Aberrant FGF signaling promotes granule neuron precursor expansion in SHH subgroup infantile medulloblastoma
Figures
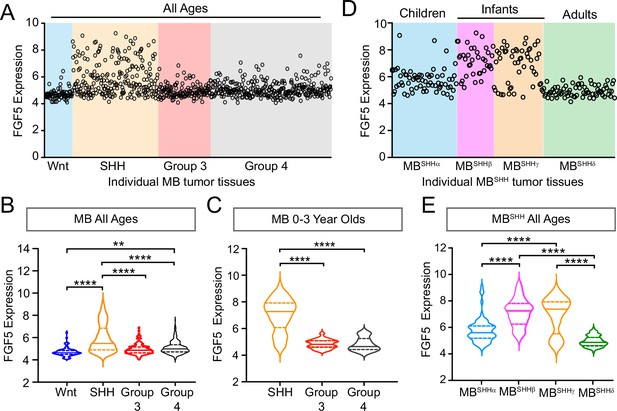
FGF5 expression is upregulated in MBSHH tumors from infant patients.
(A) Levels of FGF5 expression in human medulloblastoma (MB) tumors of all ages from GEO expression dataset #GSE85217 (Cavalli et al., 2017). (B, C) Statistical analysis of FGF5 expression levels associated with MB tumor subtypes from patients across all ages (B) and 0–3 years old MB patients (C). **p<0.01, ****p<0.0001. (D, E) The graph represents FGF5 expression levels in human MBSHH tumors of all ages from GEO expression dataset #GSE85217 (D) and corresponding plots (E) showing statistically higher FGF5 expression in tumors from infants with MBSHH compared to tumors from children or adults with MBSHH. ****p<0.0001.
-
Figure 1—source data 1
Raw data for counts.
- https://cdn.elifesciences.org/articles/100767/elife-100767-fig1-data1-v1.xlsx
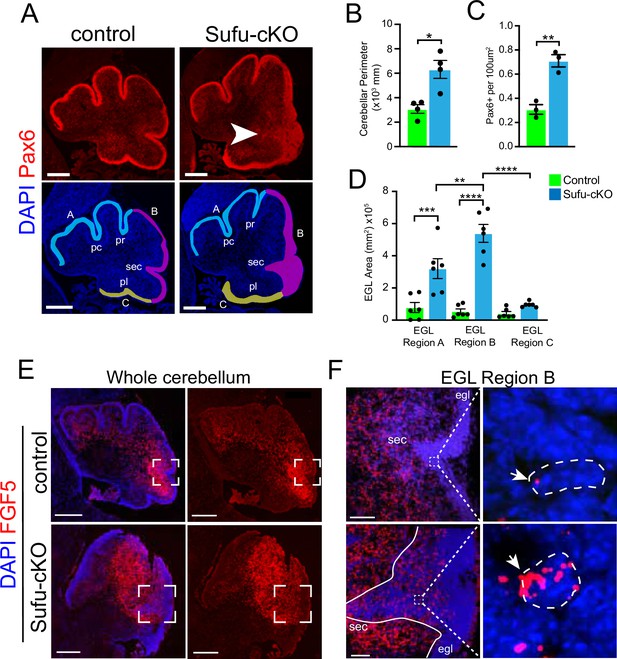
Increased Fgf5 expression coincides with region-specific expansion of granule neuron precursors (GNPs) in the P0 Sufu-cKO cerebellum.
(A) Pax6 (red) and DAPI (blue) immunofluorescence staining of the P0 Sufu-cKO and control cerebelli. Arrow points to severely expanded EGL region B in the P0 Sufu-cKO cerebellum. EGL regions are designated in DAPI-labeled sections as A (light blue), B (magenta), and C (yellow). Each region encompasses specific fissures: the preculminate (pc) and primary (pr) fissures for region A, the secondary (sec) fissure for region B, and the posterolateral (pl) fissure for region C. Scale bars: Scale bars = 250 μm. (B–D) Quantification and comparison of the cerebellar perimeter (B), total area occupied by densely packed Pax6+ cells (C), and size of specific EGL regions (D) between P0 Sufu-cKO and control cerebelli. (E) Fluorescent in situ hybridization using RNAScope probes against Fgf5 mRNA (red) shows the expansion of Fgf5 expression in the P0 Sufu-cKO cerebellum compared to controls. Sections are counterstained with DAPI to distinguish structures. Boxed areas are magnified in (F). Scale bars = 500 μm. (F) Fgf5 is ectopically expressed in cells within the EGL of the P0 Sufu-cKO cerebellum. Boxed areas within the EGL show DAPI-labeled cells expressing visibly high levels of Fgf5, identified as punctate labeling (arrowheads), in the EGL of P0 Sufu-cKO cerebellum compared to controls. Scale bars = 50 μm. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
-
Figure 2—source data 1
Raw data for counts.
- https://cdn.elifesciences.org/articles/100767/elife-100767-fig2-data1-v1.xlsx
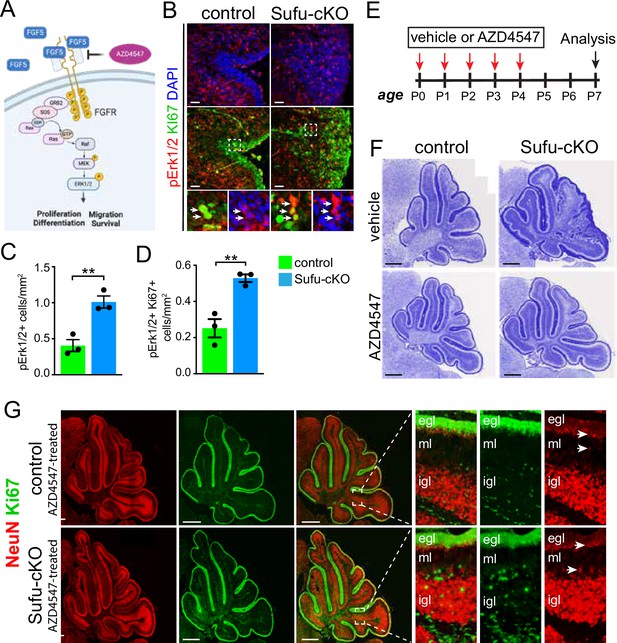
Ectopic activation of FGF signaling in the external granule layer (EGL) of P0 Sufu-cKO cerebellum.
(A) Schematic diagram showing the activation of FGF signaling activity upon binding of FGF5 to extracellular domains of FGFR via the MAPK signal transduction pathway. Created with BioRender. (B) Double-immunofluorescence staining with Ki-67 (green) and phospho-Erk1/2 (pErk1/2; red), a marker of activated MAPK signaling in the P0 Sufu-cKO and control cerebelli. Boxed regions show pErk1/2+and Ki-67+ cells (arrowheads) in the control and Sufu-cKO EGL. Scale bars = 50 μm. (C, D) Quantification of pErk1/2+ cells (C) and double-labeled pErk1/2+and Ki-67+ cells (D) in the P0 Sufu-cKO and control EGL region B. **p<0.01. (E) Experimental design of rescue studies performed by intraventricular administration of FGFR1-3 pharmacological inhibitor, AZD4547, or vehicle controls. (F) Nissl staining of the P7 control and Sufu-cKO treated with either AZD4547 or vehicle, 2 days after treatment. Scale bars = 500 μm. (G) NeuN and Ki-67 double immunofluorescence staining of the P7 control and Sufu-cKO treated with AZD4547. Boxed regions show localization and organization of NeuN+ and Ki-67+ cells in distinct cerebellar layers. Arrows point to areas of the EGL and IGL where NeuN+ cells are beginning to be expressed.
-
Figure 3—source data 1
Raw data for counts.
- https://cdn.elifesciences.org/articles/100767/elife-100767-fig3-data1-v1.xlsx
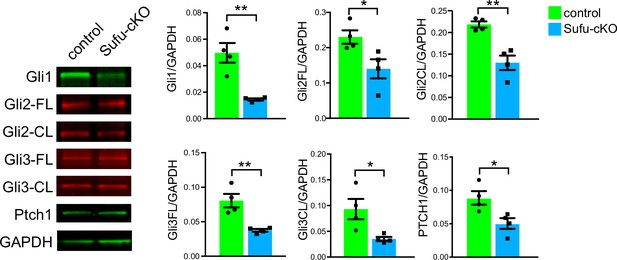
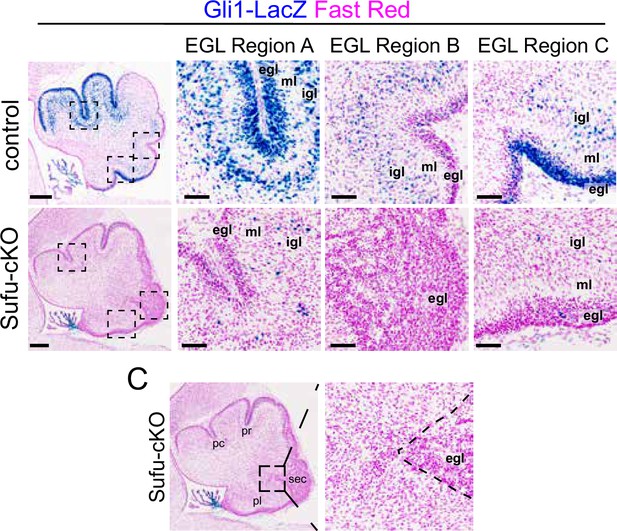
Reduced SHH signaling activity in the P0 Sufu-cKO cerebellum.
Western blot analysis of cerebellar protein lysates from P0 control and Sufu-cKO mice showing significantly lower levels of total and cleaved versions of Gli transcription factors in the P0 Sufu-cKO cerebellum. *p<0.05, **p<0.01. β-Galactosidase activity (blue), representing the Gli1-LacZ transgene, is largely absent in areas adjacent to the external granule layer (EGL) along the secondary (sec) fissure of the P0 Sufu-cKO cerebellum.
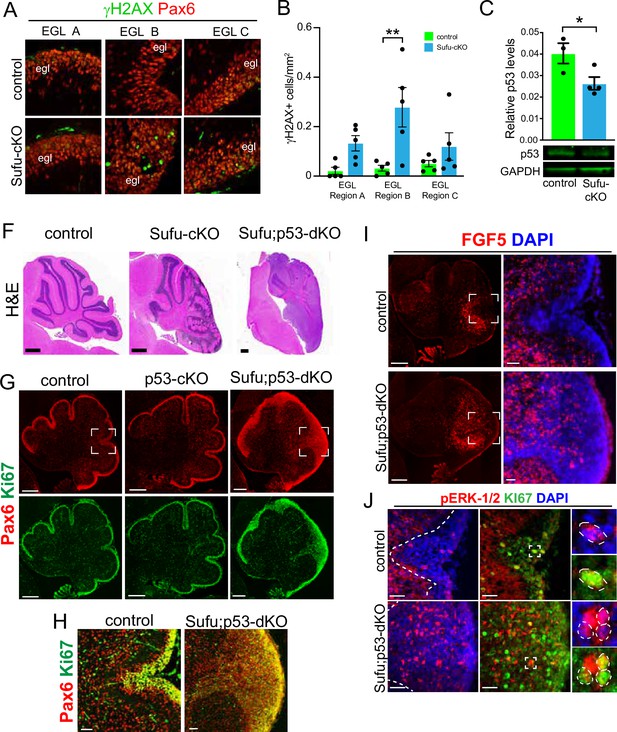
Evidence of pre-neoplastic lesions and high rates of cell death in Sufu-cKO granule neuron precursors.
(A) Double-immunofluorescence staining with Pax6 (red) and γH2AX (green), a marker for double-strand DNA breaks in specific external granule layer (EGL) regions of the P0 Sufu-cKO and control cerebella. (B) Quantification of γH2AX+ cells in each cerebellar region of P0 control and Sufu-cKO mice. **p<0.01. (C) Western blot analysis of Trp53 protein levels in P0 control and Sufu-cKO cerebellar protein lysates. *p<0.05. (F) H&E staining of P60 control, Sufu-cKO, Sufu;Trp53-dKO cerebella. Scale bars = 500 μm. (G, H) Double-immunofluorescence staining against Pax6 (red) and Ki-67 (green) in the P0 control, Trp53-cKO, and Sufu;Trp53-dKO cerebellum (G). Boxed regions in (G) are magnified in (H), demonstrating the expansion of the EGL in the P0 Sufu;Trp53-dKO cerebellum compared to littermate controls. Scale bars = 200 μm (A) and 50 μm (B). (I) Fluorescent in situ hybridization using RNAScope probes against Fgf5 mRNA (red) and DAPI labeling in the P0 Sufu;Trp53-dKO and control cerebellum. Boxed areas are enlarged to show ectopic localization of Fgf5+ cells in the EGL of the Sufu-Trp53-dKO cerebellum, unlike in controls. Scale bars = 200 μm and 50 μm (boxed area). (J) Double-immunofluorescence staining with Ki-67 (green) and phospho-Erk1/2 (pErk1/2; red) in the P0 Sufu;Trp53-dKO and control cerebelli. Boxed regions show cells double-labeled with pErk1/2+and Ki-67+ cells in the control and Sufu;Trp53-dKO EGL region B. Scale bars = 25 μm.
-
Figure 4—source data 1
Raw data for counts.
- https://cdn.elifesciences.org/articles/100767/elife-100767-fig4-data1-v1.xlsx
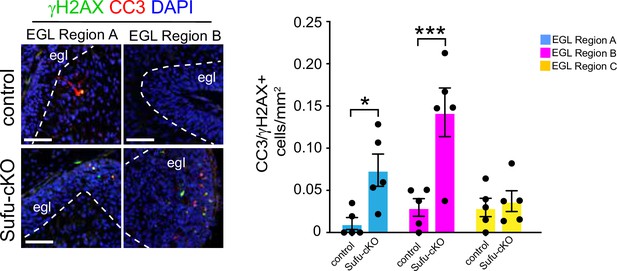
Evidence of pre-neoplastic lesions and high rates of cell death in Sufu-cKO granule neuron precursors.
Double-immunofluorescence staining with γH2AX (green) and cleaved-caspase 3 (CC3; red), a marker for apoptotic cells, and DAPI labeling in regions A and B. Scale bars = 50 μm. Graph shows quantification of the density of cells labeled with CC3 and γH2AX within each EGL regions. *p<0.05, ***p<0.001.
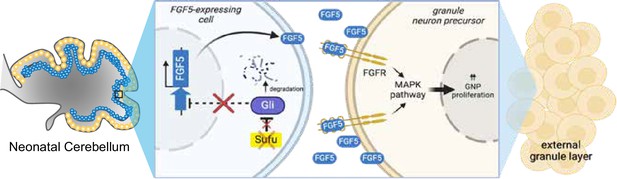
Loss of Sufu function drives excess proliferation of granule neuron precursors via FGF signaling activation.
The schematic diagram models how Sufu loss of function (LOF) facilitates the expansion of granule neuron precursors (GNPs) (yellow cells) in the external granule layer (EGL) at the early stages of cerebellar development. We hypothesize that Fgf5-expressing cells (blue cells, yet to be identified) send FGF5 signals to GNP to proliferate and that ectopic expression of Fgf5 in the absence of SUFU is responsible for the uncontrolled expansion of EGL localized GNPs. Created with BioRender..

Comparative expression of FGF ligands, FGF5, FGF10, FGF12, and FGF19, across all MB subgroups.
FGF12 expression is not significantly different, while FGF5, FGF10, and FGF19, show distinct upregulation in MBSHH subgroup (MBWNT n=70, MBSHH n=224, MBGR3 n=143, MBGR4 n=326).
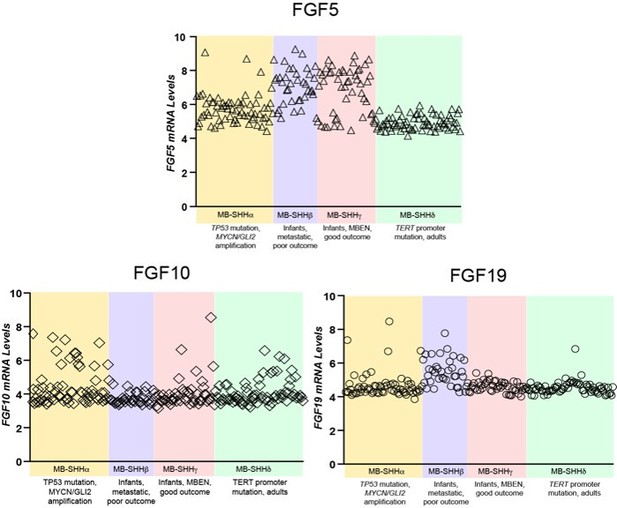
Comparative expression of FGF5, FGF10, and FGF19 in different MBSHH subtypes.
FGF5 specifically show mRNA relative levels above 6 in 81% of MBSHH infant patient tumors (n=80 MBSHHα and MBSHHγ tumors) unlike 35% of MBSHHα (n=65) or 0% of MBSHHδ (n=75) tumors.

Expression of FGF5 and FGF8 in the P4 mouse cerebellum (Allen Brain Atlas, https://developingmouse.brain-map.org).
Tables
| MBWNT n=70 | MBSHHα n=66 |
|---|---|
| MBSHH n=224 | MBSHHβ n=35 |
| MBGR3 n=143 | MBSHHγ n=47 |
| MBGR4 n=326 | MBSHHδ n=77 |