FAM150A and FAM150B are activating ligands for anaplastic lymphoma kinase
Figures
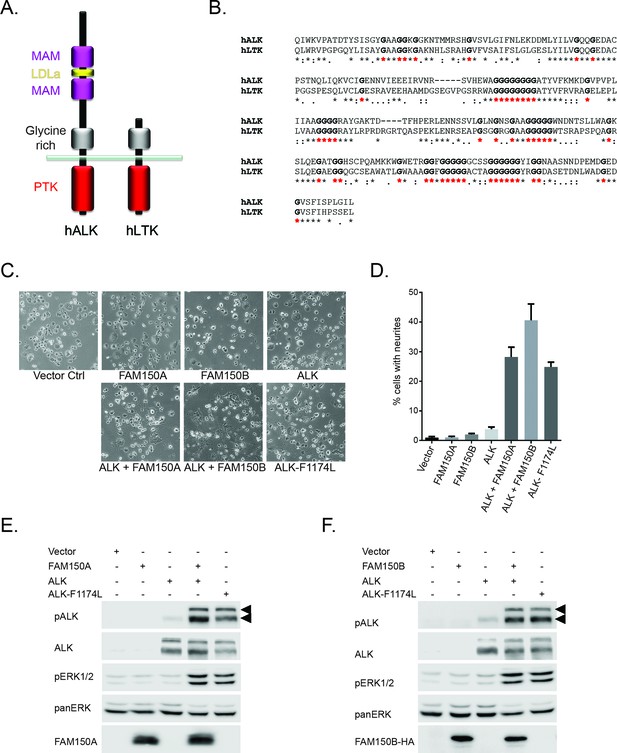
FAM150A and FAM150B activate ALK.
(A) Schematic overview of human anaplastic lymphoma kinase (ALK) and leukocyte tyrosine kinase (LTK) protein domain structures. ALK and LTK share a membrane proximal extracellular glycine-rich region (GR, grey), transmembrane and an intracellular tyrosine kinase domain (red). In addition, the extracellular region of ALK contains two MAM domains (purple) and an LDLa-motif (yellow). (B) Alignments of the GR of ALK and LTK, conserved runs of glycine residues are highlighted in bold with red asterisks. (C) Neurite outgrowth in PC12 cells expressing either vector control, FAM150A, FAM150B, ALK, FAM150A and ALK, FAM150B and ALK or ALK-F1174L quantified in (D). Experiments were performed in triplicate and each sample within an experiment was performed in duplicate (error bars indicate SD). (E) Whole cell lysates from PC12 cells expressing either vector control, FAM150A, ALK, FAM150A and ALK or ALK-F1174L were analyzed by immunoblot analysis of ALK, pALK-Y1604 (arrowheads), FAM150A and ERK1/2. Pan-ERK was employed for equal loading. (F) Whole cell lysates from PC12 cells expressing either vector control, FAM150B, ALK, FAM150B and ALK or ALK-F1174L were analyzed by immunoblot analysis of ALK, pALK-Y1604 (arrowheads), HA (FAM150B) and pERK1/2. Pan-ERK was employed for equal loading.
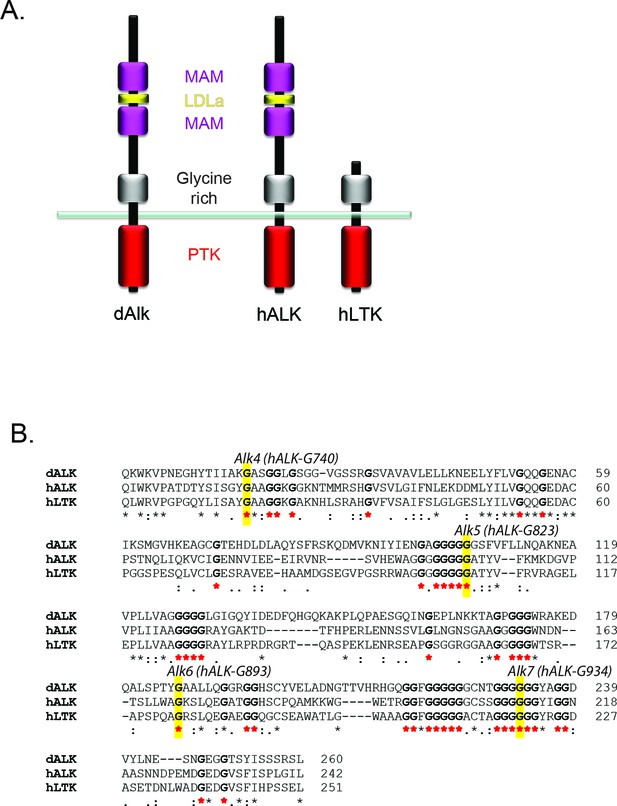
Glycine residues in the glycine-rich region of Drosophila ALK are critical for function.
(A) Schematic overview of anaplastic lymphoma kinase (ALK) and leukocyte tyrosine kinase (LTK) protein domain structure. ALK and LTK share a membrane proximal extracellular glycine-rich region (grey), transmembrane and an intracellular tyrosine kinase domain (red). In addition, the extracellular region of ALK contains two MAM domains (purple) and an LDLa-motif (yellow). (B) Alignment of the glycine-rich region of ALK and LTK, conserved runs of glycine residues are highlighted in bold with red asterisks. Highlighted in yellow are single glycine residues mutated to either aspartic acid or glutamate that result in complete loss of function in Drosophila ALK (Englund et al., 2003), and the conserved residue in human ALK is indicated.
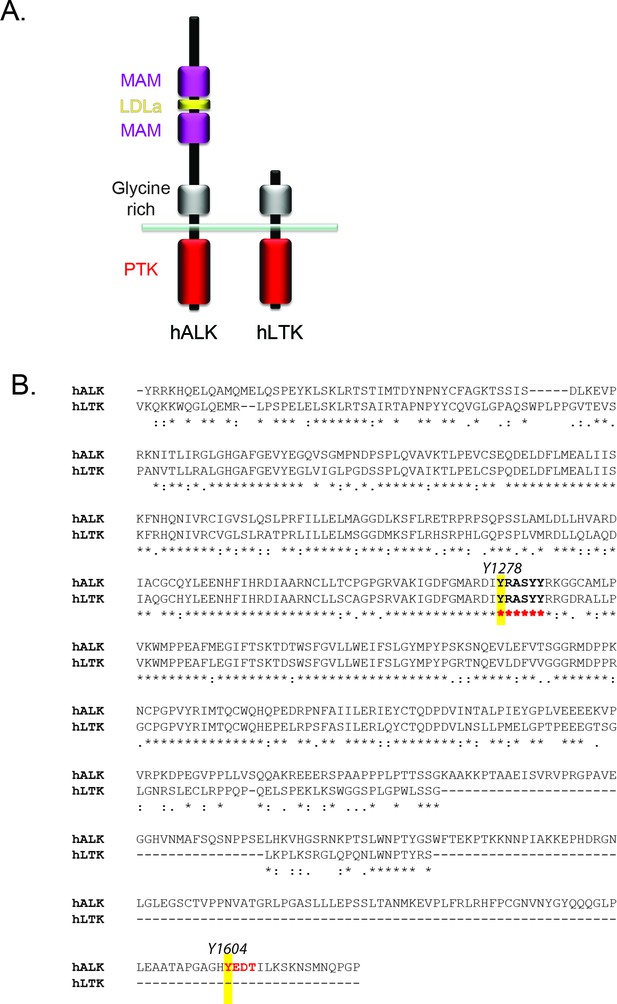
Conservation of phosphoepitopes in the intracellelular domains of ALK and LTK.
(A) Schematic overview of anaplastic lymphoma kinase (ALK) and leukocyte tyrosine kinase (LTK) protein domain structure. (B) Alignment of the intracellular region of ALK and LTK, highlighting in yellow the Y1278 phosphoepitope in the activation loop which shares significant homology between ALK and LTK, and the Y1604 phosphoepitope of ALK which is not found in LTK.
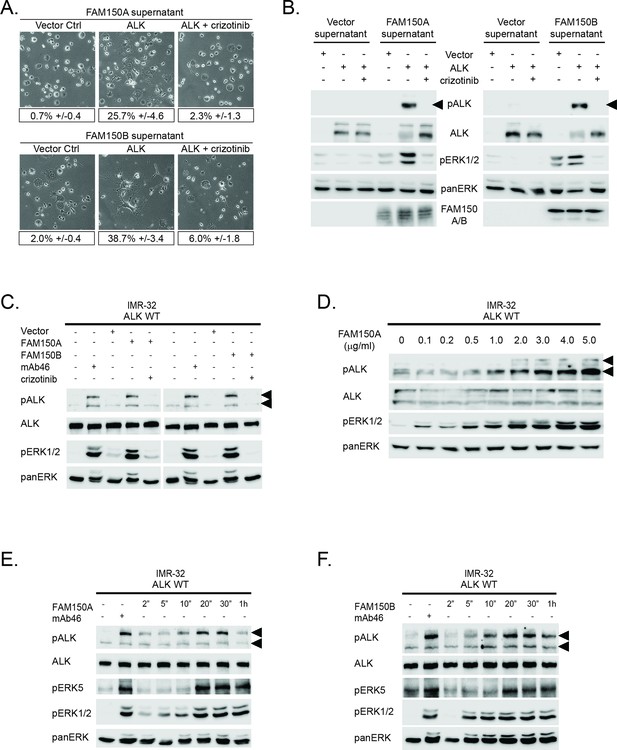
Conditioned medium containing either FAM150A or FAM150B activates endogenous ALK.
(A) Neurite outgrowth in PC12 cells expressing either vector control or anaplastic lymphoma kinase (ALK) were cultured in medium from Human Embryonic Kidney (HEK) 293 cells transfected with either FAM150A or FAM150B, quantified below. Experiments were performed in triplicate and each sample within an experiment was performed in duplicate. Values represent mean ± SD from at least three independent experiments. (B) Whole cell lysates from PC12 cells expressing either vector control or ALK stimulated with medium from HEK293 cells transfected with vector control, FAM150A or FAM150B were analyzed by immunoblot. Analysis was carried out in the presence or absence of 250 nM crizotinib. Detection of ALK activation was visualized with pALK-Y1604 (arrowheads), ALK and pERK1/2 in whole cell lysates. The presence of FAM150A in supernatants was confirmed with anti-FAM150A antibodies, while the presence of FAM150B-HA was confirmed with anti-HA antibodies. Pan-ERK was employed for equal loading. (C) IMR32 cells harboring a wild-type ALK receptor were stimulated for 20 min with medium from HEK293 cells transfected with either vector control, FAM150A or FAM150B prior to analysis by immunoblot. Analysis was carried out in the presence or absence of 250 nM crizotinib. Stimulation with the ALK activating antibody mAb46 was employed as positive control. Detection of ALK activation was visualized with ALK, pALK-Y1604 (arrowheads) and pERK1/2. Pan-ERK was employed for equal loading. (D) IMR32 cells harboring a wild-type ALK receptor stimulated with increased amounts of recombinant His-tagged FAM150A purified from Sf21 cells. Detection of ALK activation was visualized with ALK, pALK-Y1278 (arrowheads) and pERK1/2. Pan-ERK was employed for equal loading. (E) Time course of IMR32 cells stimulated with FAM150A conditioned medium. Stimulation with ALK activating antibody mAb46 was employed as positive control. Detection of ALK activation was visualized with ALK, pALK-Y1604 (arrowheads), pERK5 and pERK1/2. Pan-ERK was employed for equal loading. (F) Time course of IMR32 cells harboring a wild type ALK receptor stimulated with FAM150B conditioned medium. Stimulation with ALK activating antibody mAb46 was employed as positive control. Detection of ALK activation was visualized with ALK, pALK-Y1604 (arrowheads), pERK5 and pERK1/2. Pan-ERK was employed for equal loading.
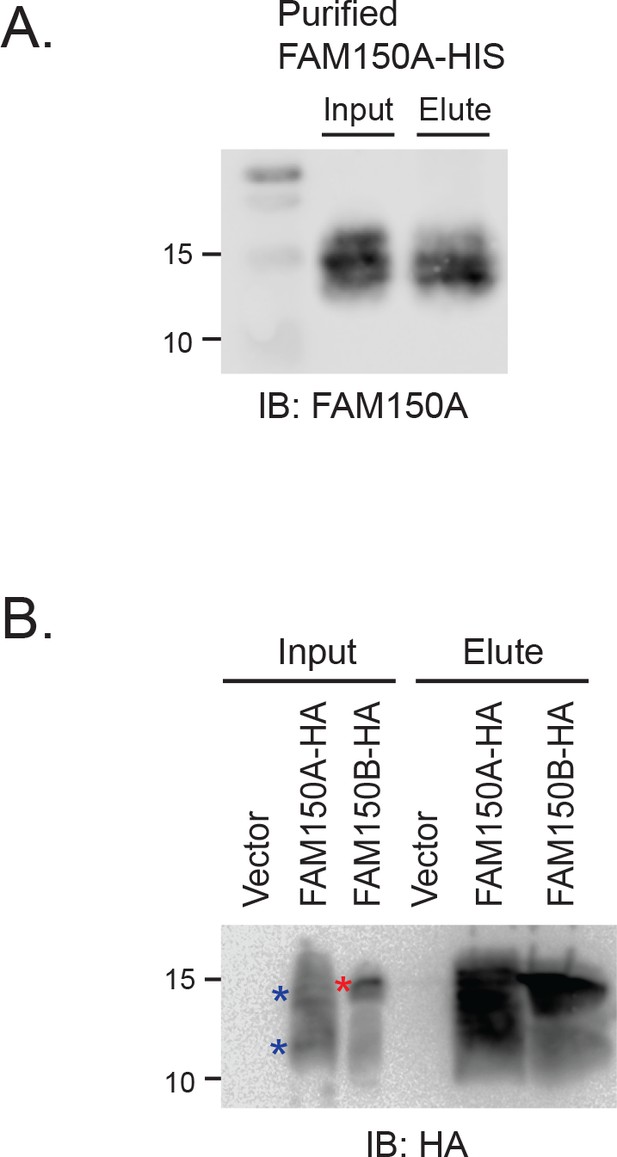
FAM150 proteins interact with heparin.
(A) Purified FAM150A-HIS was incubated with heparin-agarose overnight at 4º°C prior to extensive washing. Bound proteins were eluted, separated on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by immunoblotting with anti-FAM150A. Purified FAM150A-His (5 µg) was employed as input control. (B) Conditioned media from cells transfected with pcDNA3 control, pTT5-FAM150A-HA, or pcDNA3-FAM150B-HA was incubated with heparin-agarose for 3 hr at 4º°C prior to extensive washing. Bound proteins were eluted, separated on 13% SDS-PAGE and analyzed by immunoblotting with anti-HA. Conditioned media (50 µl) was employed as input control. *blue asterisk indicates FAM150A-HA, *red asterisk indicates FAM150B-HA in conditioned medium input.
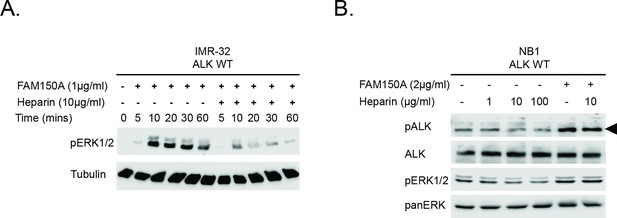
Investigation of the effect of Heparin and FAM150A on ALK activation.
(A) IMR32 cells were treated with FAM150A alone in the presence or absence of 10 µg/ml heparin alone for the times indicated, prior to analysis by immunoblot. Anaplastic lymphoma kinase (ALK) signaling was visualized with pERK1/2. Tubulin was employed for equal loading. (B) NB1 cells were treated with FAM150A alone or heparin alone or with FAM150A and heparin together at the indicated concentrations for 10 min, prior to analysis by immunoblot. Detection of ALK activation was visualized with ALK, pALK-Y1604 (arrowhead) and pERK1/2. Pan-ERK was employed for equal loading.
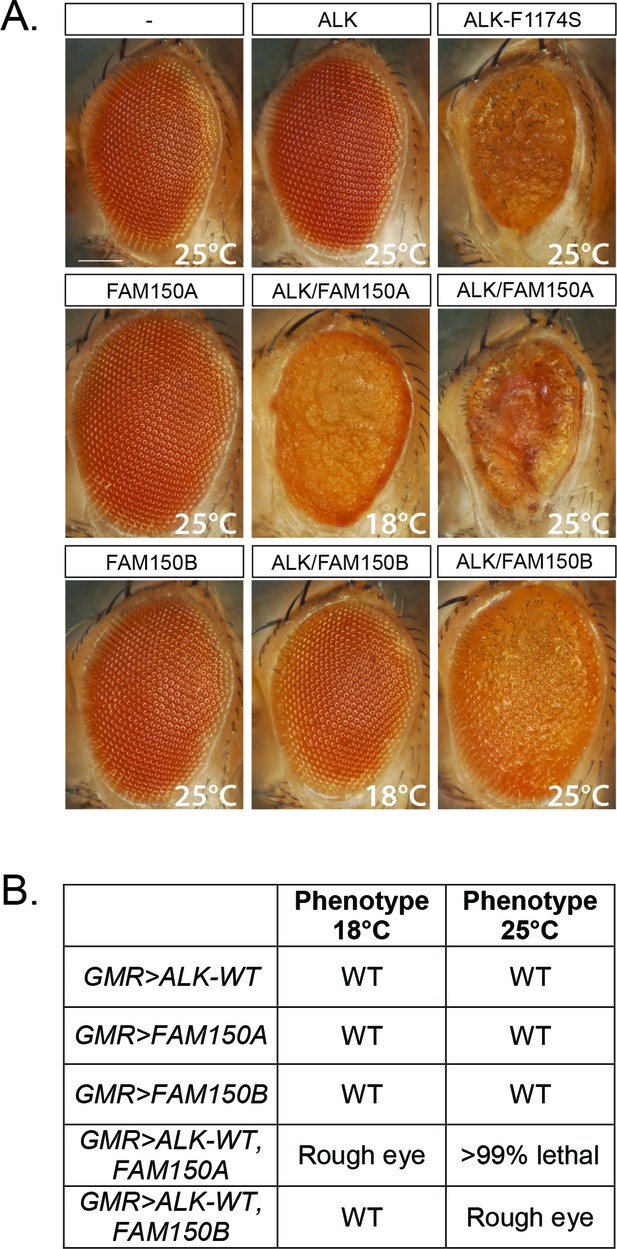
Expression of either FAM150A or FAM150B is sufficient for the activation of wild-type ALK.
(A) Ectopic expression of either FAM150A or FAM150B together with the wild-type human anaplastic lymphoma kinase (ALK) receptor in the Drosophila eye with the GMR-Gal4 driver disrupts the highly organized pattern of ommatidia of the Drosophila eye and generates a rough eye phenotype. Images of adult Drosophila eyes ectopically expressing wild-type ALK in the presence of either FAM150A or FAM150B are shown. Controls expressing either wild-type ALK, FAM150A or FAM150B alone do not display a rough eye phenotype. The constitutively active ALK-F1174S mutant was employed as positive control. (B) Phenotypes observed in adult flies upon GMR-Gal4 driven expression of either FAM150A or FAM150B together with the wild-type ALK at two different temperatures, 18°C and 25°C.
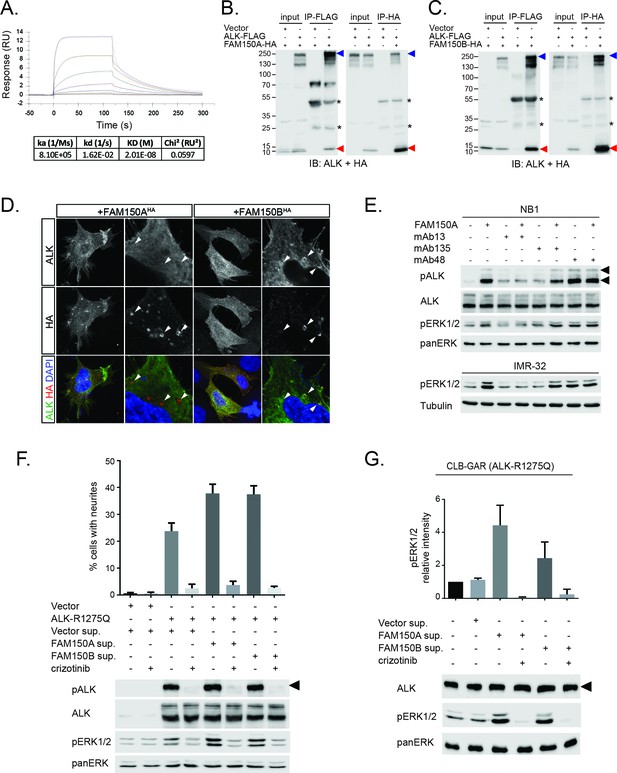
FAM150A and FAM150B bind to ALK and further activate signaling mediated by the R1275Q ALK neuroblastoma mutation.
(A) Binding kinetics of purified FAM150A to extracellular domain of anaplastic lymphoma kinase (ALK-ECD-Fc) in a Biacore surface plasmon resonance (SPR) analysis. (B) FAM150A immunoprecipitates with human ALK. Immunoprecipitation with either anti-FLAG(DYKDDDDK)(ALK) or anti-HA (FAM150A) was performed and the resulting immunoprecipitates immunoblotted for the presence of ALK (blue arrowheads) and FAM150A (red arrowheads), *indicates immunoglobulin light and heavy chains. (C) FAM150B immunoprecipitates with human ALK. Immunoprecipitation with either anti-FLAG (ALK) or anti-HA (FAM150B) was performed and the resulting immunoprecipitates immunoblotted for the presence of ALK (blue arrowheads) and FAM150B (red arrowheads),*indicates immunoglobulin light and heavy chains. (D) Human Embryonic Kidney (HEK) 293 cells expressing ALK were incubated with either control or HA-tagged FAM150A or HA-tagged FAM150B conditioned medium prior to analysis by immunohistochemistry. Both HA-tagged FAM150A and HA-tagged FAM150B bind to ALK-expressing cells. Higher magnification panels indicate intracellular vesicles positive for both ALK and HA-tagged FAM150A/B. (E) NB1 and IMR32 neuroblastoma cells were treated with 2 µg/ml monoclonal antibodies (mAB13, mAb48 or mAb135) prior to stimulation with FAM150A. Detection of ALK activation was visualized with pALK-Y1604 (arrowheads) and pERK1/2 in whole cell lysates. Pan-ERK or tubulin were employed for equal loading. (F) Whole cell lysates from PC12 cells expressing either vector control or ALK-R1275Q were stimulated with medium from HEK293 cells transfected with vector control, FAM150A or FAM150B prior to analysis by immunoblot. Analysis was carried out in the presence or absence of 250 nM crizotinib. Detection of ALK activation was visualized with pALK-Y1604 (arrowheads) and pERK1/2 in whole cell lysates. Pan-ERK was employed for equal loading. Neurite outgrowth was performed in triplicate and each sample within an experiment was performed in duplicate (error bars indicate SD). (G) CLB-GAR cells harboring the ALK-R1275Q mutant were stimulated for 30 min with medium from HEK293 cells transfected with either vector control, FAM150A or FAM150B prior to analysis by immunoblot. Analysis was carried out in the presence or absence of 250 nM crizotinib. Detection of ALK signaling activation was visualized with pERK1/2. Pan-ERK was employed for equal loading. pERK1/2 intensity was analyzed from three independent experiments (error bars indicate SD).
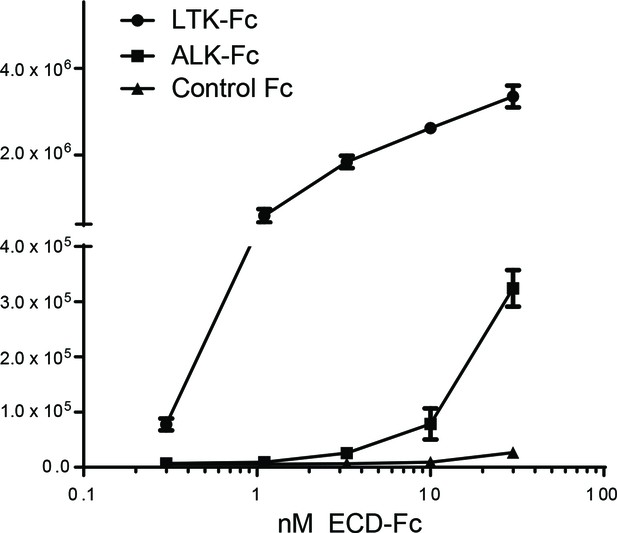
FAM150A binds to the extracellular domain of human ALK by ELISA.
Purified FAM150A was coated on plates and binding of extracellular domain of ALK (ALK-ECD-Fc) was detected by anti-Fc HRP conjugate. LTK-ECD-Fc was used as a binding positive control. FAM150A binds specifically to the ECD of human ALK.
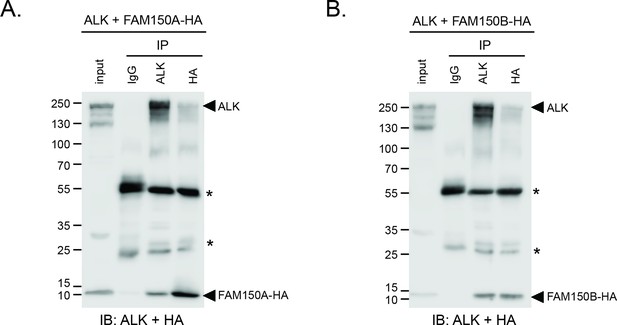
ALK interacts with both FAM150A and FAM150B.
IgG control immunoprecipitations. (A) FAM150A coimmunoprecipitates with human anaplastic lymphoma kinase (ALK). Immunoprecipitation with either anti-IgG control, anti-ALK or anti-HA (FAM150A) was performed and the resulting immonoprecipitates immunoblotted for the presence of ALK and FAM150A-HA. (B) FAM150B coimmunoprecipitates with human ALK. Immunoprecipitation with either anti-IgG control, anti-ALK or anti-HA (FAM150B) was performed and the resulting immonoprecipitates immunoblotted for the presence of ALK and FAM150B-HA. * indicates immunoglobulin light and heavy chains.
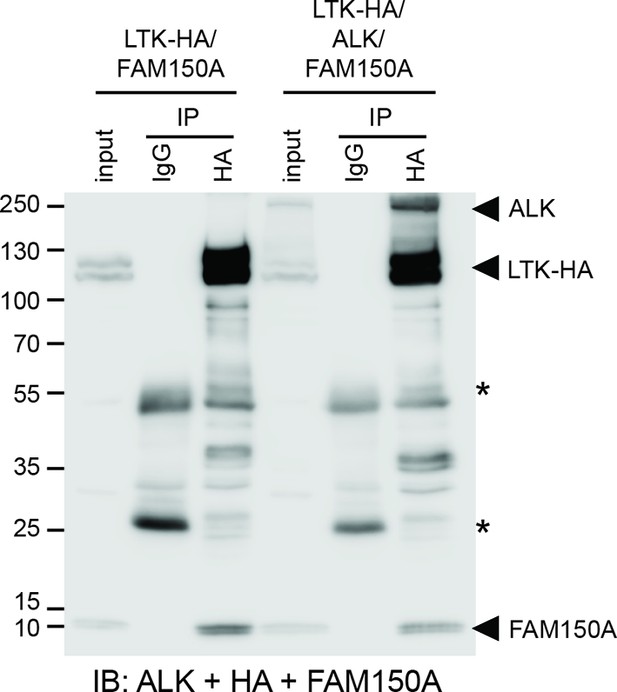
ALK and LTK interact in the presence of FAM150A. FAM150A and anaplastic lymphoma kinase (ALK) coimmunoprecipitate with leukocyte tyrosine kinase (LTK).
Immunoprecipitation with either anti-IgG control or anti-HA (LTK) was performed and the resulting immonoprecipitates immunoblotted for the presence of ALK (mAb135), LTK (HA) and FAM150A. *indicates immunoglobulin light and heavy chains.
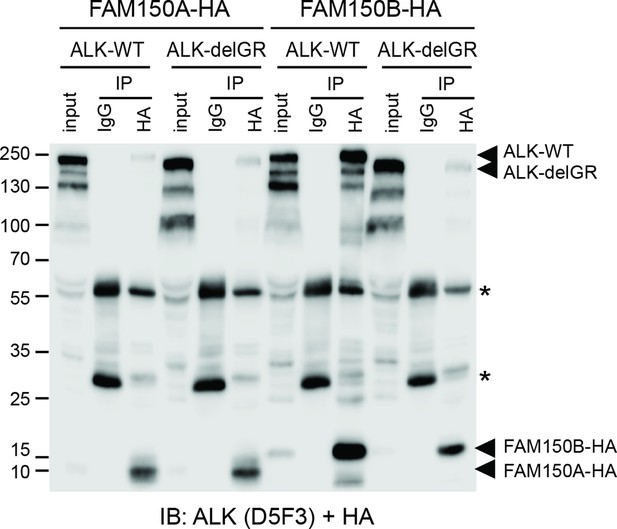
Effect of deletion of the glycine rich domain of ALK on FAM150A and FAM150B binding.
HA-tagged FAM150A coimmunoprecipitates both wild-type anaplastic lymphoma kinase (ALK-WT) and ALK lacking the glycine-rich region (ALK-delGR). In contrast, HA-tagged FAM150B is able to immunoprecipitate ALK-WT, but not ALK-delGR. Input indicates 1/50th whole cell lysate, * indicates immunoglobulin light and heavy chains.
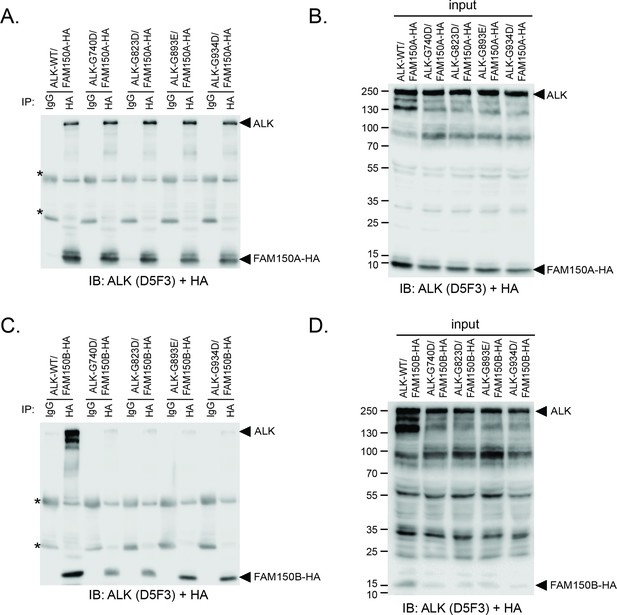
Effect of glycine mutations in the glycine-rich domain of ALK on FAM150A and FAM150B binding.
(A) FAM150A coimmunoprecipitates with wild-type anaplastic lymphoma kinase (ALK-WT) and with ALK bearing mutations in conserved glycines 740 (G740D), 823 (G823D), 893 (G893E) and 934 (G934D) residing in the extracellular glycine-rich region (GR) of ALK. Immunoprecipitation with either anti-IgG control, anti-ALK (D5F3) or anti-HA (FAM150A) was performed. (B) Control blot indicating that the various ALK glycine mutants are expressed.(C) FAM150B coimmunoprecipitates with ALK-WT but not with ALK bearing mutations in conserved glycines 740 (G740D), 823 (G823D), 893 (G893E) and 934 (G934D) residing in the extracellular GR of ALK. Immunoprecipitation with either anti-IgG control, anti-ALK (D5F3) or anti-HA (FAM150B) was performed. (D) Control blot indicating that the various ALK glycine mutants are expressed. Immunoblots were analyzed for the presence of ALK (D5F3) and FAM150A-HAor FAM150B-HA (HA).* indicates immunoglobulin light and heavy chains.
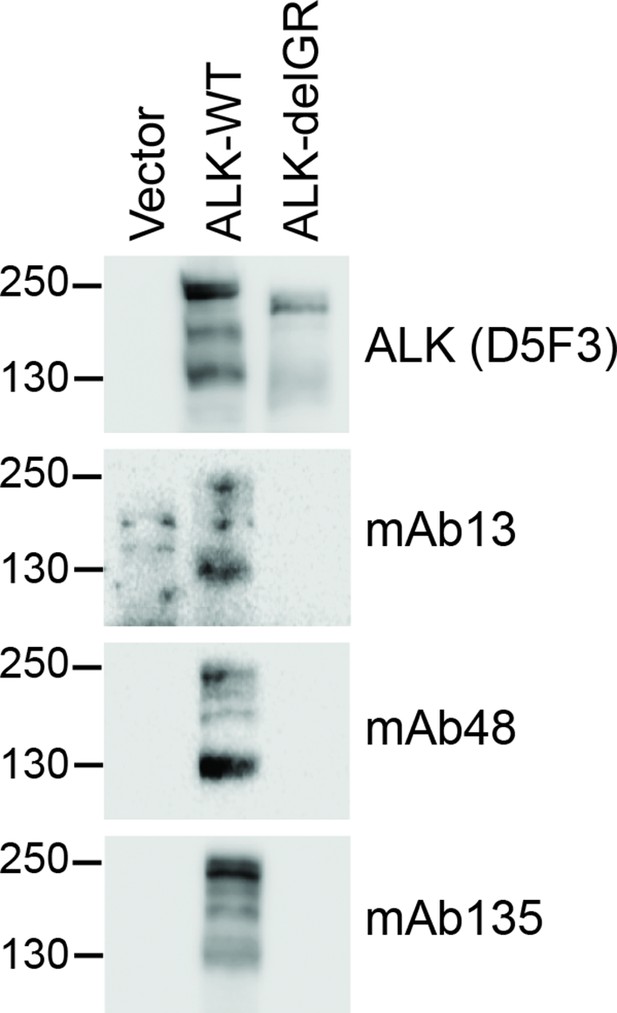
Identification of monoclonal antibodies recognising the glycine-rich region of the ALK ECD.
Monoclonal antibodies raised against anaplastic lymphoma kinase (ALK) (ALK D5F3, mAb13, mAb48 and mAb135) were tested for their ability to recognize the glycine-rich region (GR) of ALK. Among those tested, mAb13, mAb48 and mAb135 were found to recognize ALK-WT, but not ALK in which the GR had been deleted (ALK-delGR).
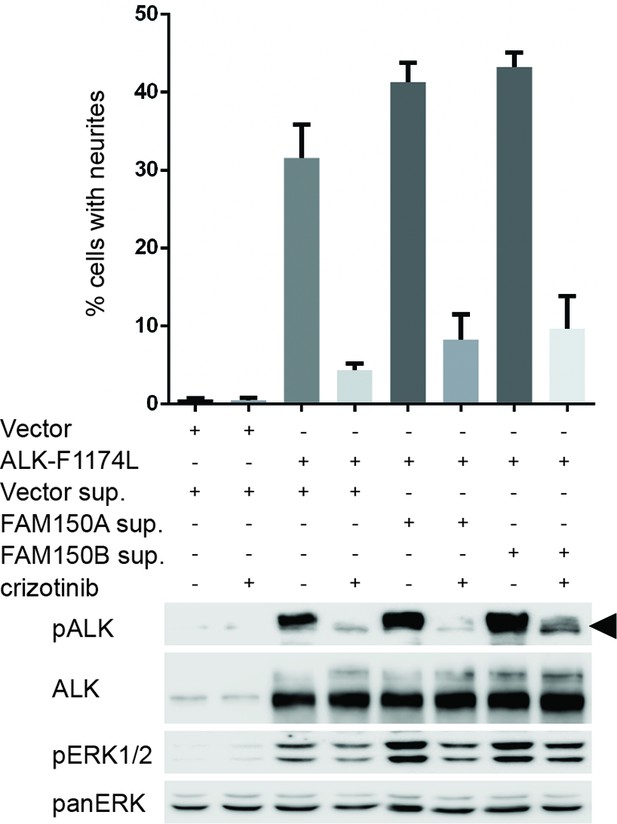
FAM150A and FAM150B bind to ALK and further activate signaling mediated by the ALK-F1174L neuroblastoma mutant.
Whole cell lysates from PC12 cells expressing either vector control or anaplastic lymphoma kinase (ALK)-F1174L were stimulated with medium from Human Embryonic Kidney (HEK) 293 cells transfected with vector control, FAM150A or FAM150B prior to analysis by immunoblot. Analysis was carried out in the presence or absence of 250 nM crizotinib. Detection of ALK activation was visualized with pALK-Y1604 (arrowhead) and pERK1/2 in whole cell lysates. Pan-ERK was employed for equal loading. Neurite outgrowth was performed in triplicate and each sample within an experiment was performed in duplicate (error bars indicate SD)





