A pain-mediated neural signal induces relapse in murine autoimmune encephalomyelitis, a multiple sclerosis model
Figures
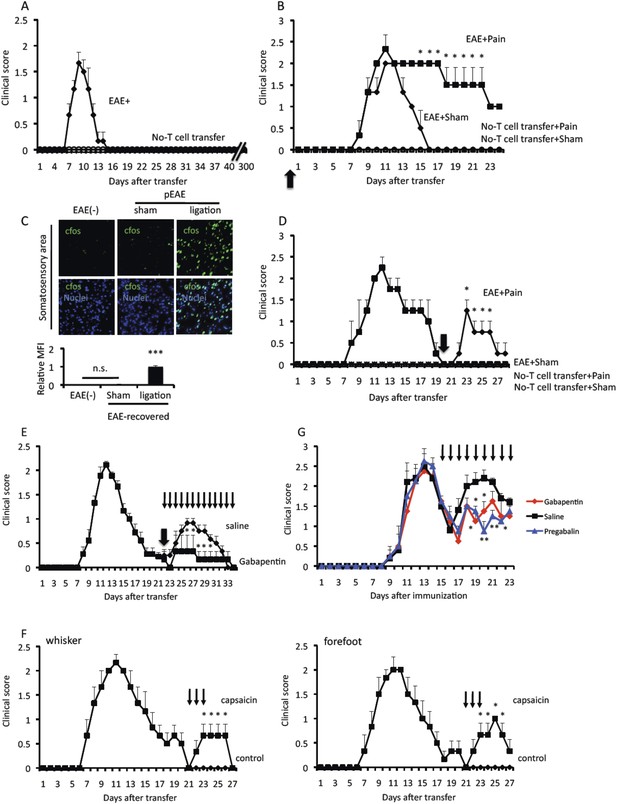
Pain induction causes EAE relapse.
Pathogenic CD4+ T cells isolated from EAE mice were intravenously transferred into wild type C57BL/6 mice. (A) EAE development without pain induction (n = 8–10 per group). (B) EAE development with or without pain induction on the same day of T cell transfer (day 0, thick arrow) (n = 3–5 per group). (C) Immunohistochemical staining for cfos in the somatosensory area of wild type mice and EAE-recovered mice and EAE-recovered mice with pain induction (n = 2–3 per group) (top). Quantification of the histological analysis of the ACC (bottom). (D) EAE development with or without pain induction 20 days (thick arrow) after T cell transfer in EAE-recovered mice (n = 3–5 per group). (E) EAE development in an EAE relapse induced by pain model in the presence or absence of Gabapentin (day 22–34, thin arrows). Pain was induced 22 days after the T cell transfer in EAE-recovered mice (thick arrow) (n = 3–5 per group). (F) EAE development with or without capsaicin treatment (day 21–23, thin arrows) in the whiskers or forefeet (n = 3–5 per group). (G) EAE development in a relapsing-remittent model in the presence Gabapentin (red), Pregabalin (blue), or saline (black) everyday from day 15 after immunization (thin arrows) (n = 4–5 per group). Mean scores ± SEM are shown. *, p < 0.05, **, p < 0.01, ***, p < 0.001, n.s., not significant. Experiments were performed at least 3 times; representative data are shown.
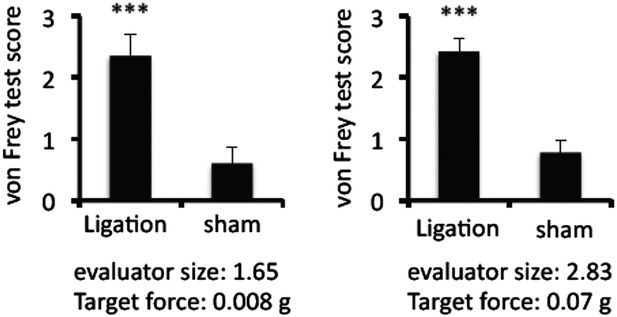
Ligation of the middle branch of trigeminal neurons increased von Frey test values.
Von Frey test scores in the presence or absence of ligation (n = 8–10 per group). Mean scores ± SEM are shown. ***, p < 0.001. Experiments were performed at least 3 times; representative data are shown.
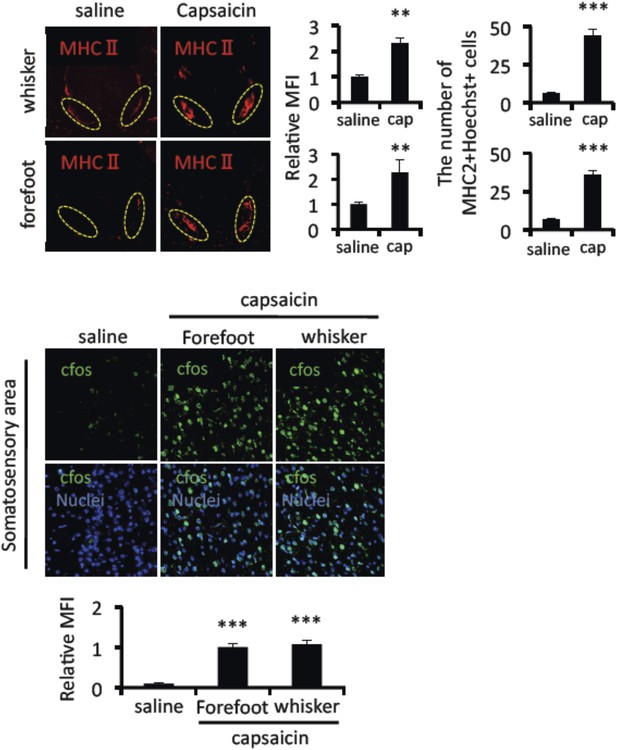
Mice treated with capsaicin enhanced the accumulation of MHC class II+CD11b+ cells and cfos expression in neurons of the somatosensory area without pain induction.
Immunohistochemical staining for MHC class Ⅱ in the L5 cord with administration of capsaicin in the whiskers or forefeet region (n = 4 per group). Dotted circles show accumulated cells at the ventral vessels (left). Quantification of the histological analysis around the ventral vessels based on MFI in dotted circles (middle) and cell number of the accumulated MHC class II+ cells in dotted circles by using serial frozen sections stained with anti-MHC class II antibody and Hoechst (right). Immunohistochemical staining for cfos in the somatosensory area of EAE-recovered mice with capsaicin treatment (8 hr) in the whiskers or forefeet (n = 3–5 per group). Quantification of the histological analysis of the somatosensory area based on cfos MFI (right bottom). Mean scores ± SEM are shown. **, p < 0.01; ***, p < 0.001. Experiments were performed at least 3 times; representative data are shown.
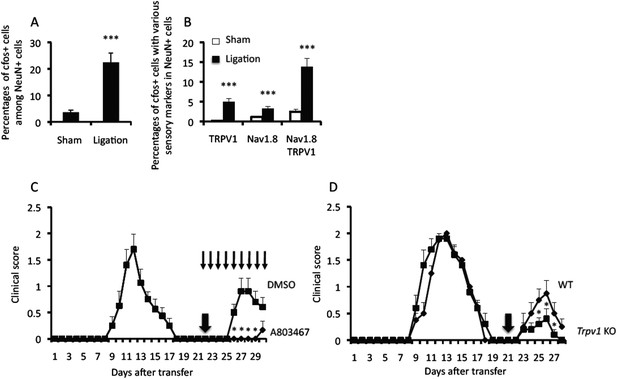
Sensory activation is involved in EAE relapse.
(A) Percentages of cfos+ cells among NeuN+ cells in the presence or absence of ligation in the trigeminal ganglions of wild type mice (n = 2–3 per group). (B) Percentages of cfos+ cells with sensory markers TRPV1 and/or Nav1.8 in the presence or absence of ligation of the trigeminal ganglions of wild type mice (n = 2–3 per group). (C) EAE development with or without A803467 treatment (day 22–30, thin arrows) in the presence of pain induction 22 days (thick arrow) after T cell transfer in EAE-recovered mice (n = 4–5 per group). (D) EAE development in TRPV1 deficient mice in the presence of pain induction 21 days (arrow) after T cell transfer in EAE-recovered mice (n = 4–5). Mean scores ± SEM are shown. *, p < 0.05, ***, p < 0.001, n.s., not significant. Experiments were performed at least 3 times; representative data are shown.
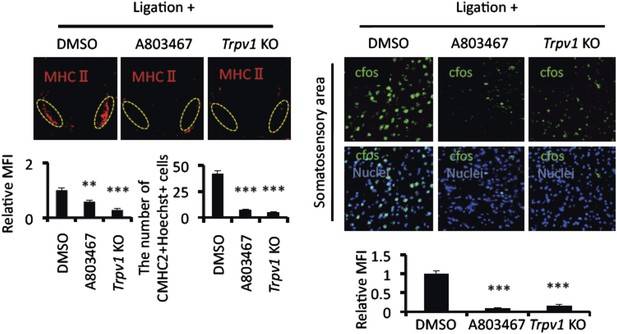
Mice treated with A803467 and TRPV1-deficient hosts suppressed the accumulation of MHC class II+CD11b+ cells and cfos expression in neurons of the somatosensory area after pain induction.
Immunohistochemical staining for MHC class II in the L5 cord with pain induction (8 hr) and treatment with A803467 in wild type or TRPV1-deficient EAE-recovered mice (left top) (n = 3–5 per group). Dotted circles show accumulated cells at the ventral vessels. Quantification of the histological analysis around the ventral vessels based on MFI in dotted circles (left bottom) and cell number of the accumulated MHC class II+ cells in dotted circles by using serial frozen sections stained with anti-MHC class II antibody and Hoechst (bottom right). Immunohistochemical staining for cfos in the somatosensory area of EAE-recovered mice with pain induction (8 hr) and treatment with A803467 administration in wild type or TRPV1-deficient EAE-recovered mice (top right) (n = 3–5 per group). Quantification of the histological analysis of the somatosensory area based on cfos MFI (right bottom). Mean scores ± SEM are shown. **, p < 0.01; ***, p < 0.001. Experiments were performed at least 3 times; representative data are shown.
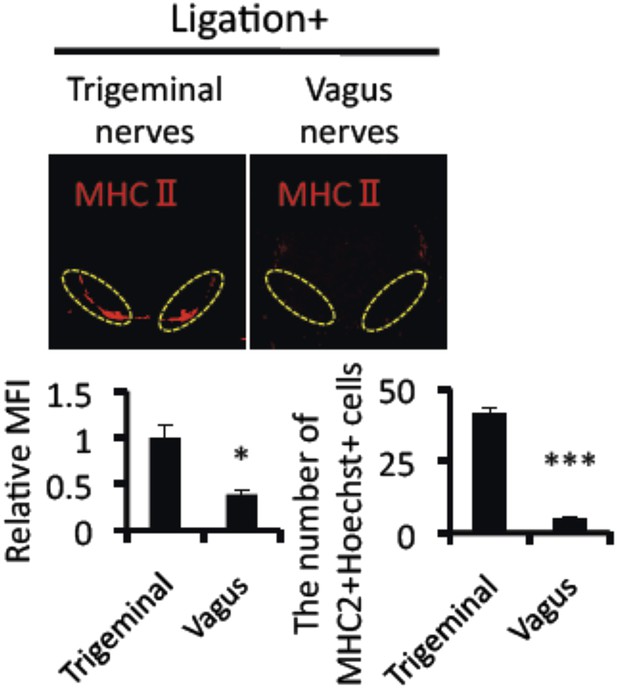
Mice treated with ligation via vagus nerves did not induce the accumulation of MHC class II+CD11b+ cells.
Immunohistochemical staining for MHC class II in the L5 cord with ligation (8 hr) via vagus or trigeminal nerves 21 days after T cell transfer in EAE-recovered mice (n = 4–5 per group). Dotted circles show accumulated cells at the ventral vessels (top). Quantification of the histological analysis around the ventral vessels based on MFI in dotted circles (left bottom) and cell number of the accumulated MHC class II+ cells in dotted circles by using serial frozen sections stained with anti-MHC class II antibody and Hoechst (right bottom). Mean scores ± SEM are shown. *, p < 0.05, **, p < 0.01, ***, p < 0.001, n.s., no significance. Experiments were performed at least 3 times; representative data are shown.
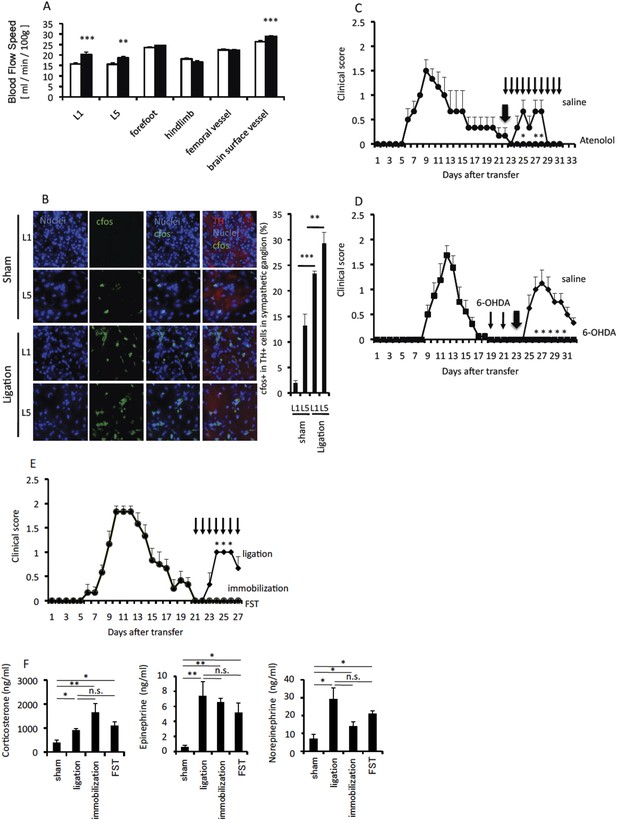
Sympathetic activation is involved in pain-mediated EAE relapse.
Pain was induced in wild type C57BL/6 mice or EAE-recovered mice 20 days after pathogenic T cell transfer. (A) Blood flow speeds in blood vessels of various organs were measured 2 days after pain induction (closed bars) or in sham operated mice (open bars) (n = 3–5 per group). (B) Activation of sympathetic neurons in the first or fifth lumbar sympathetic ganglia was evaluated by cfos expression. Immunohistochemical staining for tyrosine hydroxylase (TH) is also shown (red) (n = 3 per group). Quantification of the histological analysis of the somatosensory area based on cfos MFI (right). (C) Pathogenic CD4+ T cells isolated from EAE mice were intravenously transferred into wild type C57BL/6 mice. Pain was induced 22 days later (thick arrow), and EAE development was evaluated with or without atenolol treatment every day from day 22 in EAE-recovered mice (thin arrows) (n = 3–5 per group). (D) EAE development with or without 6-OHDA treatment at day 19 and day 21 (thin arrows) upon pain induction at day 23 (thick arrow) after T cell transfer in EAE-recovered mice (n = 3–5 per group). (E) EAE development with pain induction (diamonds), immobilization stress (circles), or forced swimming (FST) (triangles) every day from 21 days after T cell transfer in EAE-recovered mice (thin arrows) (n = 4–5 per group). (F) Serum concentrations of corticosterone, norepinephrine, and epinephrine with pain, immobilization stress or forced swimming 21 days after T cell transfer in EAE-recovered mice (n = 4–5 per group). Mean scores ± SEM are shown. *, p < 0.05, **, p < 0.01, ***, p < 0.001, n.s., not significant. Experiments were performed at least 3 times; representative data are shown.
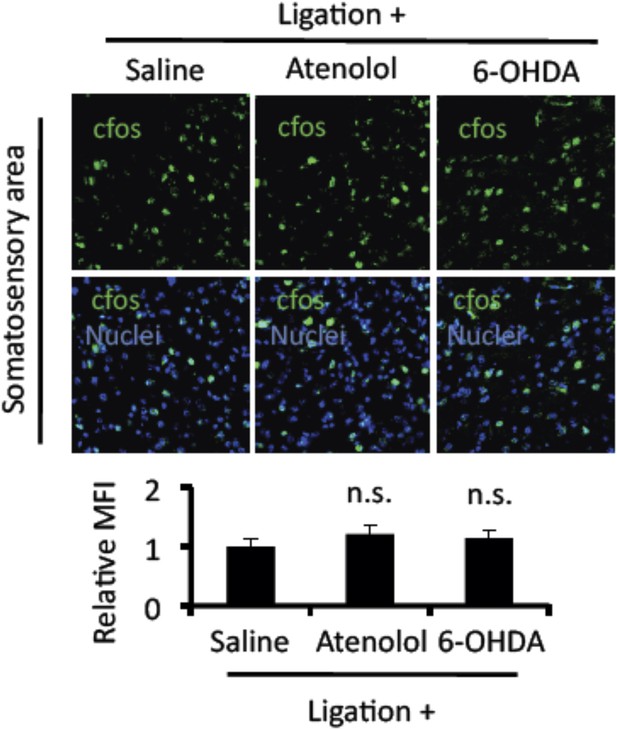
Mice treated with atenolol and 6-OHDA did not suppress cfos expression in neurons of the somatosensory area after pain induction.
Immunohistochemical staining for cfos in the somatosensory area of EAE-recovered mice with pain induction and treatment with atenolol or 6-OHDA administration (top) (n = 3–5 per group). Quantification of the histological analysis of the somatosensory area (bottom). Mean scores ± SEM are shown. n.s., not significant. Experiments were performed at least 3 times; representative data are shown.
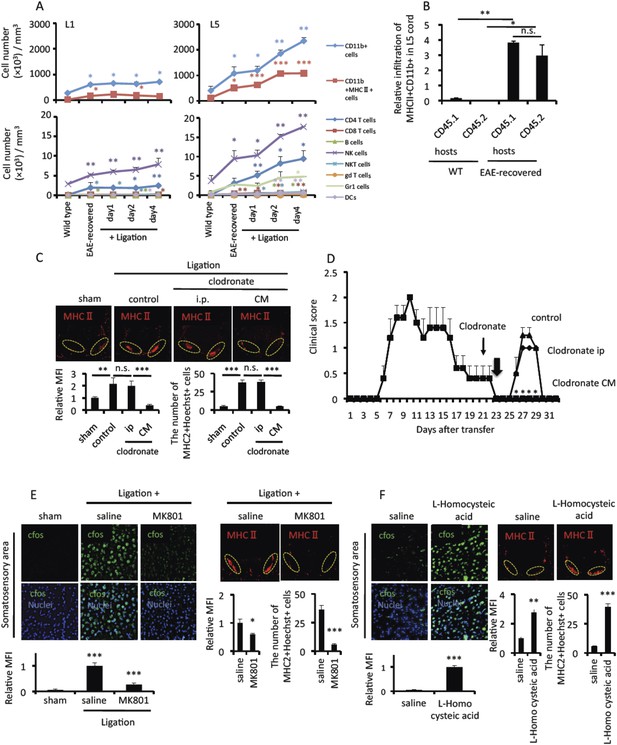
Pain induction accumulates MHC class II+CD11b+ cells at the ventral vessels of L5 via the anterior cingulate cortex in EAE-recovered mice.
(A) Pathogenic CD4+ T cells isolated from EAE mice were intravenously transferred into wild type C57BL/6 mice. Pain was induced 20 days later in EAE-recovered mice (EAE-recovered mice). The L1 or L5 cord was isolated at days 0, 1, 2, and 4 after pain induction (n = 3–4 per group), and the corresponding number of cells was evaluated using a flow cytometer. Because L1 spinal cord has about sevenfold bigger volume than L5 cord according to an 11.7 tesla MRI (Mori et al., 2014) (L1 average volume, 7.1 mm3 and L5 average volume, 1.0 mm3), immune cell numbers of L1 cord were divided by 7.1. Upper Figures, CD11b+ cells (blue); CD11b+MHC class II+ cells (red). Lower Figures, CD4+TCR+ cells (blue); CD8+TCR+ cells (red); B220+ cells (green); NK1.1+TCR- cells (violet); NK1.1+TCR+ cells (sky blue); γδTCR+ cells (orange); Gr1+ cells (light green); CD11c+ (light violet). Cell numbers ± SEM are shown. Statistical comparisons were made with wild type. (B) Relative infiltration of MHC class II+CD11b+ cells in the L5 cord of C57BL/6-SJL mice based on parabiosis experiments using C57BL/6 mice (CD45.2+) and C57BL/6-SJL mice (CD45.1+) in the presence (EAE-recovered) or absence (WT) of EAE development induced by MOG-specific pathogenic CD4+ T cell transfer (n = 4–5 per group). 30 days after the T cell transfer, L5 cords of EAE-recovered C57BL/6-SJL hosts were evaluated. CD45.2+ cells are peripheral derived cells from another body. (C) Immunohistochemical staining for MHC class II in the L5 cord with or without pain induction and treatment with clodronate liposome administration in the cisterna magna (CM) or the peritoneal cavity (ip) (top) (n = 3 per group). Dotted circles show accumulated cells at the ventral vessels (top). Quantification of the histological analysis around the ventral vessels based on MFI in dotted circles (left bottom) and cell number of the accumulated MHC class II+ cells in dotted circles by using serial frozen sections stained with anti-MHC class II antibody and Hoechst (right bottom). (D) EAE development was evaluated with clodronate liposome administration into the cisterna magna (CM) or the peritoneal cavity (ip) day 21 after pathogenic T cell transfer (thin arrows) in the presence of pain induction in EAE-recovered mice (n = 3–5 per group). Pain was induced 23 days later (thick arrow). (E) Immunohistochemical staining for cfos in the somatosensory area of EAE-recovered mice with pain induction and MK801 administration to the somatosensory area (left top) (n = 3 per group). Quantification of the histological analysis of the somatosensory area based on cfos MFI (bottom). Immunohistochemical staining for MHC class II in the L5 cord with pain induction and MK801 administration to the somatosensory area (right top) (n = 3 per group). Dotted circles show accumulated cells at the ventral vessels (top). Quantification of the histological analysis around the ventral vessels based on MFI in dotted circles (bottom left) and cell number of the accumulated MHC class II+ cells in dotted circles by using serial frozen sections stained with anti-MHC class II antibody and Hoechst (bottom right). (F) Immunohistochemical staining for cfos in the somatosensory area of EAE-recovered mice with L-Homocysteic acid administration to the somatosensory area (left top) (n = 3 per group). Quantification of the histological analysis of the somatosensory area based on cfos MFI (left bottom). Immunohistochemical staining for MHC class II in the L5 cord with L-Homocysteic acid administration to the somatosensory area (right top) (n = 3 per group). Dotted circles show accumulated cells at the ventral vessels. Quantification of the histological analysis around the ventral vessels based on MFI in dotted circles (left bottom) and cell number of the accumulated MHC class II+ cells in dotted circles by using serial frozen sections stained with anti-MHC class II antibody and Hoechst (right bottom). Mean scores ± SEM are shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Experiments were performed at least 3 times; representative data are shown.
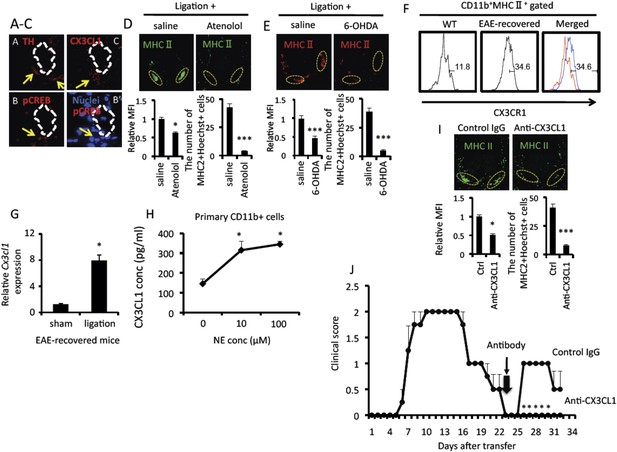
Pain mediated CX3CL1 expression via the sympathetic pathway is critical for the accumulation of activated MHC class II+CD11b+ cells at L5 ventral vessels in EAE-recovered mice.
Immunohistochemical staining for (A) TH (tyrosine hydroxylase), phospho-CREB (B), merging of phospho-CREB with nuclei (B′) or CX3CL1 (C) in the ventral side of the L5 cord using serial sections (n = 3–5 per group). White dotted polygons indicate the shape of the L5 ventral vessel. Arrows show TH, phosphor-CREB, and CX3CL1 staining around the ventral vessels. (D and E) Immunohistochemical staining for MHC class II in the L5 cord with pain induction and treatment with atenolol (D) or 6-OHDA (E) (top) (n = 3 per group). Dotted circles show accumulated cells at the ventral vessels. Quantification of the histological analysis around the ventral vessels based on MFI in dotted circles (left bottom) and cell number of the accumulated MHC class II+ cells in dotted circles by using serial frozen sections stained with anti-MHC class II antibody and Hoechst (right bottom). (F) The expression of CX3CR1 on CD11b+MHC class II+ cells of the spinal cord in healthy wild type mice (WT, left and red in right) and EAE-recovered mice (EAE-recovered, middle and blue in right) (n = 4 per group). (G) CX3CL1 mRNA expression in the ventral vessels was investigated by real time PCR with or without pain induction in EAE-recovered mice (n = 3–5 per group). (H) CX3CL1 expression was enhanced in the presence of norepinephrine. Primary CD11b+ cells in wild type mice (n = 2) were stimulated with norepinephrine for 24 hr. Culture supernatants were collected and assessed using an ELISA specific for mouse CX3CL1. (I) Immunohistochemical staining for MHC class II in the L5 cord with pain induction and treatment with an anti-CX3CL1 antibody, which was injected into the cisterna magna (top) (n = 3–5 per group). Dotted circles show accumulated cells at the ventral vessels. Quantification of the histological analysis around the ventral vessels based on MFI in dotted circles (left bottom) and cell number of the accumulated MHC class II+ cells in dotted circles by using serial frozen sections stained with anti-MHC class II antibody and Hoechst (right bottom). (J) Pathogenic CD4+ T cells isolated from EAE mice were intravenously transferred into wild type C57BL/6 mice. Pain was induced 23 days later (thick arrow), and EAE development was evaluated with or without anti-CX3CL1 antibody injection into the cisterna magna in EAE-recovered mice on day 23 (thin arrow) (n = 4–5 per group). Mean scores ± SEM are shown. *, p < 0.05; ***, p < 0.001. Experiments were performed at least 3 times; representative data are shown.
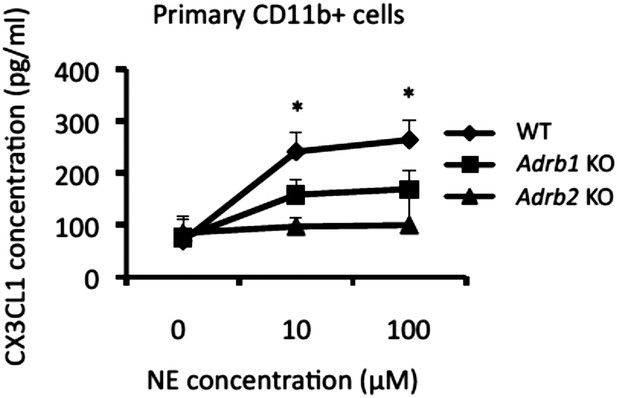
CX3CL1 expression in CD11b+ cells was enhanced in the presence of norepinephrine in a manner dependent on β1 and β2 receptors.
Primary CD11b+ cells isolated from β1 and β2 receptor deficient mice (n = 2 per group) were stimulated with norepinephrine for 24 hr. Culture supernatants were collected and assessed using an ELISA specific for mouse CX3CL1. Mean scores ± SEM are shown. *, p < 0.05. Experiments were performed at least 3 times; representative data are shown.
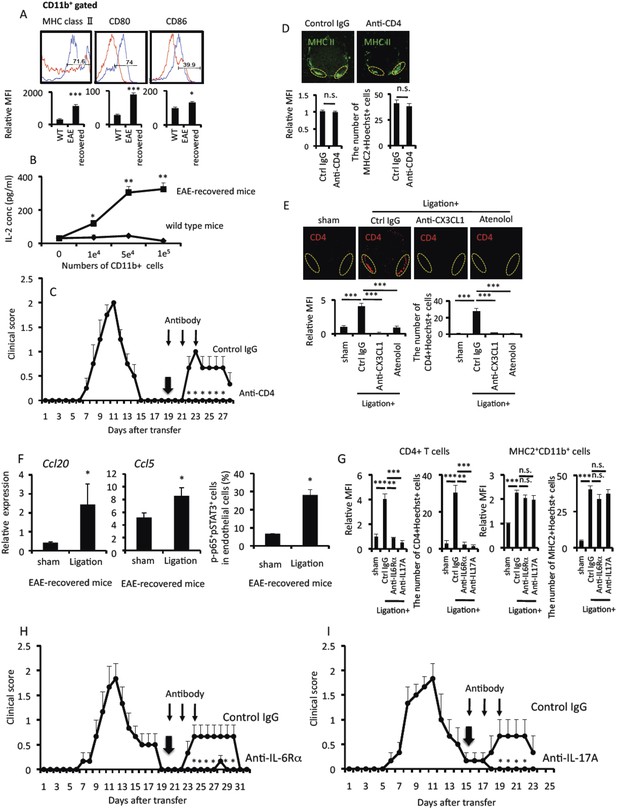
Pain-mediated accumulation of MHC class II+CD11b+ cells and CD4+ T cells at L5 ventral vessels is important for the development of EAE relapse.
(A) The expression of MHC class II, CD80, and CD86 on CD11b+ cells in the spinal cord of wild type mice (red) and EAE-recovered mice (blue) (top) (n = 4 per group). Mean Fluorescence Intensity (bottom). (B) CD11b+ cells isolated from EAE-recovered mice but not wild type mice have the potential of antigen presentation to CD4+ T cells without peptide addition (n = 2 per group). Culture supernatants were collected and assessed using an ELISA specific for mouse IL-2. (C) Pathogenic CD4+ T cells isolated from EAE mice were intravenously transferred into wild type C57BL/6 mice. EAE development was evaluated 19 days later with or without intra-peritoneal injection of an anti-CD4 antibody (days 19, 21, and 23, thin arrows) and pain induction (day 19, thick arrow) in EAE-recovered mice (n = 4–5 per group). (D) Immunohistochemical staining for MHC class II in the L5 cord with pain induction (8 hr) and treatment with anti-CD4 antibody (using the same hosts as (C)) (top) (n = 4–5 per group). Dotted circles show accumulated cells at the ventral vessels. Quantification of the histological analysis around the ventral vessels based on MFI in dotted circles (bottom left) and cell number of the accumulated MHC class II+ cells in dotted circles by using serial frozen sections stained with anti-MHC class II antibody and Hoechst (bottom right). (E) Immunohistochemical staining for CD4 in the L5 cord with or without pain induction (8 hr) and treatment with anti-CX3CL1 antibody or atenolol (top) (n = 3–5 per group). Dotted circles show accumulated cells at the ventral vessels. Quantification of the histological analysis around the ventral vessels based on MFI in dotted circles (left bottom) and cell number of the accumulated CD4+ cells in dotted circles by using serial frozen sections stained with anti-CD4 antibody and Hoechst (right bottom). (F) CCL20 and CCL5 mRNA expressions at L5 ventral vessels was evaluated with or without pain induction in EAE-recovered mice (left and middle) (n = 3 per group). Quantification of the histological analysis for p65+pSTAT3+ endothelial cells around ventral vessels based on serial frozen sections in EAE-recovered mice with or without pain induction (right) (n = 3 per group). (G) Immunohistochemical staining for CD4 and MHC class II in the L5 cord with pain induction and treatment with anti-IL-6Rα antibody or anti-IL-17A antibody (n = 4–5 per group). Quantifications of the histological analysis around ventral vessels based on serial frozen sections (CD4 [left] and MHC class II [right]) are shown. (H) Pathogenic CD4+ T cells isolated from EAE mice were intravenously transferred into wild type C57BL/6 mice. EAE development was evaluated from day 0–32 after pathogenic T cell transfer with or without anti-IL-6 receptor α antibody treatment (days 20, 22, and 24, thin arrows) upon pain induction (day 20, thick arrow) in EAE-recovered mice (n = 4–5 per group). (I) Pathogenic CD4+ T cells isolated from EAE mice were intravenously transferred into wild type C57BL/6 mice. EAE development was evaluated 15 days later with or without intra-peritoneal injection of an anti-IL-17A antibody treatment (days 15, 17, and 19, thin arrows) or pain induction (day 15, thick arrow) in EAE-recovered mice (n = 4–5 per group). Mean scores ± SEM are shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Experiments were performed at least 3 times; representative data are shown.
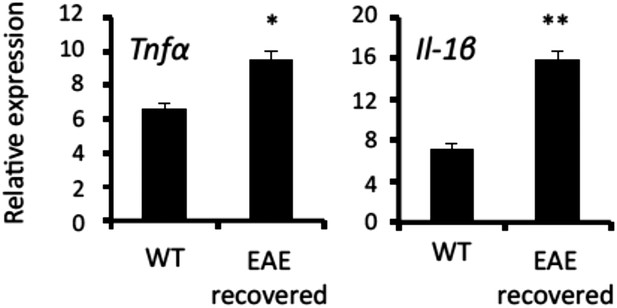
MHC class II+CD11b+ cells in the CNS of EAE-recovered mice expressed TNFα and IL-1β.
Expression of TNFα, and IL-1β in MHC class II+CD11b+ cells in the L5 cord of EAE-recovered mice (n = 3 per group). Mean scores ± SEM are shown. *, p < 0.05; **, p < 0.01. Experiments were performed at least 3 times; representative data are shown.





