement 1 |
| pRS413 MYO2 | CEN, HIS3 | (Catlett and Weisman, 1998) | Figure 2—figure supplement 1, Figure 4—figure supplement 1 |
| pRS413 myo2-N1304D | CEN, HIS3 | (Catlett et al., 2000) | Figure 2—figure supplement 1, Figure 4—figure supplement 1 |
| pRS416 KOG1 | CEN, URA3 | (Jin et al., 2014) | Figure 4—figure supplement 1 |
| pRS313 | CEN, HIS3 | (Sikorski and Hieter, 1989) | Figure 4—figure supplement 1 |
| pRS415 | CEN, LEU2 | (Sikorski and Hieter, 1989) | Figure 4—figure supplement 1 |
| pRS415 VAC17 | CEN, LEU2 | (Jin et al., 2009) | Figure 4—figure supplement 1 |
| pRS313 KOG1 | CEN, HIS3 | (Nakashima et al., 2008) | Figure 4—figure supplement 1 |
| pRS313 kog1-105 | CEN, HIS3 | (Nakashima et al., 2008) | Figure 4—figure supplement 1 |





