Down-regulation of TORC2-Ypk1 signaling promotes MAPK-independent survival under hyperosmotic stress
Figures
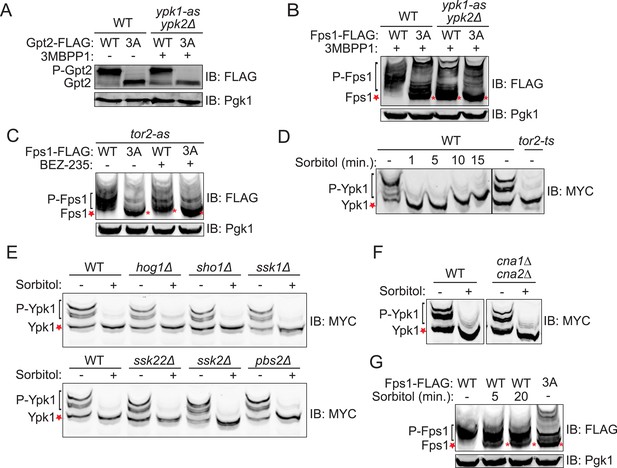
Fps1 (but not Gpt2) is phosphorylated by Ypk1.
(A) Wild-type (BY4741) or ypk1-as ypk2Δ (yAM135-A) cells expressing plasmid borne Gpt2-3xFLAG (pAX238) or Gpt23A-3xFLAG (pAX244) were grown to mid-exponential phase and then treated with vehicle (−) or 10 μM 3-MB-PP1 (+) for 90 min. Cells were harvested, extracts prepared, resolved by SDS-PAGE, and blotted as in ‘Materials and methods’. (B) Wild-type cells expressing either Fps1-3xFLAG (yGT21) or Fps13A-3xFLAG (yGT22) from the FPS1 promoter at the normal chromosomal locus, or ypk1-as ypk2Δ cells expressing either Fps1-3xFLAG (yAM281) or Fps13A-3xFLAG (yAM284-A) from the FPS1 promoter at the normal chromosomal locus, were grown to mid-exponential phase and treated as in (A) with vehicle or 3-MB-PP1 for 60 min. Cells were harvested, extracts prepared, resolved by Phos-tag SDS-PAGE, and blotted as in ‘Materials and methods’. Unphosphorylated Fps1 (red asterisk). (C) A tor2-as strain (yKL5) expressing Fps1-3xFLAG (pAX274) or Fps13A-3xFLAG (pAX275) was grown to mid-exponential phase and then treated with vehicle (−) or 2 μM BEZ-235 (+) for 30 min. Cells were harvested, extracts prepared, resolved and analyzed as in (B). (D) Wild-type (BY4741) or tor2-29ts (JTY5468) cells expressing Ypk17A-myc (pFR252) were grown at 30°C (left panel) or 26°C (right panel) to mid-exponential phase, then diluted into fresh YPD in the absence (−) or presence of 1 M sorbitol (final concentration). After the indicated times (1–15 min), culture samples were collected, lysed and the resulting extracts resolved by Phos-tag SDS-PAGE and analyzed by immunoblotting with anti-myc mAb 9E10, as described in ‘Materials and methods’. (E) As in (D), except for the genotype (strain) expressing Ypk17A-myc (pFR252), which were, aside from the wild-type control, hog1Δ (YJP544), sho1Δ (JTY5540), ssk1Δ (JTY5541), ssk22Δ (JTY5539), ssk2Δ (JTY5538) or pbs2Δ (JTY5537), and the treatment with 1 M sorbitol was for 1 min. (F) Wild-type (BY4741) or otherwise isogenic cna1∆ cna2∆ (JTY5574) cells expressing Ypk17A-myc (pFR252) were grown to mid-exponential phase then diluted into fresh YPD in the absence (−) or presence (+) of 1 M sorbitol (final concentration). After 1 min, the cells were collected, lysed and the resulting extracts resolved by Phos-tag SDS-PAGE and analyzed by immunoblotting with anti-myc mAb 9E10, as described in ‘Materials and methods’. (G) Wild-type cells expressing either Fps1-3xFLAG (yGT21) or Fps13A-3xFLAG (yGT22) from the chromosomal FPS1 locus, were diluted into fresh YPD in the absence (−) or presence of 1 M sorbitol (final concentration) for the indicated times and then extracts of the cells prepared and analyzed as in (B).
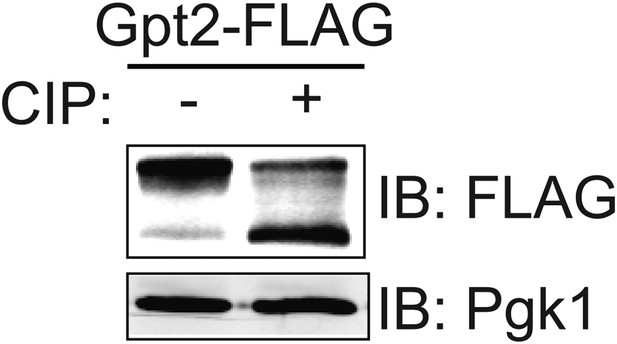
Gpt2 is a phosphoprotein in vivo.
Wild-type (BY4741) cells expressing Gpt2-3xFLAG (pAX238) were grown to mid-exponential phase. The cells were harvested, lysed and trichloroacetic acid extracts prepared as described in the ‘Materials and methods’. The precipitated proteins were resolubilized, treated with calf intestinal phosphatase (CIP), resolved by SDS-PAGE, and analyzed by immunoblotting, all as described in the ‘Materials and methods’.
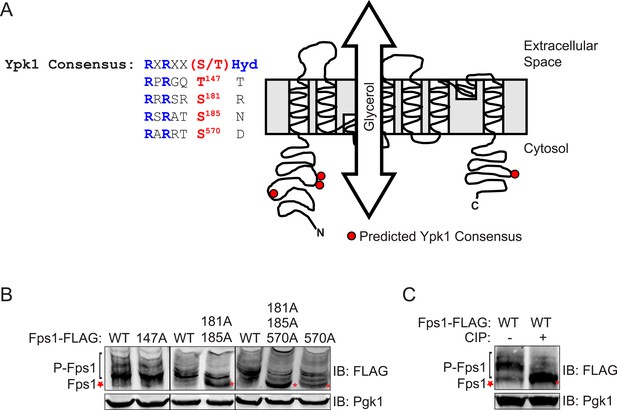
Fps1 is phosphorylated at three predicted Ypk1 sites in vivo.
(A) Diagram of Fps1 (adapted from Lee et al., 2013) with approximate location of predicted Ypk sites indicated as red circles. Primary sequence context of predicted sites are shown at left. (B) Extracts from Fps1-3xFLAG (yGT21), Fps1(T147A)-3xFLAG (yAM310-A), Fps1(S181A S185A)-3xFLAG (yAM301-A), Fps1(S570A)-3xFLAG (yGT24) or Fps13A-3xFLAG (yGT22) expressing strains were prepared, resolved by Phos-tag SDS-PAGE (see ‘Materials and methods’), and blotted as in Figure 1B. Red asterisks, unphosphorylated Fps1. (C) Cells expressing Fps1-3xFLAG (yGT21) were grown, extracts prepared, treated with phosphatase, as in ‘Materials and methods’, and analyzed on a Phos-tag gel, as described in Figure 1B.
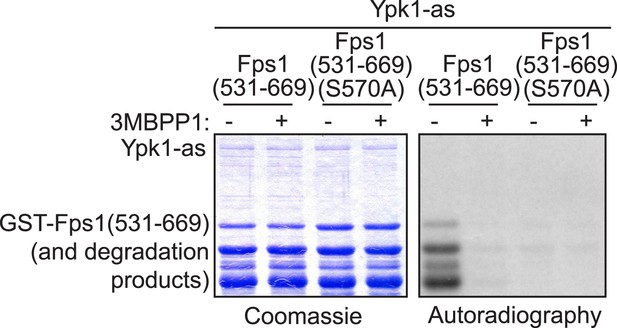
A fragment carrying one of the in vivo Ypk1-dependent sites in Fps1 is phosphorylated by purified Ypk1 in vitro exclusively on the same site.
GST-Fps1(531-669) (pBT7) and GST-Fps1(531-669 S570A) (pAX135) were purified from Escherichia coli and incubated with [γ-32P]ATP and Ypk1-as, purified from S. cerevisiae, in the absence or presence of 3-MB-PP1. The products were then resolved by SDS-PAGE and analyzed by Coomassie blue dye staining and autoradiography, as indicated, using procedures described in ‘Materials and methods’.
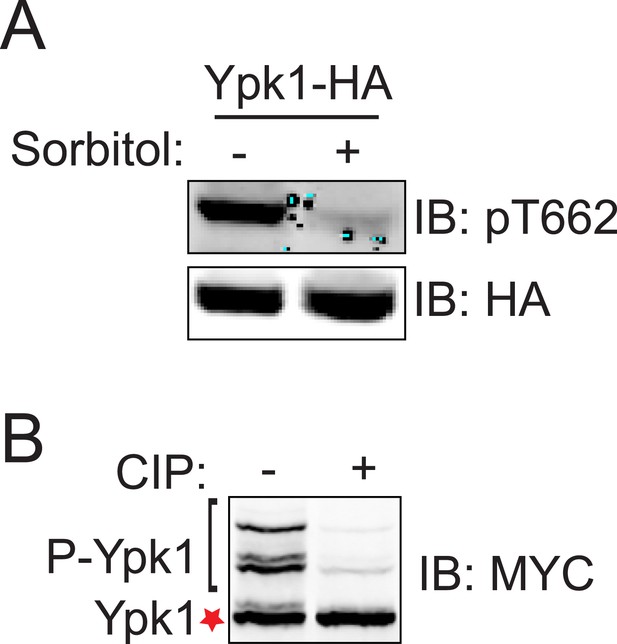
Modification at T662 and isoforms of Ypk17A both accurately report authentic in vivo phosphorylation.
(A) A ypk1∆ strain (JTY6142) expressing Ypk1-HA (pPL215) was grown to mid-exponential phase and diluted into fresh medium in the absence (−) or presence (+) of 1 M sorbitol (final concentration). After 1 min, the cells were collected by centrifugation for 5 min and lysed. The resulting extracts were resolved by SDS-PAGE and analyzed by immunoblotting with anti-pYpk1(T662) antibody and anti-HA antibody, as described in ‘Materials and methods’. (B) Cells (BY4741) expressing Ypk17A-myc (pFR252) were grown, extracts prepared, treated with phosphatase, and analyzed as Figure 1D.
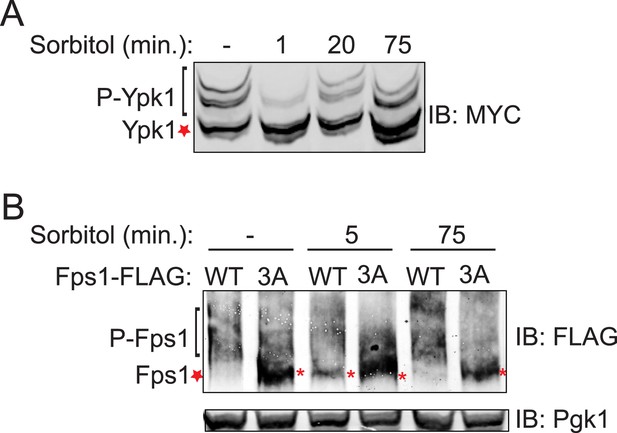
Hyperosmotic shock induced loss of Ypk1 and Fps1 phosphorylation is transient.
(A) Wild-type (BY4741) cells expressing Ypk17A-myc (pFR252) were grown at 30°C to mid-exponential phase then diluted into fresh YPD in the absence (−) and presence (+) of 1 M sorbitol (final concentration) for the indicated time periods. Extracts were prepared, resolved and blotted as described in ‘Materials and methods’. (B) Fps1-3xFLAG (yGT21) or Fps13A-3xFLAG (yGT22) expressing cells were treated with 1 M sorbitol for indicated time points and Fps1-3xFLAG was resolved and analyzed as in Figure 1B.
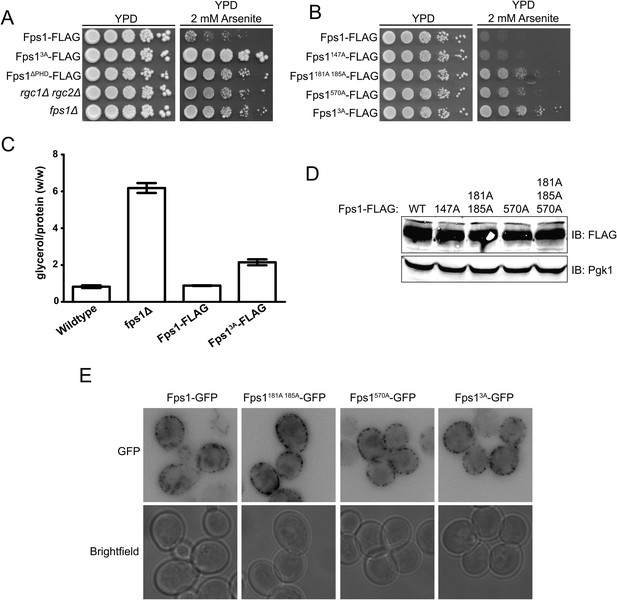
Phosphorylation by Ypk1 opens the Fps1 channel.
(A) Cultures of Fps1-3xFLAG (yGT21), Fps13A-3xFLAG (yGT22), Fps1ΔPHD-3xFLAG (yAM307-A), rgc1Δ rgc2Δ (DL3188) and fps1Δ (yAM181-A) were adjusted to A600 nm = 1.0 and serial dilutions were then spotted onto YPD plates containing the indicated concentration of arsenite. Cells were allowed to grow for 4 days at 30°C prior to imaging. (B) As in (A), except Fps1-3xFLAG (yGT21), Fps1(T147A)-3xFLAG (yAM310-A), Fps1(S181A S185A)-3xFLAG (yAM301-A), Fps1(S570A)-3xFLAG (yGT24) or Fps13A-3xFLAG (yGT22) cultures were used and cells were grown for 2 days at 30°C prior to imaging. (C) Triplicate exponentially-growing cultures of wild-type (BY4742), fps1Δ (yAM181-A), Fps1-3xFLAG (yGT21) and Fps13A-3xFLAG (yGT22) strains were harvested, extracted, and the glycerol and protein concentration measured as described in ‘Materials and methods’. Values represent the ratio of glycerol-to-protein (error bar, standard error of the mean). (D) Extracts from the strains in (B) were resolved by standard SDS-PAGE using 8% acrylamide gels. (E) fps1Δ (yAM181-A) cells expressing Fps1-GFP (pAX290), Fps1(S181A S185A)-GFP, (pAX294), Fps1(S570A)-GFP (pAX293) or Fps13A-GFP (pAX295) were viewed by fluorescence microscopy as described in ‘Materials and methods’. Representative fields are shown.
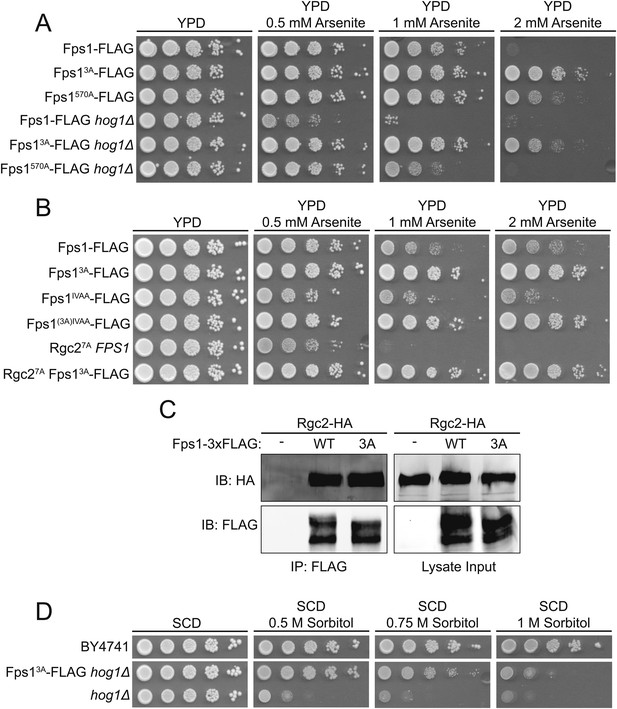
TOR Complex 2 (TORC2)-dependent Ypk1-mediated regulation of Fps1 is independent of Hog1 and Rgc1 and Rgc2.
(A) Cultures of Fps1-3xFLAG (yGT21), Fps1570A-3xFLAG (yGT24), Fps13A-3xFLAG (yGT22), Fps1-3xFLAG hog1Δ (yAM275), Fps1570A-3xFLAG hog1Δ (yAM291-A) and Fps13A-3xFLAG hog1Δ (yAM278) strains were adjusted to A600 nm = 1.0 and serial dilutions were then spotted onto YPD plates containing the indicated concentration of arsenite. Cells were allowed to grow for 2 days at 30°C prior to imaging. (B) As in (A), except Fps1IVAA-3xFLAG (yAM308-A), Fps1(3A)IVAA-3xFLAG (yAM309-A), Rgc27A-HA (yAM315) and Fps13A-3xFLAG Rgc27A-HA (yAM318) strains were tested. The Fps1IVAA mutation prevents Hog1 binding to and regulation of Fps1, and Rgc27A cannot be displaced from Fps1 because it cannot be phosphorylated by Hog1; both mutations render the channel constitutively open and make cells arsenite sensitive (Lee et al., 2013). (C) Fps1-3xFLAG (yAM271-A) or Fps13A-3xFLAG (yAM272-A) strains were co-transformed with PMET25-Rgc2-HA (p3151) and PMET25-Fps1-3xFLAG (pAX302) or PMET25-Fps13A -3xFLAG (pAX303) plasmids. After Rgc2-HA and Fps1-3xFLAG expression, Fps1 was immuno-purified with anti-FLAG antibody-coated beads (see ‘Materials and methods’). The bound proteins were resolved by SDS-PAGE and the amount of Rgc2-HA present determined by immunoblotting with anti-HA antibody. (D) Wild-type (BY4741), hog1Δ (YJP544) or Fps13A-3xFLAG hog1Δ (yAM278) strains were grown and serial dilutions of these cultures plated onto synthetic complete medium lacking tryptophan with 2% dextrose and the indicated concentration of sorbitol. Cells were grown for 3 days prior to imaging.
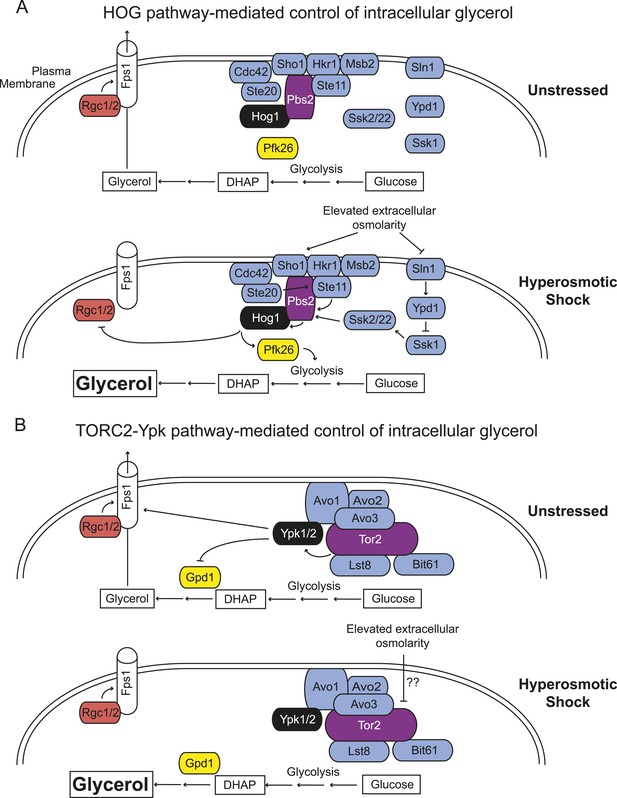
Saccharomyces cerevisiae has two independent sensing systems to rapidly increase intracellular glycerol upon hyperosmotic stress.
(A) Hog1 MAPK-mediated response to acute hyperosmotic stress (adapted from Hohmann, 2015). Unstressed condition (top), Hog1 is inactive and glycerol generated as a minor side product of glycolysis under fermentation conditions can escape to the medium through the Fps1 channel maintained in its open state by bound Rgc1 and Rgc2. Upon hyperosmotic shock (bottom), pathways coupled to the Sho1 and Sln1 osmosensors lead to Hog1 activation. Activated Hog1 increases glycolytic flux via phosphorylation of Pkf26 in the cytosol and, on a longer time scale, also enters the nucleus (not depicted) where it transcriptionally upregulates GPD1 (de Nadal et al., 2011; Saito and Posas, 2012), the enzyme rate-limiting for glycerol formation, thereby increasing glycerol production. Activated Hog1 also prevents glycerol efflux by phosphorylating and displacing the Fps1 activators Rgc1 and Rgc2 (Lee et al., 2013). These processes act synergistically to elevate the intracellular glycerol concentration providing an osmolyte to counterbalance the external high osmolarity. (B) Unstressed condition (top), active TORC2-Ypk1 keeps intracellular glycerol level low by inhibition of Gpd1 (Lee et al., 2012) and because Ypk1-mediated phosphorylation promotes the open state of the Fps1 channel. Upon hyperosmotic shock (bottom), TORC2-dependent phosphorylation of Ypk1 is rapidly down-regulated. In the absence of Ypk1-mediated phosphorylation, inhibition of Gpd1 is alleviated, thereby increasing glycerol production. Concomitantly, loss of Ypk1-mediated phosphorylation closes the Fps1 channel, even in the presence of Rgc1 and Rgc2, thereby promoting glycerol accumulation to counterbalance the external high osmolarity. Schematic depiction of TORC2 based on data from Wullschleger et al. (2005); Liao and Chen (2012); Gaubitz et al. (2015).
Additional files
-
Supplementary file 1
Yeast strains used in this study.
- https://doi.org/10.7554/eLife.09336.011
-
Supplementary file 2
Plasmids used in this study.
- https://doi.org/10.7554/eLife.09336.012





