Developmental compartments in the larval trachea of Drosophila
Figures
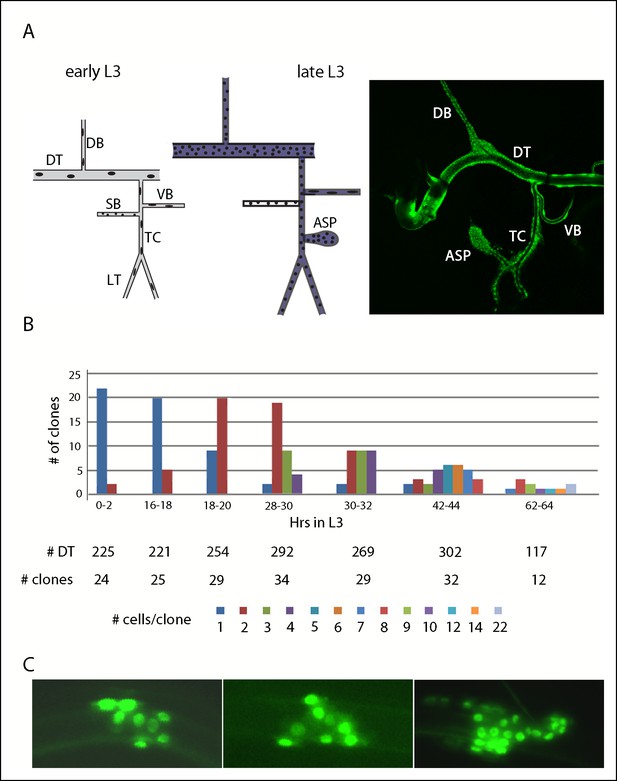
Cell divisions in the second tracheal metamere during the third instar.
(A) Drawings of the Tr2 branches before (early L3) and after (late L3) the onset of cell divisions; dorsal trunk (DT), dorsal branch (DB), visceral branch (VB), spiracular branch (SB), transverse connective (TC), lateral trunk (LT), air sac primordium (ASP), region in which btl-Gal4 is expressed (purple). (right panel) Nuclei in Tr2 visualized by fluorescence of GFP expressed by the btl-Gal4 driver. (B) Bar graph representing clones of indicated sizes in the DT at the indicated times post L2-L3 molt; the numbers of DTs examined, numbers of cells in the clones and color code are listed below. (C) Three representative DT clones showing cell proliferation.
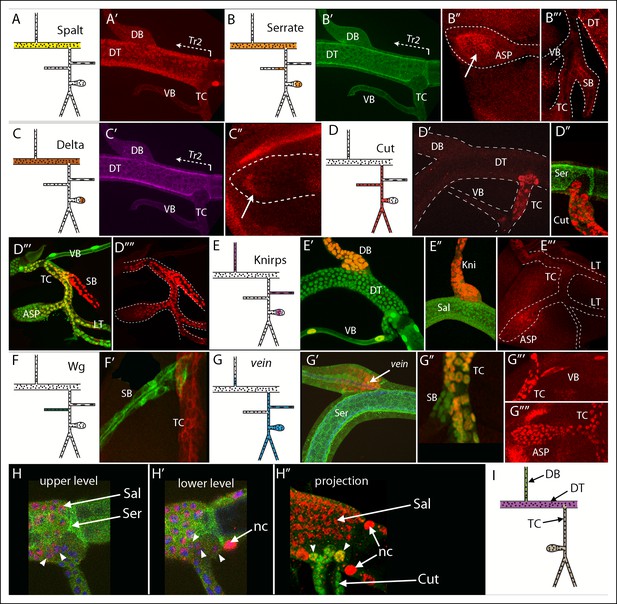
Patterns of gene expression in the second tracheal metamere of third instar larvae.
(A-–C) A Tr2 preparation stained for Sal, Ser and Delta; (A) Sal expression was specific to the DT; (A’) anti-Sal antibody stained the DT, but not DB, TC or VB; (B) Ser was expressed in the DT, ASP and SB; anti-Ser antibody stained the DT but not DB, TC or VB (B’), the distal ASP (B”) and the SB (B”’); (C) Delta was expressed in the DT and ASP; (C’) anti-Delta antibody stained the DT specifically and the distal ASP; (D) Cut was expressed in the TC, SB, ASP and LT; anti-Cut antibody stained the TC (D’) but not Ser-expressing DT cells (D”), and the SB, LT and proximal ASP (D”’, D””; Cut expression in the myoblasts was erased manually in the image file to highlight tracheal expression only); (E) Knirps was expressed in the DB, VB and ASP; anti-Knirps antibody stained nuclei in the DB and VB (E’) but not Sal-expressing DT cells (E”) and stained the medial region of the ASP (E”’); nuclei in (D”’), (E’) and (G”) contained GFP (btl-Gal4 UAS-nlsGFP); (F) wg was expressed in the SB; (F’) wg expression in the SB indicated by GFP fluorescence (wg-Gal4 UAS-GFP) but not in the TC (btl-CD8:Cherry); (G) vein was expressed in the proximal DB, TC, VB, ASP and LT; vein expression indicated by staining for vein-lacZ (red) in DB adjacent to Ser-expressing DT cells (G’), in the TC but not SB (G”), and in the VB, TC and ASP (G”’, G””). (H, H’) Sal expression (red, nuclear) and Ser expression (green, non-nuclear) detected in DT cells (arrows) in upper level and lower level optical sections; arrowheads indicate 5 cells in the TC domain that express Sal but not Ser; nc (node cell), DAPI-stained nuclei (blue); (H”) projection image showing Sal (red) and Cut (green) expressing TC cells; 2 cells in the TC domain (arrowheads) stained for both Sal and Cut. (I) Drawing with the DT, DB and TC expression domains indicated in purple, green and brown, respectively. The clonal analysis has not established whether the VB and SB are expression domains distinct from the TC.
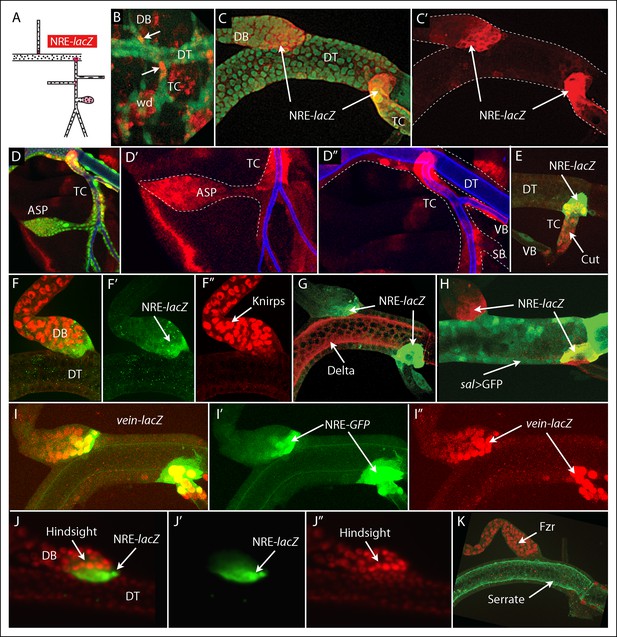
Discrete regions of Notch activation in the second tracheal metamere.
(A) Drawing of a late L3 Tr2 metamere with red areas indicating the regions that express the Notch reporter NRE-lacZ; (B) Tr2 of a stage 14–16 embryo with tracheal nuclei marked with GFP fluorescence (btl-Gal4 UAS GFP), stained with anti-β-galactosidase antibody to identify cells expressing NRE-lacZ at DT/DB and DT/TC junctions (arrows); (C, C’) NRE-lacZ expression (red) at DT/DB and DT/TC junctions of L3 Tr2; Low (D) and high magnification (D’ D”) images showing NRE-lacZ expression in the ASP, TC and VB; (E) Double stained preparation shows coincidence of NRE-lacZ and Cut expression in the TC; (F-–F”) images showing coincidence of NRE-lacZ and Knirps expression in the DB; (G) image showing juxtaposition of NRE-lacZ expression in the DB and TC with Delta; (H) image showing sal expression juxtaposed with NRE-lacZ expression in the DB and overlapping with NRE-lacZ expression in the TC; (I–I”) images showing coincidence of NRE-GFP and vein-lacZ expression at the DT/DB and DT/TC junctions; (J–J”) images showing juxtaposition of DB cells that express high levels of NRE-lacZ with cells that express the Notch target Hindsight; (K) the Notch target Fzr is expressed in DB cells adjacent to cells that express high levels of NRE-lacZ.
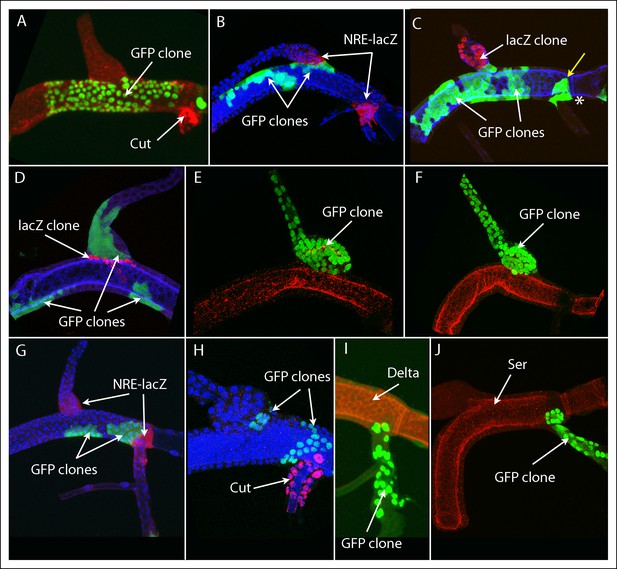
Marked clones that meet but do not cross into or from the dorsal trunk.
(A-–D) Marked clones in the DT line the DT/DB junction. (A) large patch of GFP-expressing cells abuts the DT/DB junction; Cut expression in TC (red); clone induction by regimen Experiment E (Table 1). (B) several GFP expressing clones in DT (Expt. A), one of which abuts the DT/DB junction defined by NRE-lacZ expression (red). (C) several GFP expressing clones in DT (white arrows), one of which abuts the DT/DB junction and is juxtaposed to a LacZ-expressing DB clone; a single cell clone expressing GFP in the TC (yellow arrow) abuts the DT/TC junction and a GFP-expressing TC clone (*); Ser expression (blue); (Expt. G). (D) LacZ-expressing DT clone abuts the DT/DB junction and is juxtaposed to a GFP-expressing DB clone; Ser expression (blue); (Expt G). (E, F) GFP expressing DB clones that abut the DT/DB junction (Expt. F). (G–J) GFP expressing clones that abut the DT/TC junction that was also defined by expression of NRE-lacZ (G; Expt. B), Cut (H; Expt. E), Delta (I; Expt. D) and Ser (J; Expt. F). (B, G, H) DAPI (blue).
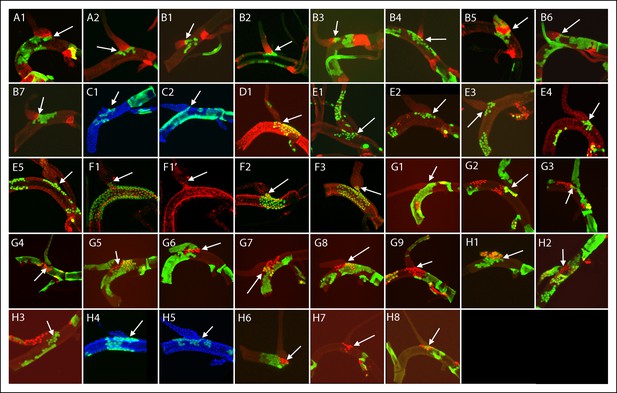
Dorsal trunk clones at the DT/DB border.
GFP-expressing clones induced by regimens A-H; arrows point to DT/DB junction. (A, B) NRE-LacZ (red); (C, H4,5) DAPI (blue); (D) anti-Delta (red); (E) anti-Cut (red); (F) anti-Ser (red); the abnormal morphology of the DT/DB junction was characteristic of the Minute genotype; (F1) all M cells in the clone expressed Ser; (F1’) red channel only showing limit of Ser expression at DT/DB junction. (G, H) Dual clone regimens produced clones that expressed GFP (green), LacZ (red) or both (yellow); clones appear in the projection images in (H1, H2) to be juxtaposed at DT/DB junction but are not.
Dorsal branch clones at the DT/DB border.
GFP-expressing clones induced by regimens B-H; arrows point to DT/DB junction. (B) NRE-LacZ (red); (C) DAPI (blue); (D) anti-Delta (red); (E) anti-Cut (red); (G, H) Dual clone regimens produced clones that expressed GFP (green), LacZ (red) or both (yellow).
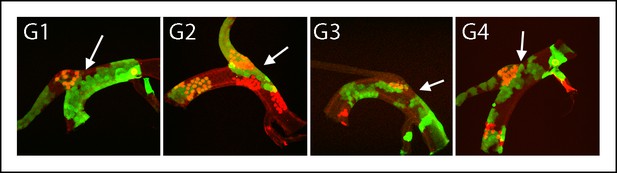
Dorsal trunk and dorsal branch clones abutting the same DT/DB border.
GFP- and LacZ-expressing clones induced by regimen G; arrows point to DT/DB junction. Regimen G produced clones that expressed GFP (green), LacZ (red) or both (yellow).
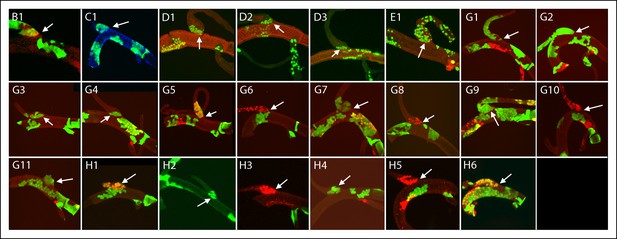
Dorsal trunk clones at the DT/TC border.
GFP-expressing clones induced by regimens A-H; arrows point to DT/TC junction. (A, B) NRE-LacZ (red); (C) DAPI (blue); (D) anti-Delta (red); (E) anti-Cut (red); (F) anti-Ser (red); (F1, F1”) images of upper- and bottom-most optical sections; (F1’) higher magnification image from (F) showing coincidence of GFP and Ser expression; the abnormal morphology of the DT/TC junction was unique to this sample; all M cells in the clone expressed Ser; (G, H) Dual clone regimens produced clones that expressed GFP (green), LacZ (red) or both (yellow). Panel (D3), which is the same specimen as panel (D1, Figure 4—figure supplement 1), is a projection image of sections representing one surface that illustrates the GFP labeled DT clone at the DT/TC border.
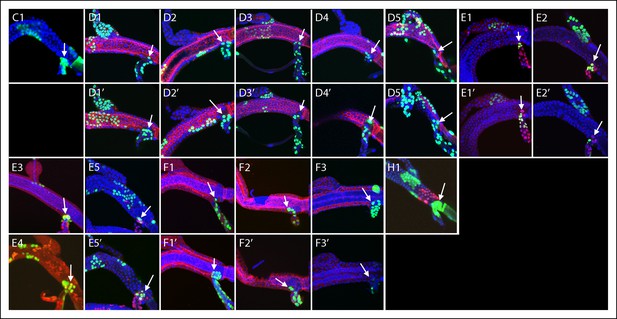
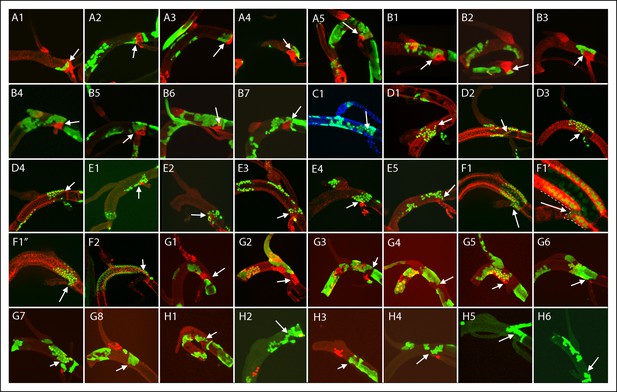
Transverse connective clones abutting the DT/TC border.
GFP-expressing clones induced by regimens C, D, E, F, H; arrows point to DT/TC junction. DAPI (blue); (D) anti-Delta (red); (E) anti-Cut (red); (F) anti-Ser (red); (H) Dual clone regimen produced clones that expressed GFP (green) and LacZ (red). (D3) is also shown in Figure 4 (I), (F1) is also shown in Figure 4 (J); both are included here to show separate projection images of upper and lower layers.
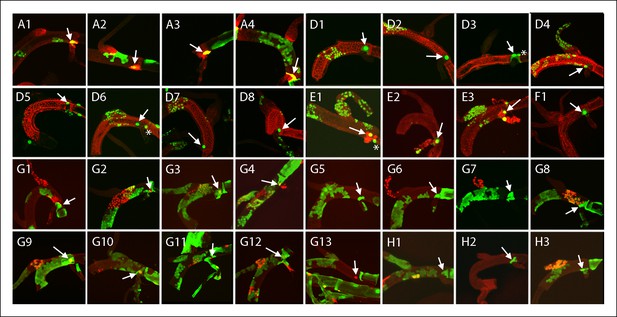
Large marked cells in the TC domain of the DT.
Panel labels refer to marking regimens for clones; arrows indicate the one or two large marked cells in the TC domain and (*) indicates node cells. (A) NRE-LacZ (red); (D) anti-Delta (red); (E) anti-Cut (red); (F) anti-Ser (red); (G, H) dual clone regimens produced clones that expressed GFP (green), LacZ (red) or both (yellow).
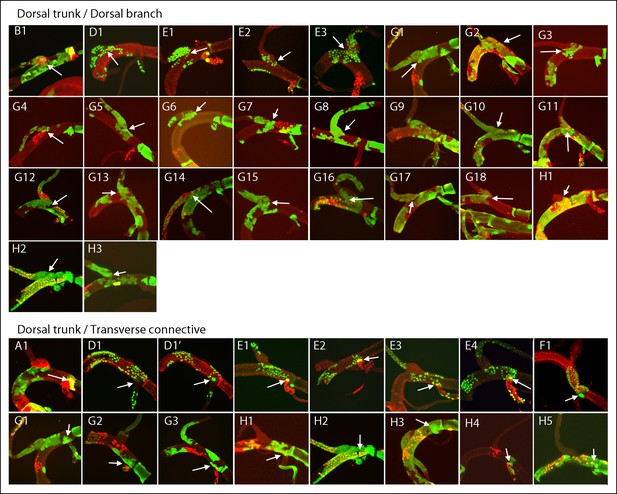
Marked patches of cells that straddle the DT/DB and DT/TC borders.
Panel labels refer to marking regimens for DT/DB clones (upper panels) and DT/TC clones (lower panels); borders indicated by arrows. (A, B) NRE-LacZ (red); (D) anti-Delta (red); (E) anti-Cut (red); (F) anti-Ser (red); (G, H) dual clone regimens produced clones that expressed GFP (green), LacZ (red) or both (yellow). DT/DB: (E2) Most GFP-expressing cells in this patch are in the DB, 2–4 are in DT. (G4) GFP-expressing patch contains approximately 8 cells in the DT and a larger number in the DB; an independent LacZ-expressing (red) clone is in the DT; merging of red and green fluorescence from different optical sections (yellow). (H1) Patch contains 3–4 GFP-expressing DB cells and a larger number in DT, which also has cells expressing both GFP and LacZ (yellow). (H3) Patch contains 3–4 DT and a larger number of DB cells expressing GFP. DT/TC: (A1 also in Figure 4—figure supplement 1; H2 also H2 upper panel). In (A1, E1, E4, F1, H1, H2, H3, H4, H5), the patch of GFP-expressing DT cells included one large cell in TC domain. (D1, D1’) Projections of optical sections at different planes; a GFP-expressing clone defined the DT/TC border from DT side in (D1) and included a large TC domain cell in (D1’). (E2) GFP-expressing DT patch included one upper layer and one lower layer large TC domain cell. (E3) GFP-expressing patch in the DT contiguous with 6–8 GFP-expressing TC cells. (G1 (also G1 upper panel)) One GFP-expressing DT cell next to one GFP-expressing TC cell and one LacZ-expressing cell (red) on other surface. (G2) One GFP-expressing DT cell next to one GFP-expressing TC cell. (G3) Two GFP-expressing DT cells next to one GFP-expressing TC cell, and a LacZ-expressing TC clone (red) also abutting the border.
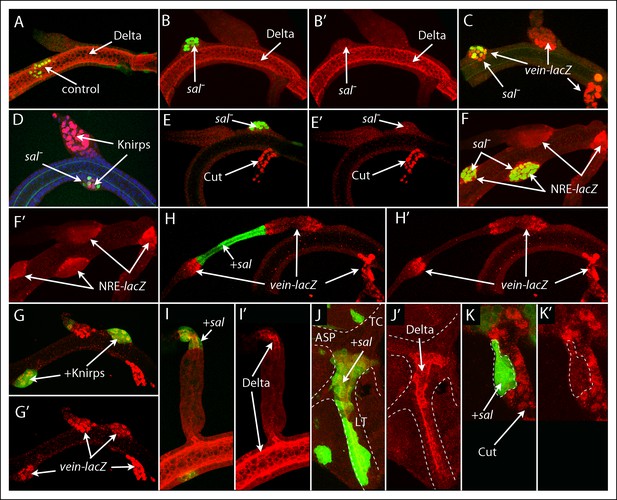
Mutant clones transform dorsal branch, dorsal trunk and transverse connective cells.
(A) Control DT clone of GFP-expressing cells with no effect on morphology or expression of Delta; (B-–F’) Clones of sal mutant DT cells generated bulges, apparently sorting out, and lacked Delta expression (B, B’), ectopically expressed vein-lacZ (C) and Knirps (D), not normally expressed by DT cells, but not Cut (E, E’) and activated the NRE-lacZ reporter (F, F’). (G, G’) Clone ectopically expressing Knirps in the DT activated vein-lacZ expression. (H, I) Clones that ectopically expressed Sal in the DB reduced the diameter of the DB and induced neighboring wild type cells to express vein-lacZ (H, H’’) and ectopically expressed Delta (I, I’’). (J, K) Clones that ectopically expressed Sal in the TC ectopically expressed Delta, which is not normally expressed in the TC (J, J’), but did not express Cut, which is normally expressed in the TC (K, K’).
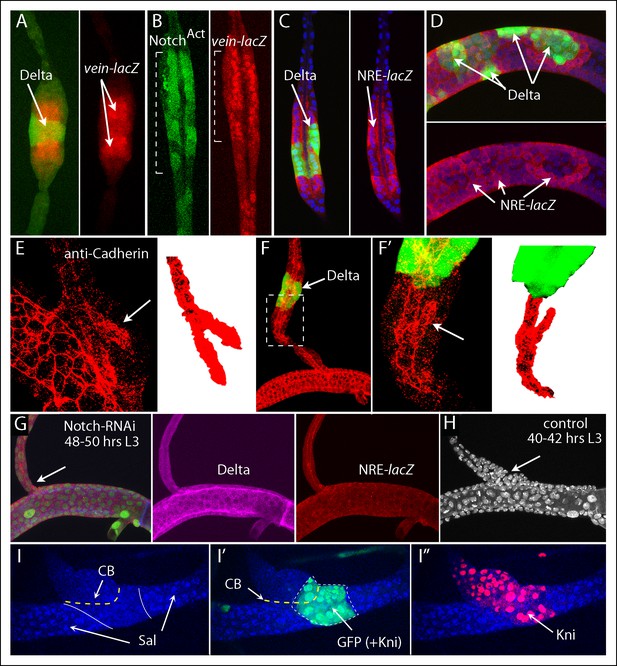
Notch signaling is required by dorsal branch cells at the junction with the dorsal trunk.
(A) Clone of DB cells that ectopically expressed Delta (green) increased the diameter of the DB and induced wild type neighbors to express vein-lacZ (red), normally expressed in the DB only by cells at the DT junction. (B) Clone of DB cells (indicated by brackets) that ectopically expressed NotchACT (green) increased the diameter of the DB and activated vein-lacZ. (C, D) Clones of DB (C) and DT (D) cells that ectopically expressed Delta (green) activated NRE-lacZ expression in adjacent cells. (E) Image of the proximal DB stained with anti-DE-Cadherin antibody; bifurcated lumen indicated by arrow (left panel) and isolated and colored red (right panel). (F) Delta expressing clone (green) in the DB induced a bifurcated luminal structure with orientation opposite to normal. (F’) Higher magnification view of boxed area of (F). (G) Expression of Notch-RNAi did not affect DT morphology or Delta expression (magenta) but reduced NRE-lacZ expression at both DT/DB and DT/TC junctions and reduced the diameter of the proximal DB (arrow). Number of DT cells was reduced to levels characteristic of normal younger larvae whose proximal DB is expanded (H). (I-I”) Clone that ectopically expressed kni, Notch-RNAi and GFP in the DT did not express Sal (I, blue) and did not sort out from DT cells (I, I”). DT/DB compartment border (CB) indicated by dashed yellow line, borders of Sal expression by solid white line (I) and border of clone by dashed white line (I').
Tables
Regimen | Marking system | # Specimens | # Marked DT | HS stage | HS | DT/DB border clones | DT/TC border clones | |||
DT side | DB side | DT DB | DT side | TC side | ||||||
A | GFP, NRE lacz | 106 | 40 | 48-50h AEL | 8' 35° | 3 | 0 | 5 | 0 | |
B | GFP, NRE-lacz | 59 | 24 | 24-32h AEL | 5' 37° | 7 | 1 | 8 | 0 | |
C | GFP | 29 | 11 | 24-32h AEL | 5' 37° | 2 | 1 | 1 | 1 | |
D | MARCM, α-Delta | 52 | 43 | 4-6h AEL | 60' 38° | 1 | 3 | 4 | 5 | |
E | MARCM, α-Cut | 48 | 34 | 4-6h AEL | 60' 38° | 6 | 2 | 6 | 5 | |
F | M MARCM, α-Ser | 251 | 30 | 4-6h AEL | 30' 38° | 3 | 2 | 2 | 3 | |
G | GFP & LacZ, α-Ser | 119 | 114 (GFP) 79 (LacZ)* | 24-26h AEL | 15' 37° | 11 | 11 | 6** | 8 | 0 |
H | GFP & LacZ | 116 | 61 (GFP) 34 (LacZ)* | 24-26h AEL | 6' 37° | 9 | 5 | 0 | 6 | 1 |
TOTAL | 780 | 361 | 42 | 25 | 6 | 40 | 15 | |||
-
Genotype A: NRElacz/hsFLP; actin>y >GAL4,UAS-GFP/ ; /MKRS B: NRElacz/hsFLP; actin>y >GAL4,UAS-GFP/ ; /MKRS C: hsFLP/Y; actin>y >GAL4,UAS-GFP/ ; /MKRS D: hsFLP122,tubGAL4,UAS-NLS-GFP/ ; tubGal80 FRT40a/FRT40a; / E: hsFLP122,tubGAL4,UAS-NLS-GFP/ ;tubGal80,FRT40a /FRT40a; / F: hsFLP,tubGAL4, UAS-GFP-NLS; / ;RpS17,tubGal80,FRT80a/FRT80a G: hsFLP/ orY; actin>y >GAL4,UAS-GFP/ ;actin>stop>lacZ-NLS/MKRS H: hsFLP/ orY; actin>y >GAL4,UAS-GFP/ ;actin>stop>lacZ-NLS/MKRS * # DTs with clones at or near the DB and TC borders ** specimens with independently marked DT and DB clones





