Multipotent versus differentiated cell fate selection in the developing Drosophila airways
Figures
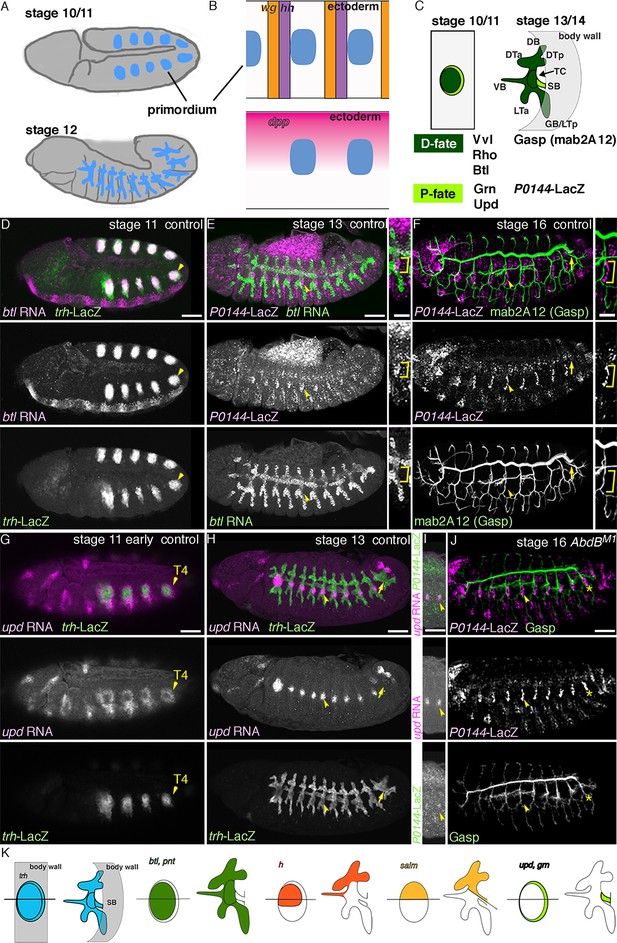
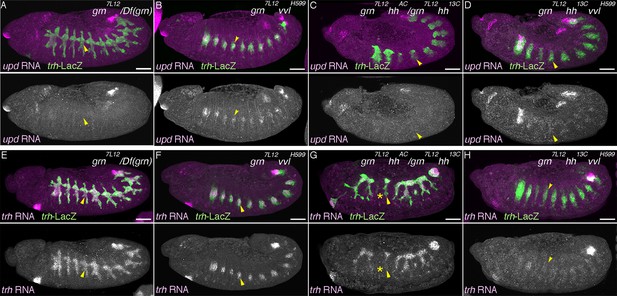
The proximo-distal cell fate organization of the Drosophila airways.
(A) A sketch of the embryonic Drosophila airways at stage 10/11 and late stage 12. (B) A representation of the ectodermal expression the secreted signaling molecules wg/WNT, hh (upper panel), and dpp (lower panel) in relation to the airway primordia at stage 10. (C–J) Expression of the P/D-fate markers at different stages of the airway development, which is summarized schematically in C, where the regional diversification of the Drosophila airway according to the proximo-distal axis is also shown in different colors. The P-fate region: spiracular branch (SB). The D-fate region: transverse connectives (TC) and six primary branches (dorsal branch/DB, dorsal trunk anterior/DTa, dorsal trunk posterior/DTp, visceral branch/VB, lateral trunk anterior/LTa, and ganglionic branch/GB/lateral trunk posterior/LTp). Expression of the D-fate markers, btl (D,E), mab2A12 (F, J) and the P-fate markers, P0144-lacZ (E, F, I, J) and upd (G-I), relative to each other or trh-lacZ (D, G, H). In this and other figures, arrowheads mark one of the 10 metameres. In E-F, enlarged picture of Tr5 is also shown, where the P-fate cells are bracketed. btl expression at stage 11 (D) is concentrated in the central parts of the primordia. At stage 13 (E), btl expression and P0144-lacZ expression (arrowheads) rarely overlap. At stage 16, mab2A12 (anti-Gasp antibody) strongly labels the lumens of the D-cells while P0144-lacZ is strongly detected in the P-cells (F, arrowheads). At stage 11 (G), upd transcript is detected at the peripheral area of each primordium (arrowheads). At stage 13 (H, I), upd is expressed in the P-cells that express P0144-lacZ (arrowheads). Note that compared to the control, where upd and P0144-lacZ are not expressed in Tr10 (arrows in F, H), the P-fate (P0144-lacZ) is established in Tr10 of AbdB mutants (asterisks in J). Scale bar is 2 μm in the enlarged panels of E-F. Scale bar in the remaining panels is 50 μm. (K) summarizes typical gene expression patterns of various marker genes in the airway primordium and in the mature airways. See text for details.
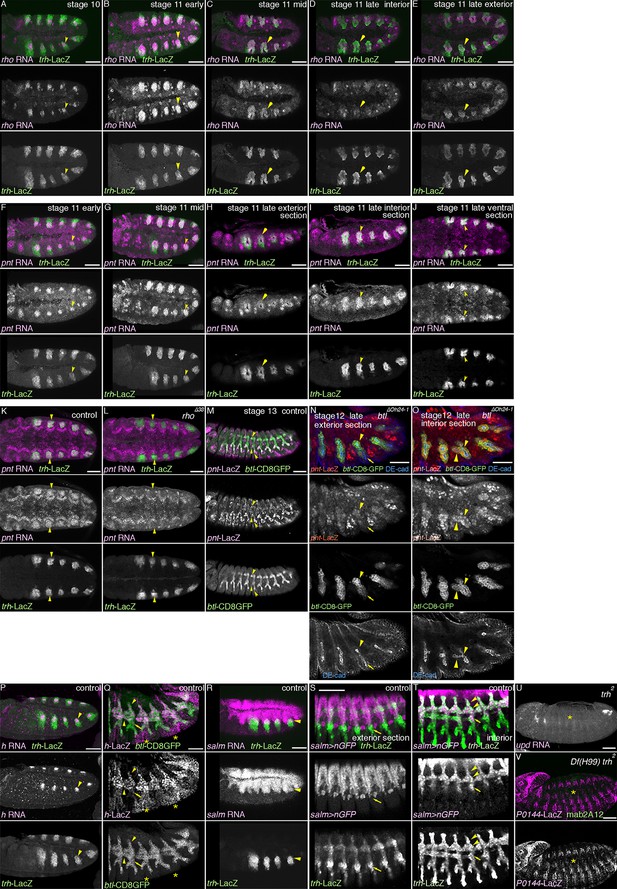
The centro-peripheral organization of the airway primordia.
Expression of rho (A-E), pnt (F-O), h (P-Q), or salm (R-T) in the airways before or after invagination. (A–E) rho expression in the trachea. At stage 10 (A), the dorso-central part of each primordium expresses rho (arrowheads). By stage 11 (B,C), when the tracheal cells invaginate, rho expression expands to cover more cells of the central/distal areas (arrowheads). At late stage 11 (E,F), rho expression in the dorsal part of the distal trachea becomes weak (D, arrowheads), while the ventral part of the distal trachea still retains strong rho expression (E, arrowheads). (F–O) pnt expression in the trachea. At early stage 11 (F), pnt is expressed in the dorso-central part of each primordium (arrowheads), which expands to the whole cental/distal areas (G-U). The proximal area expresses less pnt (H, arrowheads) than the distal area (I, arrowheads) as also evident in a section from a ventral view (J, arrowheads). At stage 11, compared to the wild type (K), pnt expression in the primordia is barely detectable in rho mutants (L, arrowheads). In the wild type (M), pnt-lacZ is expressed in the distal trachea, with its expression upregulated in a pan-tip pattern due to the Bnl/dFGF-dependent activation of Btl/dFGFR (arrowheads). In btl mutants (N,O) where this upregulation is absent, pnt-lacZ is expressed in the D-cells (arrowheads in Tr7) but is absent in the P-cells (arrows in Tr7). (P,Q) h expression in the trachea. At stage 10/11 (P), h is expressed in the dorso-central part of each primordium (arrowheads). At stage 13 (Q), expression of h-lacZ is detected in DB, DT, and VB (arroheads in Tr5), whereas TC and SB does not express it (arrows in Tr5). Note that the earlier pair-rule pattern of h-lacZ expression is also evident (asterisks). (R–T) salm expression in the trachea. At stage 10/11 (R), salm is expressed in the dorsal part of each primordium (arrowheads). At stage 13 (S,T), salm-gal4 driven UAS-nGFP labels DB, DT (arrowheads in Tr7), and part of TC and SB (arrows in Tr7). (U,V) Requirements of trh for the P/D fate selection. In trh mutants, expression of the P-fate markers upd (U, stage 13) and P0144-lacZ (V, stage 14/15) and the D-fate marker mab2A12 (V) is abolished (asterisks). Scale bar: 50 μm. DB, dorsal branch; DT, dorsal trunk; SB, spiracular branch; TC, transverse connectives; VB, visceral branch.
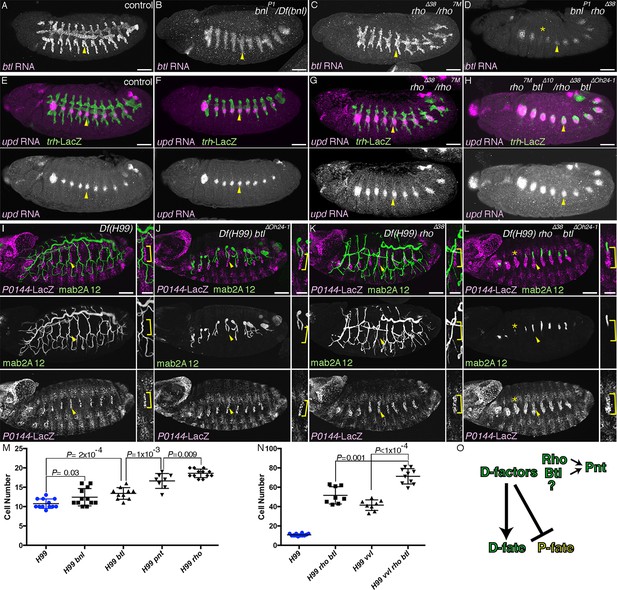
RTK activation drives the D-fate selection.
Expression of the D-fate (btl in A-D and mab2A12 in I-L) and the P-fate (upd in E-H and P0144-lacZ in I-L) markers upon loss of dEGFR and/or btl/dFGFR signaling. (A–H) Stage 12/13. (I–L) Stage 16. Compared to the control (A, E, I), in bnl mutants (B), btl mutants (F) or Df(H99) btl mutants (J), expression of the D-fate markers btl (A) and mab2A12 (J) and the P-fate markers upd (F) and P0144-lacZ (J) appears similar. Note, however, that upregulation of btl in a pan-tip pattern is abolished in bnl mutants (B) (Ohshiro et al., 2002). In rho mutants (C, G) or Df(H99) rho mutants (K), the expression area of the P-fate markers upd (G) and P0144-lacZ (K, arrowheads) is increased, leading to the decreased area of the D-fate marker expression (C, K). In rho bnl double mutants (D), rho btl double mutants (H) or Df(H99) rho btl triple mutants (L), the P-fate area is further expanded with concomitant reduction of the D-fate markers. Note that in D,L expression of the D-fate markers becomes reduced very much in a few segments (asterisk). Scale bar is 2 μm in the enlarged panels of I-L where the P-fate cells are bracketed. Scale bar in the remaining panels is 50 μm. (M,N) Scatter plots of the number of P0144-lacZ positive P-fate cells in Tr5 and Tr6 of the indicated genotypes at stage 16. All p-values were calculated by Student’s t-test. Source files are supplied in Figure 2—source data 1,2. (O) A scheme of P/D-fate selection by RTK signaling components. RTK, receptor tyrosine kinase.
-
Figure 2—source data 1
Source data for Figure 2M.
The number of P0144-lacZ-positive P-fate cells in Tr5 and Tr6 of the indicated genotypes at stage 16.
- https://doi.org/10.7554/eLife.09646.006
-
Figure 2—source data 2
Source data for Figure 2N.
The number of P0144-lacZ-positive P-fate cells in Tr5 and Tr6 of the indicated genotypes at stage 16.
- https://doi.org/10.7554/eLife.09646.007
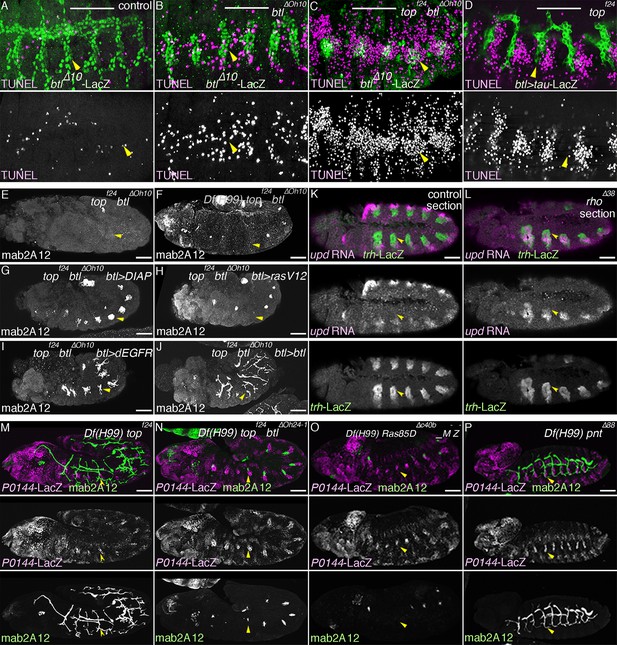
Multiple roles of dEGFR and btl/dFGFR signaling.
(A–D) RTKs promote cell survival of the tracheal cells. Single metameres in stage 13 embryos are marked with arrowheads. Compared to the control, many tracheal cells become positive for TUNEL signals in btl mutants (B). Upon loss of both RTKs, dEGFR and btl/dFGFR (C), more cells become positive for TUNEL signals, compared to either single mutants (B, D). (E–J) Branching phenotypes and rescue of dEGFR btl/dFGFR double mutants. Single metameres in stage 15 embryos are marked with arrowheads. In dEGFR btl/dFGFR double mutants (E), there is no branching and almost no mab2A12 signals. By suppressing apoptosis with Df(H99) (F) or DIAP overexpression (G), as well as by expression of RasV12 (H), dEGFR (I), or btl/dFGFR (J) mab2A12 signals are significantly restored. (K–P) Roles of RTK signaling components in the P/D fate selection. Compared to the control (K), in rho mutants (L), repression of the P fate marker upd in the D cells is retarded at stage 11/12 (arrowheads). In Df(H99) dEGFR double mutants (M), there are metameres with significantly less P0144-lacZ-positive cells than in Df(H99) rho double mutants (arrowheads). Df(H99) dEGFR btl/dFGFR triple mutants (N) additionally lose mab2A signals in some metameres (arrowheads). Ras85D maternal and zygotic mutants harboring Df(H99) (O) have similar phenotypes as Df(H99) dEGFR btl/dFGFR triple mutants (arrowheads). In Df(H99) pnt double mutants (P), P0144-lacZ-positive cells are increased in number as in Df(H99) rho double mutants (arrowheads). Scale bar: 50 μm. RTKs, receptor tyrosine kinases.
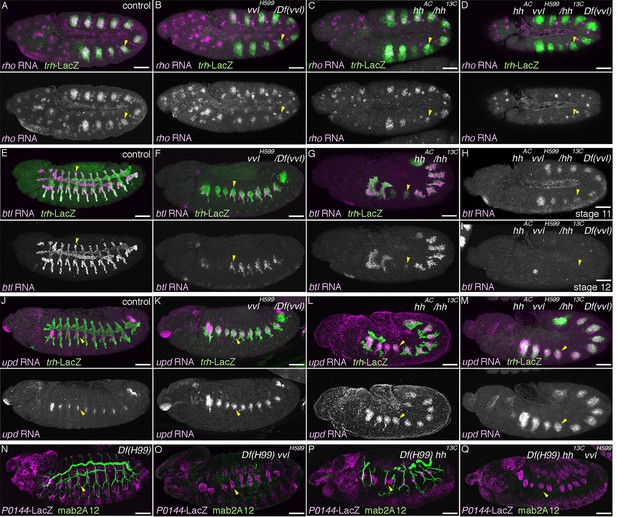
Hh and Vvl drive the D-fate selection.
Expression of the D-fate markers (rho at stage 11 in A–D, btl at stages 11-13 in E–I and mab2A12 at stages 15/16 in N–Q) and the P-fate markers (upd at stages 12/13 in J–M, P0144-lacZ in N-Q) upon loss of vvl and/or hh. Note that the genotypes in (N-Q) additionally carry the Df(H99) mutation. Compared to the control (column 1), upon loss of vvl (column 2), the expression area of the D-fate markers rho (B), btl (F), and mab2A12 (Q) is decreased while expression of the P-fate markers upd (K) and P0144-lacZ (Q) is expanded. Note that mab2A12 signals are hardly detectable in O. hh mutants (column 3) show the same trend as vvl mutants. Expression of btl (G) and mab2A12 (P) is variably lost in Tr3/4 and upd expression (P) expands to the whole distal area. In hh vvl double mutants (column 4), expression of the D-fate markers (D,I) is lost, while expression of the P-fate markers (M,Q) persist in the whole trachea. Note that weak btl expression is detectable in the primordia (H) but is lost soon after (I). Scale bar: 50 μm.
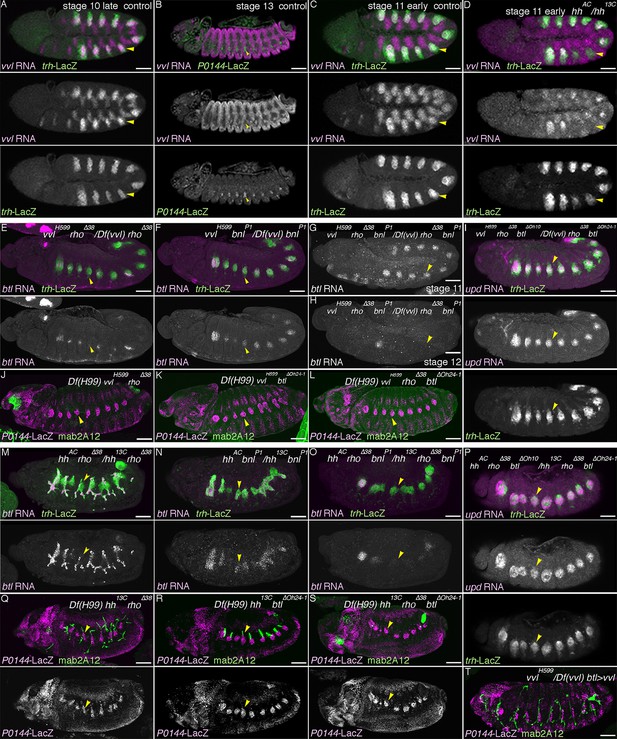
Hh, Vvl, and RTKs collaborate to drive the D-fate selection.
(A–D) vvl expression. vvl is expressed in the epidermis and in the central/distal part of each primordium/metamere (A). At stage 13 (B), tracheal vvl expression is predominant in the D-cells that are negative for P0144-lacZ. Compared to the wild type (C), vvl expression is variably decreased in hh mutants (D). (E–I) Comparison of double or triple mutants of vvl and RTKs. Loss of vvl and dEGFR signaling: vvl rho double mutants (E) or Df(H99) vvl rho triple mutants (J). Loss of vvl and btl/dFGFR signaling: vvl bnl double mutants (F) or Df(H99) vvl btl triple mutants (K). Loss of vvl and RTK signaling: vvl rho bnl triple mutants (G,H) and Df(H99) vvl rho btl quadruple mutants (L). Compared to loss of vvl and single RTK signaling components, upon loss of vvl and 2 RTK signaling factors, more cells lose expression of the D fate marker btl (E-H). Note that in vvl rho bnl triple mutants (G,H), btl expression initiates in the primordia (G) but is not maintained (H). Instead, the tracheal cells express the P-fate marker upd (I). A similar trend is seen for another pair of the P/D cell fate markers, mab2A12 and P0144-lacZ (J-L). (M–S) Comparison of double and triple loss of hh and dEGFR signaling. Loss of hh and dEGFR signaling: hh rho double mutants (M) or Df(H99) hh rho triple mutants (Q). Loss of hh and btl/dFGFR signaling: hh bnl double mutants (N) or Df(H99) hh btl triple mutants (R). Loss of hh and 2 RTK signaling components: hh rho bnl triple mutants (O) and Df(H99) vvl btl rho quadruple mutants (S). Compared to loss of hh and a single RTK signaling factor, upon loss of hh and 2 RTK signaling components, more cells lose expression of the D fate markers btl (O) and mab2A12 (R). Concomitantly, many of the tracheal cells become positive for the P-fate marker upd (P) and P0144-lacZ (S). (T) Loss of mab2A12 signal in vvl mutants is partially restored by btl-gal4 mediated overexpression of vvl. Scale bar: 50 μm. RTK, receptor tyrosine kinase.
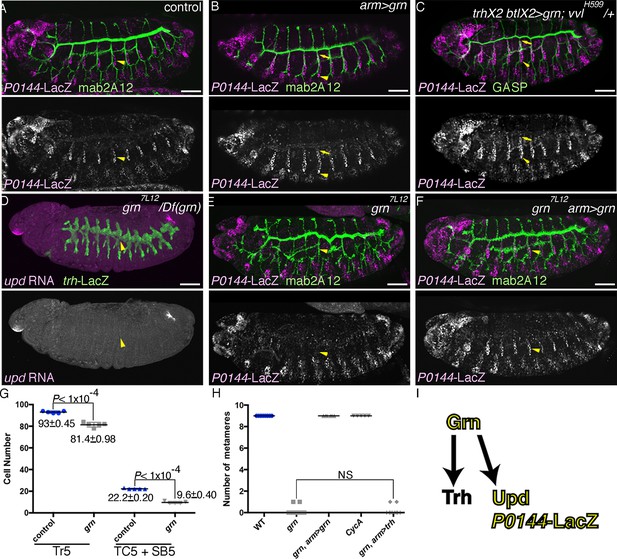
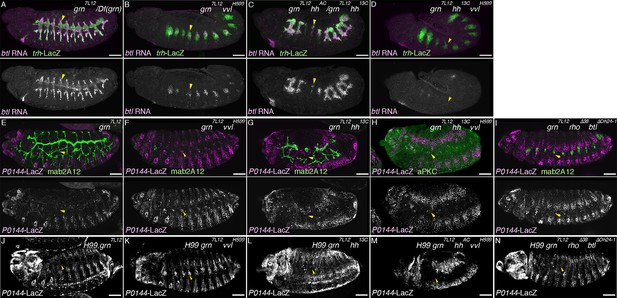
Grn promotes the P fate.
Compared to the control (A), grn overexpression with arm-gal4 (B) or two copies of btl-gal4 and trh-gal4 (C) slightly expands the area of P0144-lacZ positive cells to more distal TC areas, especially in Tr2-5 (B-C, arrows). In grn mutants, expression of the P-fate markers upd (D) or P0144-lacZ (E) disappears while arm-gal4-mediated Grn overexpression restores P0144-lacZ expression in the proximal cells (F). (G) Scatter plots of the Trh-positive cell numbers in the whole Tr5 or in the SB/TC subregion of the indicated genotypes at stage 14 (mean ± SEM, N = 5; all p-values were calculated by Student’s t-test). The cell number is decreased by around 10 cells in grn mutants in Tr5 and in the SB/TC subregion. A source file is supplied in Figure 4—source data 1. (H) Scatter plots of the number of metameres with any number of P0144-lacZ-positive cells of the indicated genotypes at stages15/16 (NS: not significant by Student’s t-test). Note that grn mutants occasionally possess a few P0144-lacZ cells in anterior metameres. A source file is supplied in Figure 4—source data 2. (I) summarizes the two functions of grn in P-fate promotion. Scale bar: 50 μm. SB, spiracular branch; SEM, standard error of the mean, TC, transverse connectives.
-
Figure 4—source data 1
Source data for Figure 4G.
The Trh-positive cell numbers in the whole Tr5 or in the SB/TC subregion of the indicated genotypes at stage 14.
- https://doi.org/10.7554/eLife.09646.012
-
Figure 4—source data 2
Source data for Figure 4H.
The number of metameres with any number of P0144-lacZ-positive cells of the indicated genotypes at stages 15/16.
- https://doi.org/10.7554/eLife.09646.013
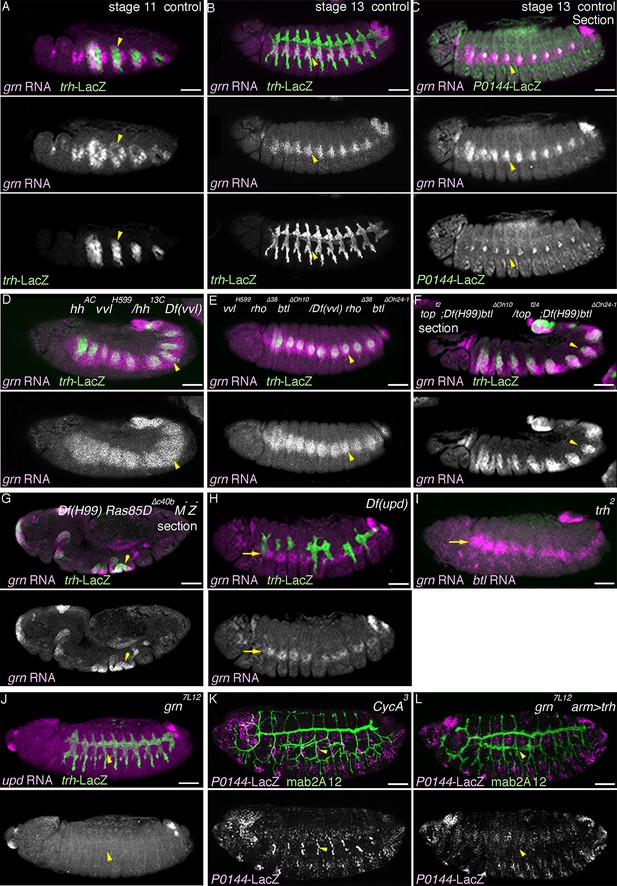
Regulation and function of grn expression.
(A–C) grn expression in wild type. At stage 11 (A), grn is preferentially expressed in the periphery of the tracheal primordia (arrowheads). At stage 13 (B,C), grn is detected in the P-cells (B) positive for P0144-lacZ (C) (arrowheads) as well as in the lateral ectoderm. (D–I) grn expression in mutants. grn expression expands to the D-cells in hh vvl double mutants (D, stage 12) or vvl rho btl triple mutants (E, stage 12/13). Loss of two RTK signaling components (F, stage 12, arrowheads in Tr7) or the downstream mediator Ras85D (G, stage 12) has weaker effects compared to hh vvl double mutants and the distal area variably becomes positive for grn (arrowheads). Note that the Df(H99) mutation is introduced in (F,G) to suppress apoptosis. In Df(upd) (H, stage 13) or trh mutants (I, stage 13), grn expression in the lateral ectoderm is largely intact, with some defects in Df(upd) mutants. Expression of the P-fate marker upd is abolished in grn mutants (J, stage 13). In CycA mutants (K, stage 15), the number of P0144-lacZ-positive cells becomes reduced but not abolished, while arm-gal4-mediated overexpression of trh does not restore P0144-lacZ expression in grn mutants (L, stage 15). Scale bar: 50 μm.
Genetic interactions of grn, hh, and vvl
Expression of the P-fate marker upd (A–D, stages 12-13) or the pan-tracheal marker trh (E–H, stages 12-13) are shown. Loss of upd expression in grn mutants (A) is partially reversed by vvl mutation (B, arrowheads) but not by hh mutation (C). In the absence of all, grn, hh, and vvl, upd expression is virtually lost (D). This accompanies severe reduction of trh expression (H, arrowheads), compared to either grn (E), grn vvl (F), or grn hh double mutants (G). Note the trh-lacZ expression in H, reflecting initial trh expression at the primordia stage. Also, expression of trh and upd in Tr1 is often detected at stage 12. Asterisks in G marks loss of trh expression in Tr3. Scale bar: 50 μm.
Genetic analysis of grn, hh, vvl, and RTK components.
Expression of the D-fate marker (btl in A–D or mab2A12 in E–I) or the P-fate marker (P0144-lacZ in E–N) is shown. Compared to grn (A), grn vvl (B), or grn hh (C) mutants, in grn hh vvl triple mutants, btl expression is largely lost as in hh vvl double mutants. Loss of P0144-lacZ expression in grn mutants (E,J) is partially restored in grn vvl double mutants (F,K) but not significantly in grn hh double mutants (G,L) or in grn rho btl triple mutants (I,N). Restoration of the P-fate in grn vvl double mutants is suppressed in grn vvl hh triple mutants (H, M). Note the presence of Df(H99) mutation in J–N. Arrowheads in E-N mark the proximal location of the tracheal cells. Scale bar: 50 μm. RTK, receptor tyrosine kinase.
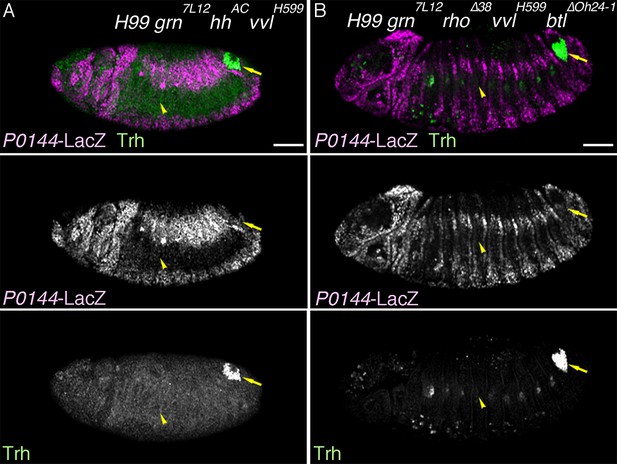
Phenotypes caused by simultaneous loss of the D- and P-factors.
Expression of pan-tracheal marker (trh) and the P-fate marker (P0144-lacZ). Either in Df(H99) grn hh vvl quadruple (A) or Df(H99) grn vvl rho btl quintuple mutants (B), the P-fate is not established and Trh expression becomes significantly reduced by stage 13/14. Arrows point persistent Trh expression in part of the posterior spiracle, which originates from a primordium specified in connection to Tr10 (Hu and Castelli-Gair, 1999). Scale bar: 50 μm.
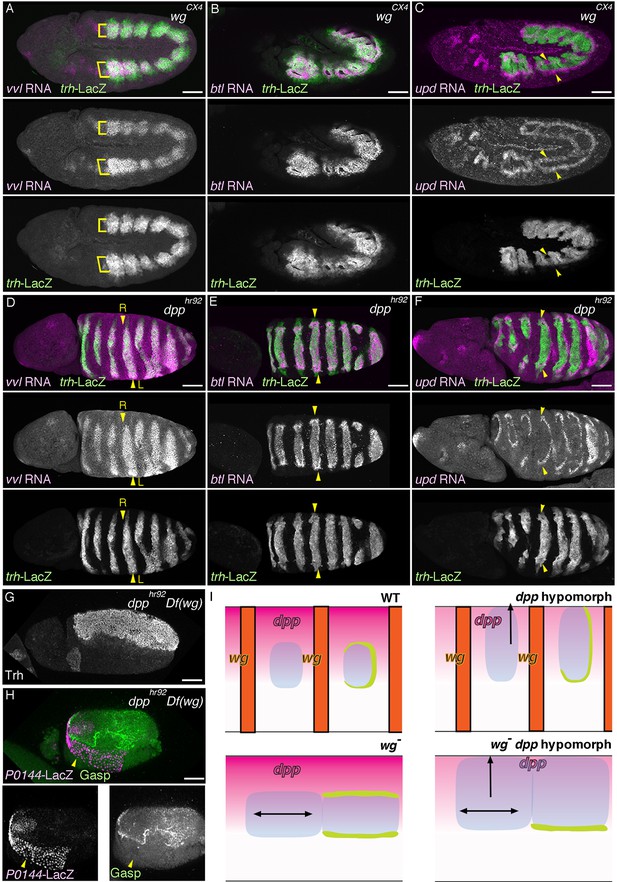
Regulation of the P/D fate selection by Wg/WNT and Dpp/BMP.
Expression regions of the D-fate markers (vvl in A, D, btl in B, E or Gasp in H) or the P-fate markers (upd in C, F or P0144-lacZ in H) are shown for wg mutants (A–C), dpp hypomorph (dpphr92) (D–F) or dpphr92 wg double mutants (H). (D-F) are dorsal views where both left (L) and right (R) sides are seen. In wg mutants, expression domains of the D-fate markers expand along the AP axis (A,B), and form a single lateral band (bracket) with occasional interruptions before the initiation of primordia invagination (A, stage 10). After invagination (B,C, stage 12), some D-fate cells remain exposed at the embryo surface to form a lateral band (B). The P-fate markers form a dorsal and a lateral band of expression at the ectoderm (C, arrowheads). In dpphr92 mutants (D-F), the airway primordia expand to the dorsal midline (D, stage 10). Concomitantly, both the D-fate (D,E) and the P-fate (F) expand to the dorsal midline. Note that the P-fate encircles the D-fate (arrowheads) and that the anterior cells often do not express the P-fate marker upd in (F, stage 11/12), probably due to the distalization by Hh that is expressed in the anterior border of each primordium. In dpphr92 Df(wg) double mutants (G,H), the whole dorsal area of the trunk/abdominal body parts become Trh positive at stage 11 (G). At stage 14/15 (H), the P-fate (arrowheads) is specified abutting the D-fate only at the ventral edge. Note that in the posterior part, the P-fate is not established as it is not established in the wild type. Scale bar: 50 μm. (I) Interpretation of data in each genotype regarding specification of the airway field (left parts) and P/D-fate selection (right parts). Expression domains of dpp and wg are colored pink and orange, respectively. In wg mutants, the airway field (light blue) expands along the AP axis (arrows) and the P-fate is established approximately as a dorsal and a ventral stripes (light green). In dpp hypomorphs (dpphr92), the airway field expands dorsally to the dorsal midline and the P-fate is established in the periphery except for the anterior and dorsal margin. In double mutants of wg and dpp hypomorph, the airway field expands both dorsally and along the AP axis, and the P-fate is only established in a ventral stripe.
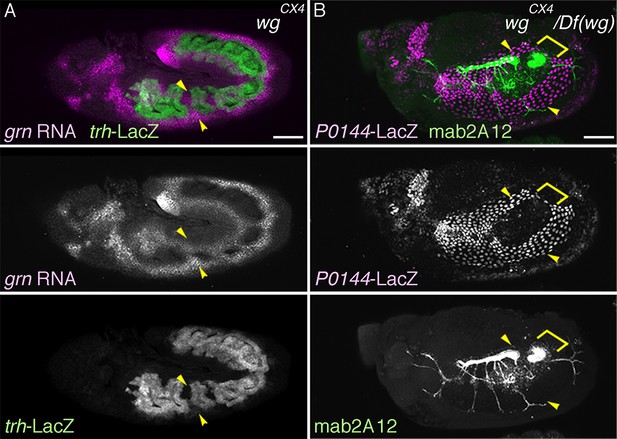
Role of wg in P/D fate selection.
Expression of the P-fate marker grn (A) or P0144-lacZ (B) or the D-fate marker mab2A12 (B) in wg/WNT mutants. Note that at stage 14/15 (B), the P-fate cells (arrowheads) that reside at the embryo surface surround the D-fate cell fraction that still remain at the surface (bracket). Scale bar: 50 μm.
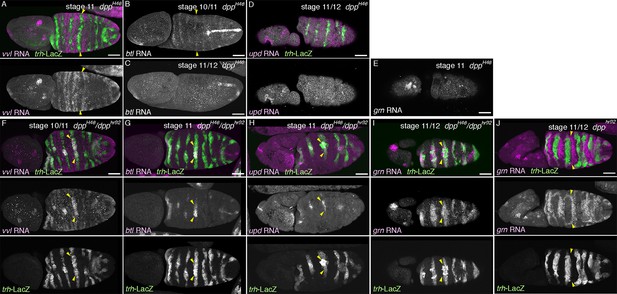
Roles of dpp in P/D fate selection.
(A–X) Expression of the D fate markers (vvl in A,F, btl in B,C,G) or the P-fate markers (upd in D,H or grn in E,I,J) in dpp null mutants (dppH46) (A-E), dppH46/dpphr92 heterozygotes (F-I) or dpphr92 homozygotes. In dppH46 mutants, expression of both the D-fate (A-C, arrowheads) and the P-fate (D,E) is not established. Note that the weak early expression of btl (B, arrowheads) is not maintained (C). In dppH46/dpphr92 mutants, both the D fate (F,G) and the P fate (H,I) are established only around the dorsal midline (arrowheads). In dpphr92 mutants, grn expression surrounding the airway cells expands to the dorsal midline (J, arrowheads). Scale bar: 50 μm.
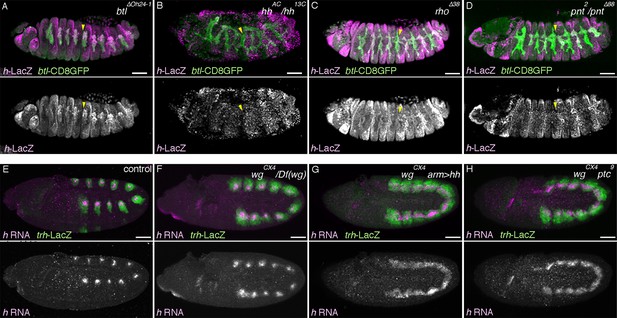
Roles of D-factors in h expression.
Expression of h-lacZ (A–D, stage 13) or h transcript (E–H, stage 10/11) is shown. h-lacZ marks the dorso-distal area of the trachea in btl mutants (A, arrowheads in Tr5) as in the wild type. This distal expression is significantly reduced (arrowheads) in hh (B), rho (C) or pnt mutants (D). Compared to the control (E), in wg mutants (F) h expression slightly expands along the AP axis with many gaps remaining. Upon overactivation of hh in wg mutants by hh overexpression (G) or ptc mutation (H), h expression expands along the AP axis to form a continuous band. Scale bar: 50 μm. AP, anterior-posterior.
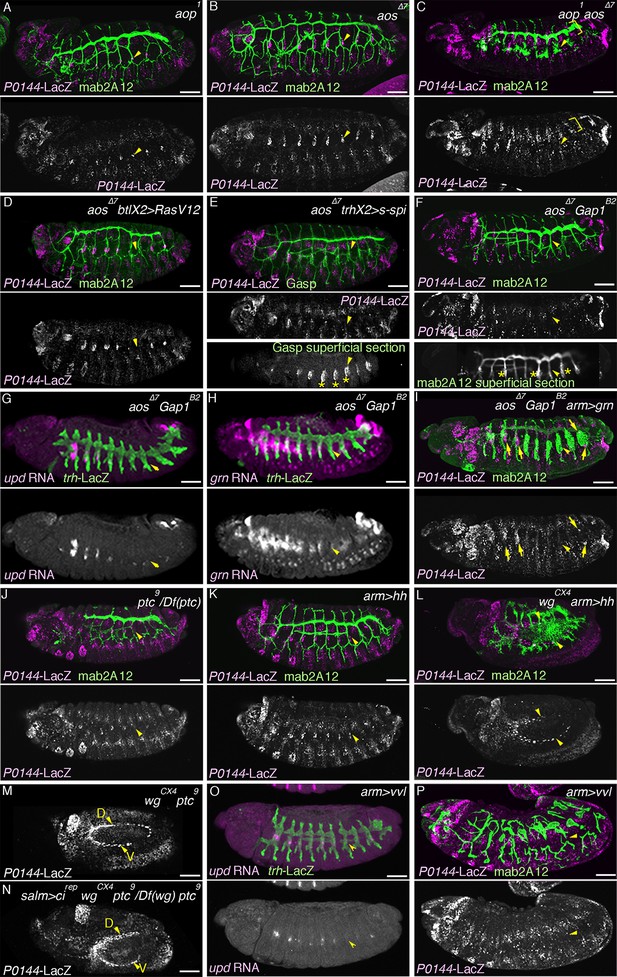
Overactivation of the D-fate determinants abrogates the P-fate specification.
(A–I) Effects of RTK signaling overactivation. Proximal areas are marked by arrowheads in Tr7. In aop mutants (A), expression of the P-fate marker P0144-lacZ is variably decreased or lost. Compared to either single mutant (A,B), in aop aos double mutants (C), P0144-lacZ expression is virtually abolished. Note that the epidermal signals in C (brackets) are from other lineages. aos mutation, combined with RasV12 overexpression by btl-gal4 (D), s-spi overexpression by trh-gal4 (E) or Gap1 mutation (F) abolish P0144-lacZ expression. Note that in E and F, after enhancement of mab2A12/Gasp signals, the epidermal surface staining is evident (asterisks). In aos Gap1 double mutants, expression of the other P-fate markers (upd in G and grn in H) is variably reduced or lost, while grn overexpression (I) partially restores P0144-lacZ expression to the proximal areas of aos Gap1 double mutants (arrows). (J–P) Effects of overactivation of hh or vvl. Overactivation of hh signaling by ptc mutation (J,M) or hh overexpression (K,L), either in the wild-type background (J,K) or wg mutant background (M,L) reduces the P0144-lacZ-positive cells. salm-gal4 mediated overexpression cirep increases the area of P0144-lacZ-positive P-fate cells in the dorsal stripe (N). The dorsal and the ventral stripes are marked with D and V in (M,N). vvl overexpression reduces or abolishes the expression region of the P-fate markers upd (O) or P0144-lacZ (P). Scale bar: 50 μm. RTK, receptor tyrosine kinase.
-
Figure 7—source data 1
Source data for Figure 7—figure supplement 2A.
The number of metameres with any number of P0144-lacZ-positive cells of the indicated genotypes at stages 15/16.
- https://doi.org/10.7554/eLife.09646.023
-
Figure 7—source data 2
Source data for Figure 7—figure supplement 2J.
The number of metameres with any number of P0144-lacZ-positive cells of the indicated genotypes at stages 15/16.
- https://doi.org/10.7554/eLife.09646.024
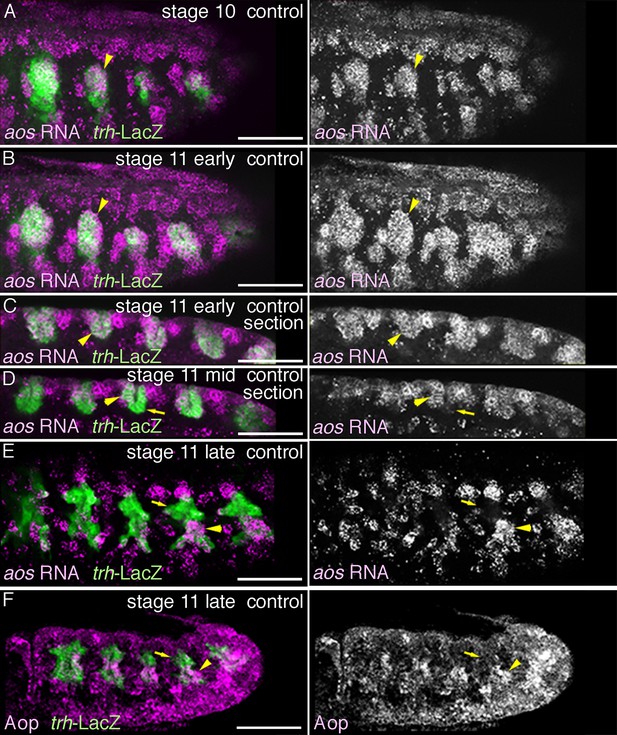
Expression of aos and Aop.
Expression of aos (A–E) or Aop (F) during stages 10-11. aos expression in the primordia (arrowheads) expands from the central part of the primordia (A) to the peripheral areas (B) to cover all the tracheal cells (C). By late stage 11, aos expression in the distal area decreases (D arrows in Tr3), while expressions of both aos and Aop are detected in the proximal area (E,F, arrowheads).
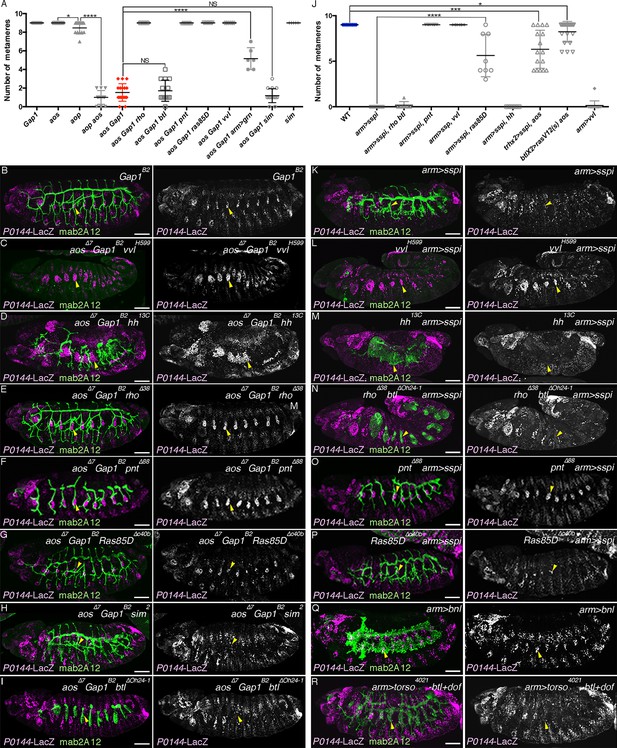
Requirement of the overactivated RTK signaling in the abrogation of the P-fate selection.
Expression of the D-fate marker mab2A12 and the P-fate marker P0144-lacZ is shown. Scatter plots of the number of metameres with any number of P0144-lacZ-positive cells of the genotypes at stages 15/16 relating to aos Gap1 mutants or overexpression of RasV12, s-spi or vvl are summarized in (A,J). All p-values were calculated by Student’s t-test. *p < 5 × 10−2, ***p < 1 × 10−3, ****p < 1 × 10-4, NS: Not significant. Source file is supplied in Figure 7—source data 1,2. In Gap1 mutants (B), P/D fate selection is comparable to aos mutants. Loss of P0144-lacZ expression in aos Gap1 double mutants is variably suppressed by mutations of vvl (C), hh (D), rho (E), pnt (F), or Ras85D (G) but not by mutations of sim (H) or btl (I). Loss of P0144-lacZ expression upon overexpression of s-spi/TGF-alpha (K) is variably suppressed by mutations of vvl (L), pnt (O) or Ras85D (P), but not by hh mutation (M) nor rho btl double mutations (N). Overexpression of Bnl/dFGF does not abolish P0144-lacZ expression (Q) while simultaneous overexpression of an active form of btl/dFGFR with its mediator, downstream of FGFR (dof) variably diminished P0144-lacZ expression (R). Note that overactivation of RTK signaling in hh mutant background (D,M) results in expansion of the field expressing airway markers. Scale bar: 50 μm. RTK, receptor tyrosine kinase.
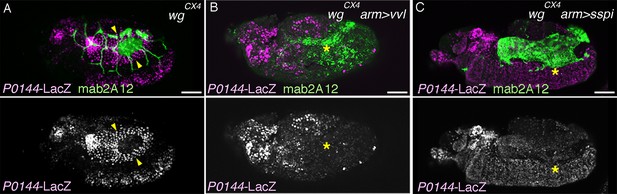
Effects of vvl or s-spi overexpression in wg/WNT mutant embryos.
Compared to single wg/WNT mutants (A, arrowheads), the P0144-lacZ-positive P-fate is not established upon arm-gal4-mediated overexpression of vvl (B, asterisks) or s-spi (C, asterisks).
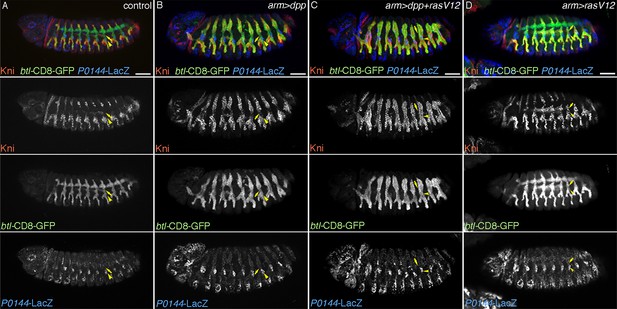
TC cells may define an intermediate fate between the more distal primary branches and the SB.
Expression of Kni is shown together with the D-fate marker btl-CD8GFP and the P-fate marker P0144-lacZ upon overexpression of dpp/BMP and/or RasV12. Arrowheads and arrows mark the P-cells and TC in Tr7, respectively. Compared to the control, dpp/BMP overexpression induces Kni expression in all the D-cells. But the Kni expression level is weaker in TC. The differential Kni expression levels in the primary branches and TC (B) become equalized by additional overexpression of RasV12 (C). Upon RasV12 overexpression alone (D), Kni expression becomes detected along the TC. Note that in all cases, P0144-lacZ-positive P-cells do not express Kni. Scale bar: 50 μm. SB, spiracular branch; TC, transverse connectives.
-
Figure 8—source data 1
Source data for Figure 8—figure supplement 1D.
The number of metameres displaying P0144-lacZ-positive cells in the indicated genotypes at stages 15/16
- https://doi.org/10.7554/eLife.09646.029
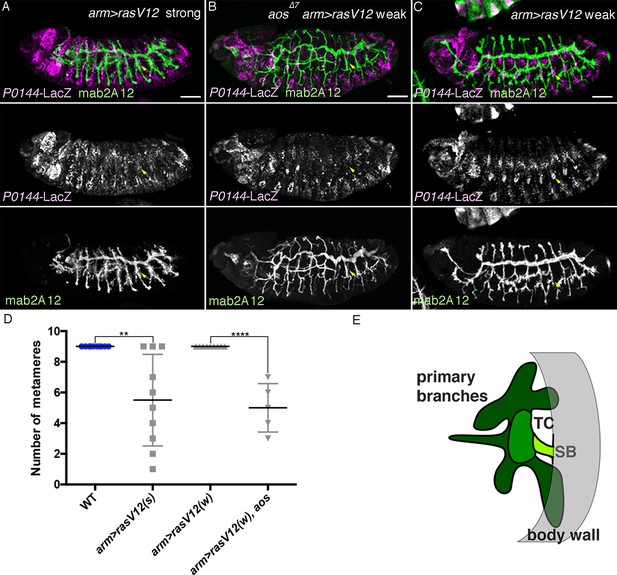
Characterization of the effects of the weaker and the stronger UAS-RasV12 lines.
Driving the stronger UAS-RasV12 line/RasV12 (s) with arm-gal4 (A) causes loss of P0144-lacZ expression (arrowheads in Tr7), while the weaker UAS-RasV12 line/RasV12 (w) (C) needs aos mutation (B) to cause loss of P0144-lacZ expression (arrowheads in Tr7). Scale bar: 50 μm. (D) Scatter plots of the number of metameres displaying P0144-lacZ-positive cells in the indicated genotypes at stages 15/16. All p-values were calculated by Student’s t-test. **p < 2 × 10−3, ****p < 1 × 10−4. A source file is supplied in Figure 8—source data 1. (E) illustrates the separation of the airway tree into three different developmental competences, SB (P-fate), TC and the remaining primary branches (both D-fates). SB, spiracular branch; TC, transverse connectives.
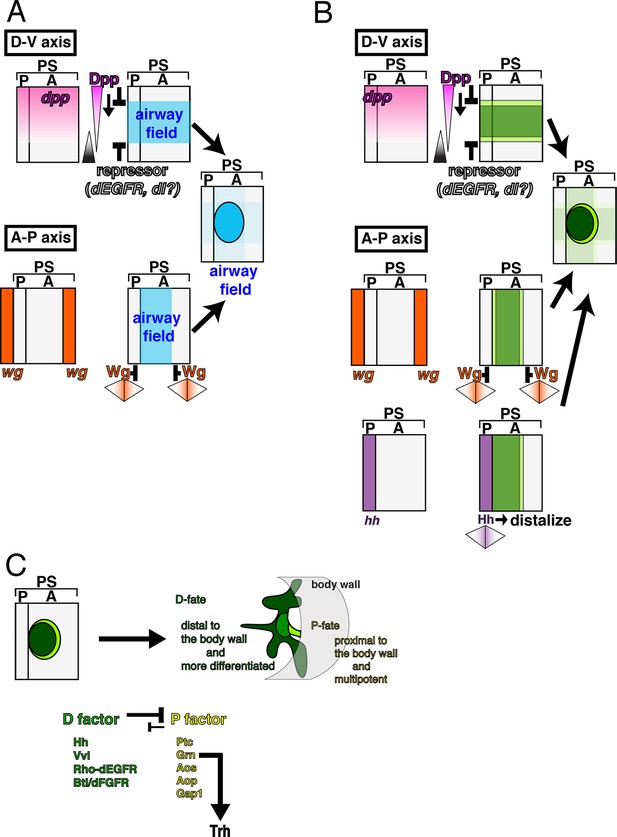
Genetic circuits explaining the early development of the Drosophila airways.
(A) Establishment of the airway field is regulated by Dpp/BMP and Wg/WNT. In each metameric unit of the Drosophila embryo (parasegment, PS) (Sanson, 2001), an airway primordium is specified just posterior to the hh expression domain (a posterior compartment of a segment, P). This is combinatorially controlled along the DV and AP axis. Dpp/BMP expressed in the dorsal region functions as both a repressor (Isaac and Andrew, 1996; Wilk et al., 1996) and an activator of the airway field (this study) while Wg/WNT functions as a repressor along the AP axis. (B) Initiation of the P/D-fate selection by Dpp/BMP, Wg/WNT, and Hh. Each airway primordium is roughly subdivided into two regions, anterior-central (D-fate, dark green) and the peripheral (P-fate, light green). The patterning cues along the AP and DV embryo axis roughly set up this radial patterning. Along the DV axis, Dpp/BMP expressed in the dorsal region functions to discriminate the P/D-fates, at least at the dorsal edge. Dpp/BMP may also discriminate the P/D-fates at the ventral edge. Alternatively, ventral cues dependent on the Dorsal TF gradient may discriminate the P/D-fates at the ventral edge. Along the AP axis, Wg/WNT expressed in transverse stripes may discriminate the P/D-fates at each edge. Hh from the anterior border stimulates the D-fate. (C) Establishing the P/D-fates by the P/D-factors. The ordered Invagination of the primordia converts the centro-peripheral patterning to the proximo-distal organization along the airway tree. By completion of invagination, D-factors establish the D-fate at the expense of the P-fate. Within the D-fate, there are two groups of cells having different responsiveness to overexpression of Dpp/BMP or Grn, e.g., TC (green) and the primary branches (dark green). See text for details. AP, anterior-posterior; DV, dorso-ventral.





