A host enzyme reduces metabolic dysfunction-associated steatotic liver disease (MASLD) by inactivating intestinal lipopolysaccharide
Figures
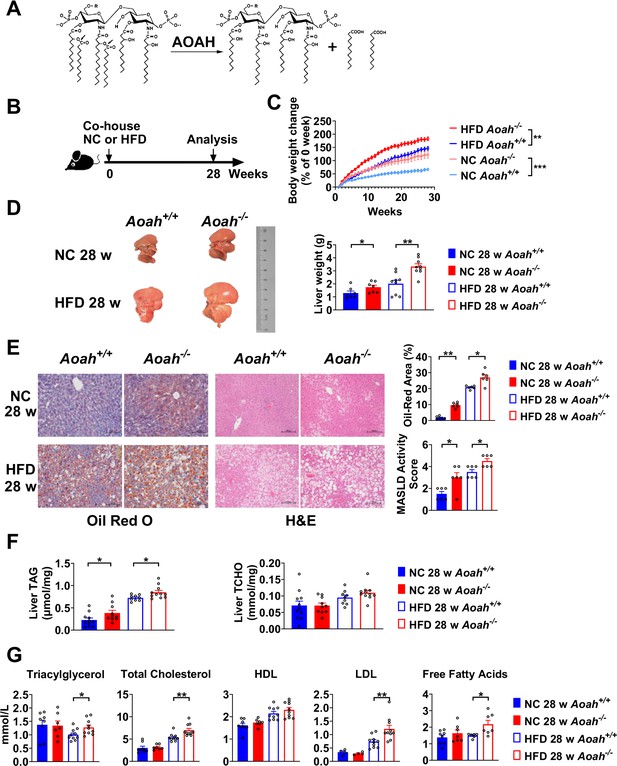
Acyloxyacyl hydrolase (AOAH) reduces hepatic lipid accumulation.
(A) AOAH cleaves the two piggyback fatty acyl chains from the lipid A moiety, inactivating lipopolysaccharides (LPS). The arrows indicate the cleavage sites. (B) Co-housed Aoah+/+ and Aoah-/- mice were fed either a normal diet (NC) or a high-fat diet plus high fructose (23.1 g/l) and glucose (18.9 g/l) in their drinking water (HFD) for 28 weeks. (C) Body weight was measured weekly for 28 weeks. Data were combined from four experiments. n=12–17. (D) Representative images of livers at 28 weeks are shown, and the liver weight was measured. n=7–9, each symbol represents one mouse. (E) Mouse livers were fixed, sectioned, and stained with Oil Red O or H&E. In Oil Red O-stained sections, the percentage of area occupied by the lipid droplets was quantified using ImageJ. H&E staining results were semi-quantitatively scored for disease severity. Data were combined from three experiments, n=6. Scare bars = 100 μm. (F) Triacylglycerol (TAG) and total cholesterol (TCHO) were measured in liver homogenates. Data were combined from at three experiments, n=9–11, each symbol represents one mouse. (G) The serum concentrations of triglyceride, TCHO, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and free fatty acids were measured. Data were combined from three experiments. n=6–10, each symbol represents one mouse. (C–G) Mann–Whitney test and two-way ANOVA were used. *p<0.05; **p<0.01; ***p<0.001.
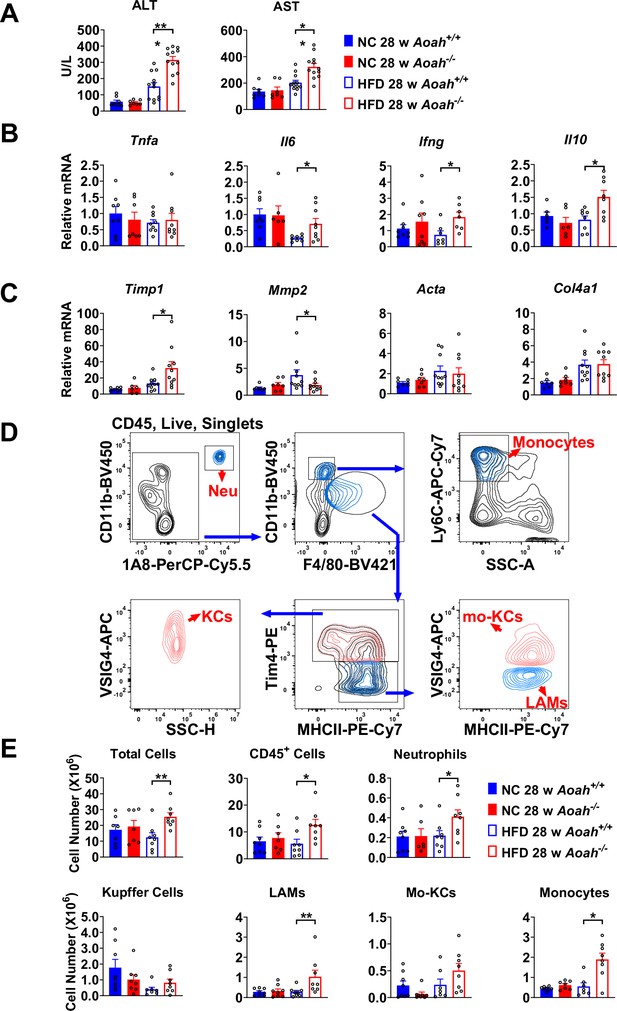
Acyloxyacyl hydrolase (AOAH) prevents hepatic inflammation and tissue injury when mice are fed high-fat diet (HFD).
(A) Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured at 28 weeks. Data were combined from three experiments, n=7–12. (B, C) Inflammation-related Tnfa, Il6, Ifng, Il10 mRNA and fibrosis-related Timp1, Mmp2, Acta, Col4a1 mRNA were measured in livers at 28 weeks. Data were combined from three experiments, n=6–10. (D) Gating strategy to identify hepatic neutrophils, monocytes, Kupffer cells, lipid-associated macrophages (LAM), and monocyte-derived Kupffer cells (Mo-KC) subsets. (E) The myeloid cell numbers in Aoah+/+ and Aoah-/- livers were calculated using FACS analysis. Data were combined from three experiments, n=6–8. Each symbol represents one mouse. Mann–Whitney test was used. *p<0.05; **p<0.01.
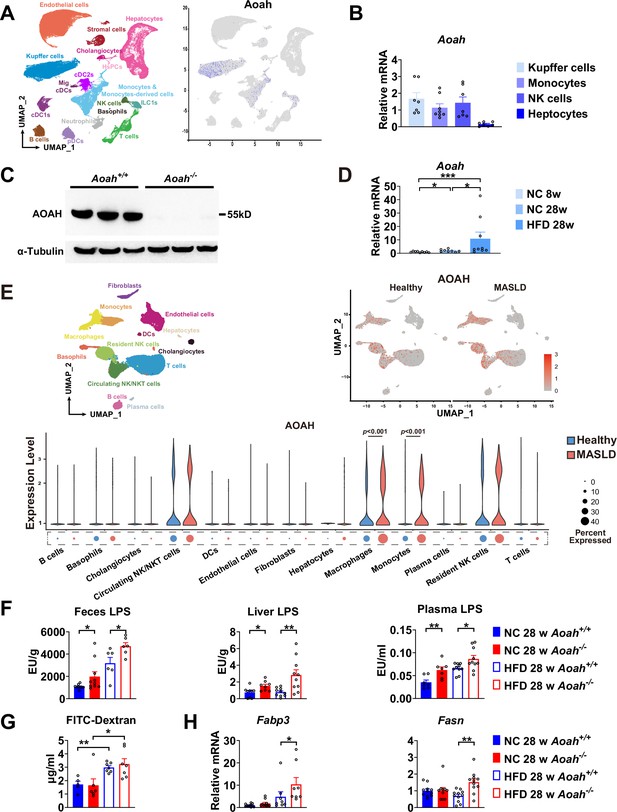
Acyloxyacyl hydrolase (AOAH) reduces hepatic lipopolysaccharides (LPS) levels and the expression of fatty acid uptake and synthesis genes.
(A) AOAH expression in mouse hepatic cells based on single-cell analysis by Remmerie et al., 2020 is shown, n=4. (B) Kupffer cells, monocytes, and NK cells were sorted using flow cytometry and hepatocytes were purified from 6- to 8-week-old mice. AOAH mRNA was measured. Data were combined from three experiments, n=7–8, each symbol represents one mouse. (C) Using western blot analysis, we confirmed that AOAH protein was present in 6–8 week-old Aoah+/+ mouse livers but not in Aoah-/- mouse liver homogenates. Similar results were obtained in two other experiments. (D) AOAH mRNA levels were measured in the livers of Aoah+/+ mice fed with a normal chow for 8 and 28 weeks, and a high-fat diet for 28 weeks. Data were combined from 2 experiments, n=5–7, each symbol represents one mouse. (E) Single-cell RNA sequencing data from livers of MASLD patients and healthy controls by Ramachandran et al., 2019. n=5/group. (F) Heat-inactivated feces suspension, liver homogenates, and plasma from Aoah+/+ and Aoah-/- mice were tested for TLR4-stimulating activity. Data were combined from at least three experiments, n=6–10, each symbol represents one mouse. (G) Gut permeability was measured. Mice were fasted for 18 h. Mice were orally gavaged with fluorescein isothiocyanate (FITC)-conjugated dextran and 4 h later, FITC fluorescence was measured in plasma. Data were combined from three experiments, n=5–7, each symbol represents one mouse. (H) At 28 weeks of normal diet (NC) or high-fat diet (HFD) feeding, liver Fabp3 and Fasn mRNAs were measured. Data were combined from three experiments, n=8–11, each symbol represents one mouse. Mann–Whitney test was used. *p<0.05; **p<0.01.
-
Figure 3—source data 1
Original tiff files of western blots for Figure 3C.
- https://cdn.elifesciences.org/articles/100731/elife-100731-fig3-data1-v1.zip
-
Figure 3—source data 2
Original tiff files containing uncropped western blots with labeling for Figure 3C.
- https://cdn.elifesciences.org/articles/100731/elife-100731-fig3-data2-v1.zip
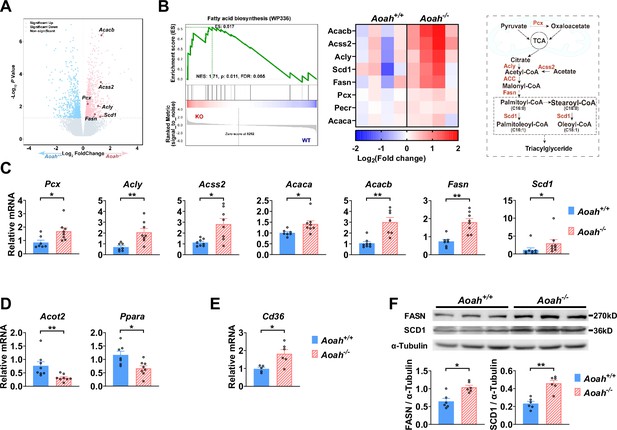
Acyloxyacyl hydrolase (AOAH) regulates the expression of hepatic fatty acid metabolism genes.
(A, B) Co-housed 6–8-week-old (i.e., young) Aoah+/+ and Aoah-/- mouse livers were subjected to RNA-seq analysis. The differentially expressed genes are shown in the volcano plot (A). The fatty acid biosynthesis pathway was found enriched using gene set enrichment analysis (GSEA) (B, left panel). The fatty acid biosynthesis-associated gene expression levels of co-housed Aoah+/+ and Aoah-/- mouse livers are shown as a heatmap, n=4 (B, middle panel). The fatty acid biosynthesis pathway is shown. The enzymes marked red had increased expression in young Aoah-/- mouse livers (B, right panel). (C–E) The hepatic expression of fatty acid synthesis (C), oxidation (D), and uptake (E) genes was measured in co-housed 6–8-week-old Aoah+/+ and Aoah-/- mice. Data were combined from three experiments, n=5–8, each symbol represents one mouse. (F) Liver homogenates from co-housed 6–8-week-old Aoah+/+ and Aoah-/- mice were subjected to western analysis. FASN, SCD1, and α-tubulin protein levels were quantitated using ImageJ. Data were combined from two experiments, n=6, each symbol represents one mouse. Mann–Whitney test was used. *p<0.05; **p<0.01.
-
Figure 4—source data 1
Original tiff files of western blots for Figure 4F.
- https://cdn.elifesciences.org/articles/100731/elife-100731-fig4-data1-v1.zip
-
Figure 4—source data 2
Original tiff files containing uncropped western blots with labeling for Figure 4F.
- https://cdn.elifesciences.org/articles/100731/elife-100731-fig4-data2-v1.zip
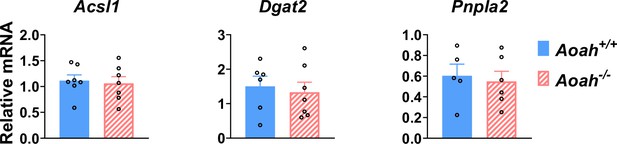
Aoah+/+ and Aoah-/- mouse livers express similar levels of triacylglycerol metabolism mRNA.
Acsl1, Dgat2, and Pnpla2 mRNA was measured in co-housed 6–8-week-old Aoah+/+ and Aoah-/- mouse livers,n=5–7, each symbol represents one mouse.
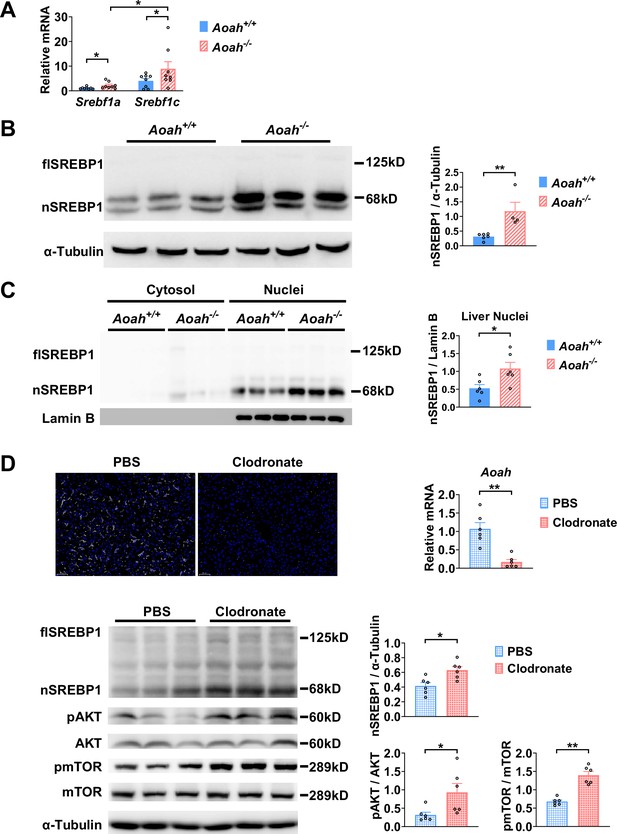
Acyloxyacyl hydrolase (AOAH) reduces hepatic SREBP1.
(A) Srebf1a and Srebf1c gene mRNA was measured in livers from Aoah+/+ and Aoah-/- mice. n=8, each symbol represents one mouse. (B) Liver homogenates from Aoah+/+ and Aoah-/- mice were subjected to western analysis. SREBP1 protein levels were quantitated using ImageJ. flSREBP1 is full-length SREBP1, which is a precursor; nSREBP1is nuclear SREBP1, which is an active form. n=4–6, each symbol represents one mouse. (C) Liver cytosol and nuclei were separated from Aoah+/+ and Aoah-/- mice and then subjected to western analysis. SREBP1 protein levels were quantitated using ImageJ. n=6, each symbol represents one mouse. (D) Aoah+/+ mice were injected i.v. with 200 μl clodronate-liposomes or PBS-liposomes. After 2 days, livers were dissected, sectioned, and stained with anti-F4/80 antibody (white) and DAPI (blue). AOAH mRNA was measured in livers. SREBP1, pAKT, AKT, pmTOR, and mTOR were measured using western blotting. n=6, each symbol represents one mouse. Mann–Whitney test was used. *p<0.05; **p<0.01.
-
Figure 5—source data 1
Original tiff files of western blots for Figure 5B–D.
- https://cdn.elifesciences.org/articles/100731/elife-100731-fig5-data1-v1.zip
-
Figure 5—source data 2
Original tiff files containing uncropped western blots with labeling for Figure 5B–D.
- https://cdn.elifesciences.org/articles/100731/elife-100731-fig5-data2-v1.zip
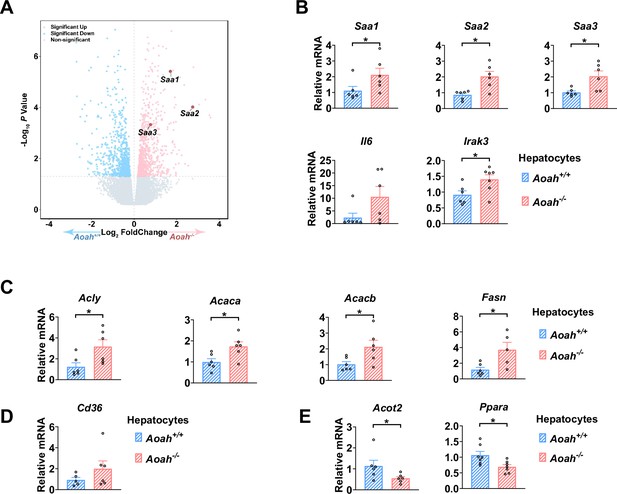
Aoah-/- mouse hepatocytes have altered expression of genes that may promote lipid storage.
(A) The differentially expressed genes between livers from co-housed 6–8-week-old Aoah+/+ and Aoah-/- mouse are shown. (B–E) Hepatocytes were isolated from co-housed 6–8-week-old Aoah+/+ and Aoah-/- mice and cell lysate was used for qPCR analysis for inflammatory (B) and fatty acid synthesis (C), uptake (D), and oxidation (E) gene expression. Data were combined from at least two experiments, n=5–7, each symbol represents one mouse. Mann–Whitney test was used. *p<0.05.
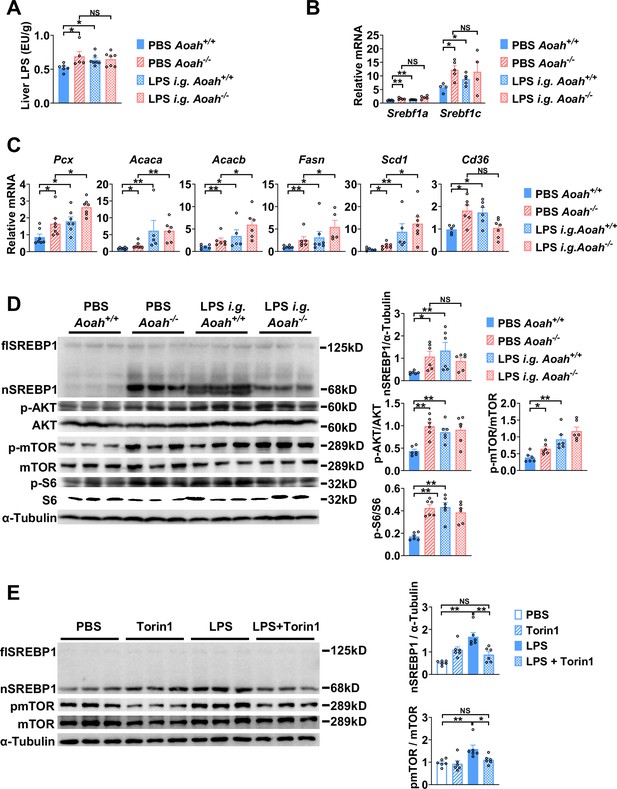
Excessive gut-derived lipopolysaccharides (LPS) increases hepatic nSREBP1 and mTOR activation.
Livers from Aoah+/+ mice, Aoah-/- mice, and Aoah+/+ mice that were orally gavaged (i.g.) with 200 μg LPS 24 h earlier were obtained. (A) Hepatic LPS levels were measured. (B) Srebf1a and Srebf1c mRNA was measured in livers. (C) The mRNA levels of fatty acid biosynthesis-related genes and Cd36 were measured using qPCR. (A–C) Data were combined from two experiments, n=5–7, each symbol represents one mouse. (D) SREBP1 protein levels and AKT-mTOR activities were measured using western and ImageJ. Livers from Aoah-/- mice and Aoah+/+ mice that were orally gavaged (i.g.) with 200 μg LPS 24 h earlier had higher nSREBP1 and mTOR activities than did those from control Aoah+/+ mice. Data were combined from two experiments, n=6, each symbol represents one mouse. (E) Primary hepatocytes were isolated from co-housed 6–8-week-old Aoah+/+ mice and treated with 10 ng/ml LPS with or without 100 nM Torin1 for 6 h. Cells were lysed for western analysis. Data were combined from two experiments, n=6. Mann–Whitney test was used. *p<0.05; **p<0.01; ***p<0.001.
-
Figure 6—source data 1
Original tiff files of western blots for Figure 6D and E.
- https://cdn.elifesciences.org/articles/100731/elife-100731-fig6-data1-v1.zip
-
Figure 6—source data 2
Original tiff files containing uncropped western blots with labeling for Figure 6D and E.
- https://cdn.elifesciences.org/articles/100731/elife-100731-fig6-data2-v1.zip
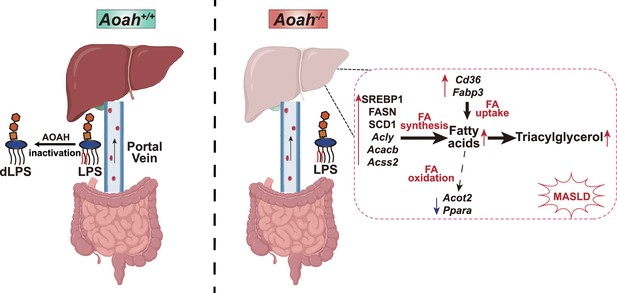
Acyloxyacyl hydrolase (AOAH) prevents metabolic dysfunction-associated steatotic liver disease (MASLD) by inactivating gut-derived lipopolysaccharides (LPS).
Gut-derived LPS may translocate via the portal vein to the liver. In Aoah+/+ mice, LPS can be deacylated by AOAH in the intestine and portal venous blood; when intact LPS reaches the liver, it can be inactivated by hepatic AOAH. In Aoah-/- mice, gut-derived LPS remains able to stimulate fat accumulation (steatosis) in the liver. LPS stimulates hepatocytes to generate nuclear SREBP1, which promotes fatty acid biosynthesis gene expression. LPS also increases the expression of fatty acid uptake genes Cd36 and Fabp3 while reducing that of fatty acid oxidation-related genes, Acot2 and Ppara. Persistent LPS stimulation renders Aoah-/- mice more likely to develop MASLD than are Aoah+/+ mice. dLPS = deacylated LPS.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/100731/elife-100731-mdarchecklist1-v1.docx
-
Supplementary file 1
Primers used for qPCR.
- https://cdn.elifesciences.org/articles/100731/elife-100731-supp1-v1.pdf