Feeding state-dependent regulation of developmental plasticity via CaMKI and neuroendocrine signaling
Figures
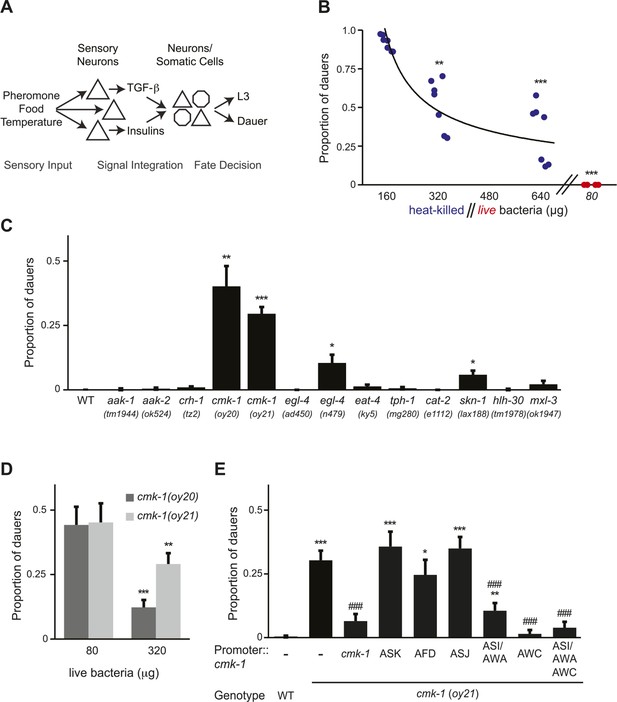
CMK-1 acts in the AWC and ASI/AWA neurons to inhibit dauer formation in fed animals.
(A) Simplified model of sensory inputs modulating TGF-β and insulin signaling in the regulation of the dauer decision. See text for details. (B) Quantification of dauer formation in wild-type animals in the presence of 6 μM ascr#3 and the indicated amounts of heat-killed (blue circles) or live (red circles) OP50 bacteria. Each filled circle represents one assay; n > 65 animals per assay, three independent experiments. Line represents best fit to the data. ** and *** indicate different from values using 160 μg of heat-killed bacteria at p < 0.01 and 0.001, respectively (ANOVA and Games-Howell post-hoc test). (C) Dauers formed by strains of the indicated genotypes in the presence of 6 μM ascr#3 and 80 μg live OP50. Each data point is the average of ≥3 independent experiments of >65 animals each. Errors are SEM. *, **, and *** indicate different from wild-type at p < 0.05, 0.01, and 0.001, respectively (ANOVA and Games-Howell post-hoc test). (D) Dauer formation in cmk-1 mutants grown with 6 μM ascr#3 and the indicated amounts of live OP50. Each data point is the average of ≥3 independent experiments of >65 animals each. Errors are SEM. ** and *** indicate different from corresponding values using 80 μg OP50 at p < 0.01 and 0.001, respectively (Student's t-test). (E) Dauers formed by strains of the indicated genotypes grown on plates containing 6 μM ascr#3 and 80 μg live OP50. Promoters used to drive wild-type cmk-1 cDNA expression were: cmk-1p—cmk-1 upstream regulatory sequences; ASK—sra-9p; AFD—ttx-1p; ASJ—trx-1p; ASI/AWA—gpa-4p; AWC—ceh-36Δp. Each data point is the average of ≥3 independent experiments of >65 animals each. For transgenic strains, data are averaged from 1–4 independent lines each. Errors are SEM. *, **, and *** indicate different from wild-type at p < 0.05, 0.01, and 0.001, respectively; ### indicates different from cmk-1(oy21) at p < 0.001 (ANOVA and Games-Howell post-hoc test).
-
Figure 1—source data 1
Dauer assay data for individual trials in Figure 1.
- https://doi.org/10.7554/eLife.10110.004
-
Figure 1—source data 2
Dauer assay data for individual trials in Figure 1—figure supplement 1.
- https://doi.org/10.7554/eLife.10110.005
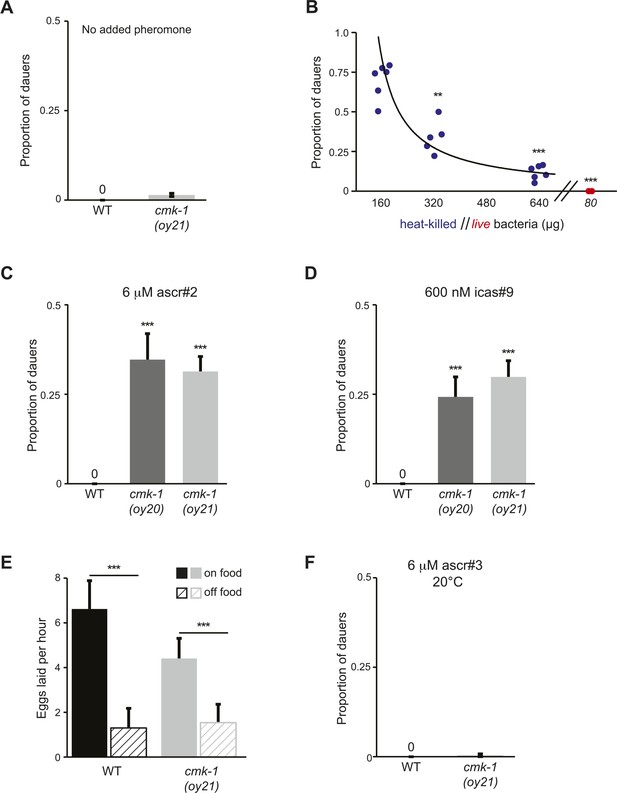
CMK-1 inhibits dauer formation in fed animals.
(A) Dauer formation in wild-type and cmk-1 mutants on 80 μg live OP50 in the absence of exogenous pheromone. n > 65 animals per assay; at least 12 independent trials. Errors are SEM. (B) Dauers formed by daf-22(m130) animals in the presence of 6 μM ascr#3 and the indicated amounts of heat-killed (blue circles) or live (red circles) OP50 bacteria. Each filled circle represents one assay; n > 65 animals per assay, three independent experiments. ** and *** indicate different from values using 160 μg of heat-killed bacteria at p < 0.01 and 0.001, respectively (ANOVA and Games-Howell post-hoc test). (C, D) Dauers formed by wild-type or cmk-1 mutants on 80 μg live OP50 and the indicated amounts of ascr#2 or icas#9. *** indicates different from wild-type at p < 0.001 (ANOVA and Games-Howell post-hoc test). n > 65 animals each, three independent experiments. Errors are SEM. (E) Egg-laying is sensitive to bacterial food in wild-type and cmk-1(oy21) mutants. Shown is the average number of eggs laid per hour by adult animals in the presence or absence of live OP50. *** indicates different between the indicated values at p < 0.001 (Student's t-test). n > 30 animals for each condition. Errors are SEM. (F) Dauers formed by wild-type or cmk-1(oy21) mutants on 80 μg of live OP50 and 6 μM ascr#3 at 20°C. For each assay: n > 65 animals each, three independent experiments. Errors are SEM.
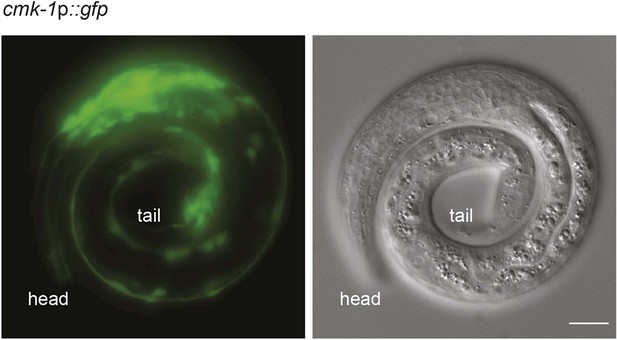
cmk-1p::gfp is expressed broadly in multiple neurons.
Shown is the expression pattern of cmk-1p::gfp in an L1 larva (left panel: GFP; right panel: DIC). Note GFP expression in multiple neurons in the head and tail. Scale bar: 10 μm.
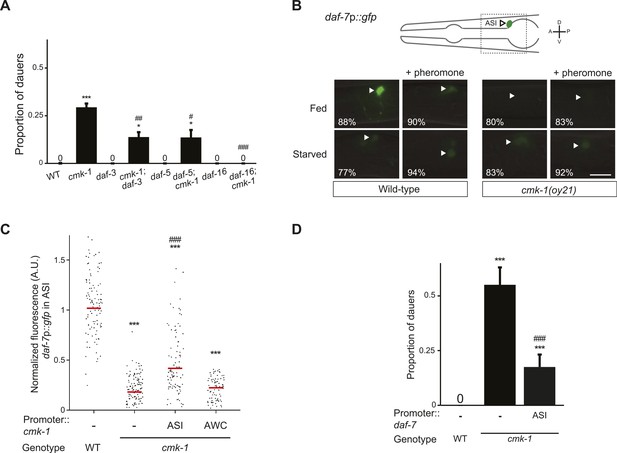
CMK-1 acts cell-autonomously to regulate daf-7 TGF-β expression in ASI.
(A) Dauers formed by the indicated strains on 80 μg live OP50 and 6 μM ascr#3. Alleles used were: cmk-1(oy21), daf-3(mgDf90), daf-5(e1385), and daf-16(mgDf50). Shown are the averages of ≥3 independent experiments with >65 animals each. Errors are SEM. (B) Representative images of daf-7p::gfp expression in L1 larvae of wild-type or cmk-1(oy21) animals under the indicated conditions. Schematic at top indicates the position of ASI cell body (lateral view); boxed region is shown in panels below. Occasional weak expression is observed in the ADL neurons. Animals were grown with plentiful live OP50 or starved for at least 6 hr in the absence or presence of 1 unit of crude pheromone (see ‘Materials and methods’). White arrowheads indicate cell bodies of ASI. Numbers in bottom left hand corners indicate the percentage of examined larvae that exhibit the shown phenotype; n > 50 each; three independent experiments. Lateral view; scale bar: 10 μm. (C) Scatter plot of fluorescence intensity of daf-7p::gfp expression in ASI in wild-type or cmk-1(oy21) mutants. Median is indicated by a red horizontal line. Animals were grown on ample live OP50 in the absence of exogenous pheromone. Each dot is the fluorescence intensity of a single neuron in a given experiment; n > 60 neurons total each, at least three independent experiments. Promoters driving wild-type cmk-1 cDNA were: ASI—srg-47p; AWC—ceh-36Δp. (D) Dauers formed by shown strains on 80 μg live OP50 and 6 μM ascr#3. The srg-47 promoter was used to drive expression of wild-type daf-7 cDNA in ASI. Shown are the averages of ≥3 independent experiments with >65 animals each. Errors are SEM. Unless indicated otherwise, * and *** indicate different from wild-type at p < 0.05 and p < 0.001, respectively, #, ##, and ### indicate different from cmk-1 at p < 0.05, 0.01, and 0.001, respectively. (ANOVA and Games-Howell post-hoc test).
-
Figure 2—source data 1
Dauer assay data for individual trials in Figure 2.
- https://doi.org/10.7554/eLife.10110.009
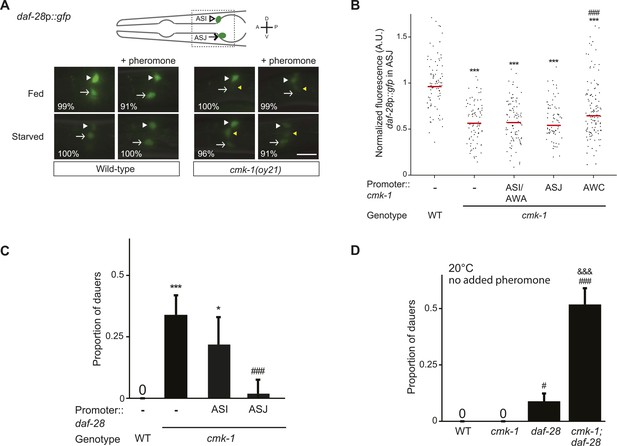
CMK-1 acts non cell-autonomously in AWC to regulate daf-28 insulin-like peptide (ILP) gene expression in ASJ.
(A) Representative images of daf-28p::gfp expression in L1 larvae of wild-type or cmk-1(oy21) animals under the indicated conditions. Schematic of worm head indicating positions of the ASI and ASJ sensory neuron cell bodies is shown at top; boxed region is shown in panels below. Animals were grown with plentiful live OP50 or starved for at least 6 hr in the absence or presence of 1 unit of crude pheromone (see ‘Materials and methods’). White arrowheads and arrows indicate cell bodies of ASI and ASJ, respectively. Yellow arrowheads indicate expression in an ectopic cell observed in ∼14% of wild-type and ∼50% of cmk-1 mutants under all conditions. Numbers in bottom left hand corners indicate the percentage of examined larvae that exhibit the shown expression patterns; n > 50 each; three independent experiments. Lateral view; scale bar: 10 μm. (B) Scatter plot of fluorescence intensity of daf-28p::gfp expression in ASJ in wild-type or cmk-1(oy21) mutants. Median is indicated by red horizontal line. Animals were grown on ample live OP50 in the absence of exogenous pheromone. Each dot is the fluorescence intensity of a single neuron in a given experiment; n > 60 neurons total each, at least three independent experiments. For transgenic strains, a representative line was selected from experiments shown in Figure 1E and crossed into the reporter strains. Promoters driving wild-type cmk-1 cDNA were: ASI/AWA—gpa-4p; AWC—ceh-36Δp; ASJ—trx-1p. (C) Dauers formed by shown strains on 80 μg live OP50 and 6 μM ascr#3. Promoters used to drive expression of wild-type daf-28 cDNA were: ASI—srg-47p and ASJ—trx-1p. Shown are the averages of ≥3 independent experiments with >65 animals each. (D) Dauers formed by the indicated strains on 80 μg OP50 at 20°C in the absence of exogenous pheromone. Alleles used were: cmk-1(oy21) and daf-28(tm2308). Shown are the averages of ≥3 independent experiments with >65 animals each. Errors are SEM. Unless indicated otherwise, * and *** indicate different from wild-type at p < 0.01 and 0.001, respectively; # and ### indicate different from cmk-1 at p < 0.05 and 0.001, respectively; &&& indicates different from daf-28 at p < 0.001 (ANOVA and Games-Howell post-hoc test).
-
Figure 3—source data 1
Dauer assay data for individual trials in Figure 3.
- https://doi.org/10.7554/eLife.10110.011
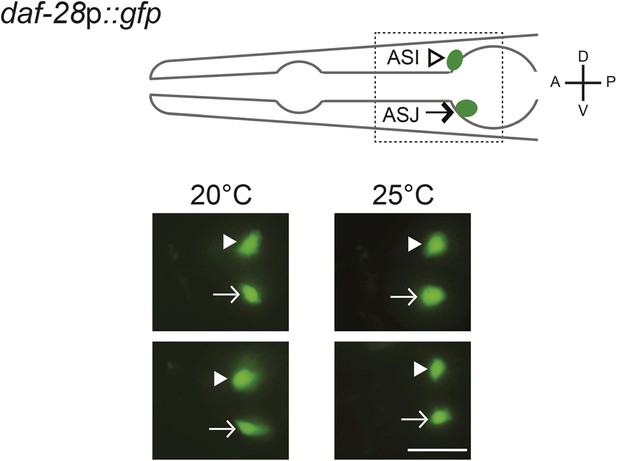
daf-28 expression is not affected by cultivation temperature.
(Top) Schematic of worm head indicating positions of the ASI and ASJ sensory neuron cell bodies. Boxed area is shown in images below. (Below) Representative images of daf-28p::gfp in developmentally synchronized L1 animals, grown on live OP50 and in the absence of exogenous pheromone, at the indicated temperatures. White arrowheads and arrows indicate cell bodies of ASI and ASJ, respectively. Lateral view; scale bar: 10 μm.
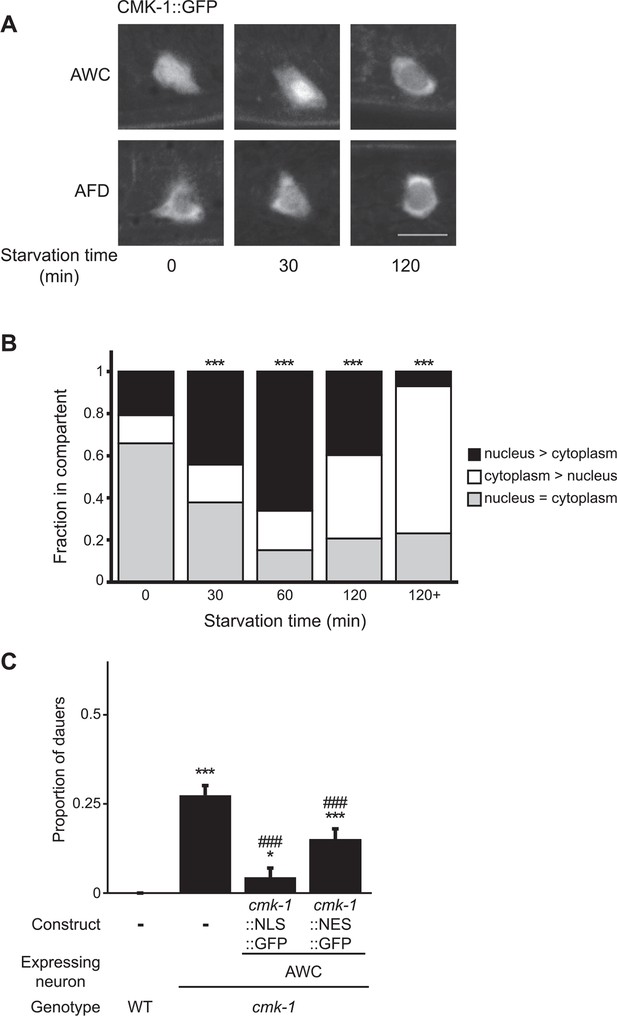
Subcellular localization of CMK-1 in AWC is regulated by feeding state.
(A, B) Representative images (A) and quantification (B) of subcellular localization of CMK-1::GFP in AWC neurons following removal from food for the indicated times. Representative images of CMK-1::GFP localization in AFD are also shown in A. Scale bar: 5 μm (A). n > 75 AWC neurons each (B). *** indicates different from distribution at 0 min at p < 0.001 (χ2 test). (C) Dauers formed by the indicated strains on 80 μg live OP50 and 6 μM ascr#3. CMK-1::NLS::GFP and CMK-1::NES::GFP were expressed in AWC under the ceh-36Δ promoter. Shown are the averages of ≥3 independent experiments with >65 animals each. For transgenic strains, data are averaged from two independent lines each. Errors are SEM. *and *** indicate different from wild-type at p < 0.05 and 0.001, respectively; ### indicates different from cmk-1(oy21) at p < 0.001 (ANOVA and Games-Howell post-hoc test).
-
Figure 4—source data 1
Dauer assay data for individual trials in Figure 4.
- https://doi.org/10.7554/eLife.10110.014
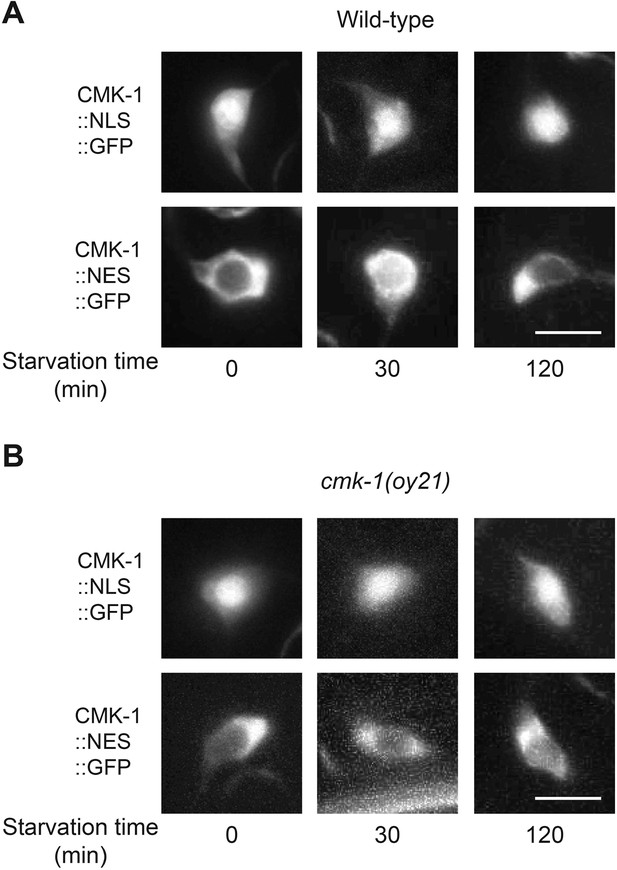
Localization of CMK-1::NLS::GFP and CMK-1::NES::GFP in AWC.
Shown is the localization of NLS::GFP or NES::GFP tagged CMK-1 protein in AWC in wild-type (A) and cmk-1(oy21) mutants (B) in the indicated conditions. Scale bar: 5 μm.
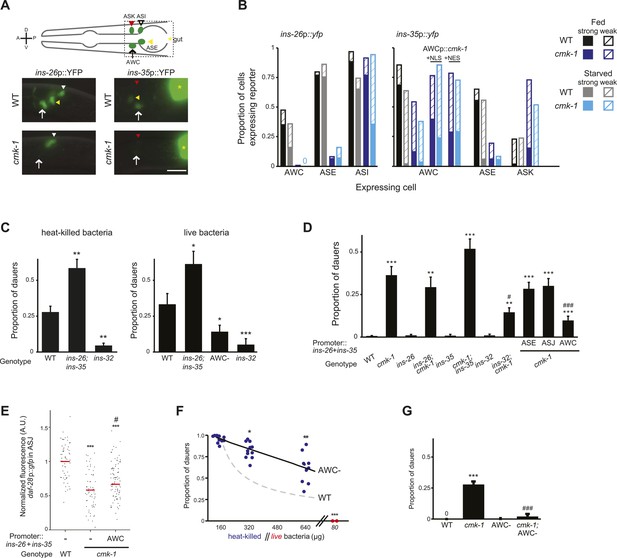
CMK-1 maintains a balance of anti- and pro-dauer signals from AWC as a function of feeding state.
(A) (Top) Schematic of worm head indicating positions of sensory neuron soma. Boxed area is shown in images below. (Below) Representative images of ins-26p::yfp and ins-35p::yfp expression in fed wild-type and cmk-1(oy21) mutants. White and yellow arrowheads indicate ASI and ASE, respectively; white arrow indicates AWC; yellow asterisk marks expression in the intestine. The location of ASK is indicated by a red arrowhead; fluorescence in ASK is weak and not visible at this exposure in shown images. Lateral view; scale bar: 10 μm. (B) Quantification of expression in each neuron type in fed and starved (>6 hr) conditions. Solid and hatched bars indicate strong and weak expression, respectively, in each cell. n > 50 animals each; three independent experiments. (C) Dauers formed by shown strains on 160 μg heat-killed OP50 and 60 nM ascr#2 (left), and 80 μg live OP50 and 6 μM ascr#3 + 600 nM ascr#5 (right). Alleles used were: ins-26(tm1983), ins-35(ok3297) and ins-32(tm6109). n > 4 assays of 65 animals each; at least three independent experiments. (D) Dauers formed by shown strains on 6 μM ascr#3 and 80 μg live OP50. ins-26 and ins-35 cDNAs were expressed in ASE, ASJ, and AWC under che-1, trx-1, and ceh-36∆ regulatory sequences, respectively. At least two independent lines were analyzed for each transgenic strain. Shown are the averages of at least three independent experiments with >65 animals each. (E) Scatter plot of fluorescence intensity of daf-28p::gfp expression in ASJ in the indicated genetic backgrounds. ins-26 and ins-35::SL2::mCherry cDNAs were expressed in AWC under the odr-1 promoter. Only animals expressing mCherry in AWC were scored. Median is indicated by red horizontal line. Each dot is the fluorescence intensity of a single neuron in a given experiment. n > 60 neurons total each, four independent experiments. (F) Quantification of dauer formation in AWC-ablated animals in the presence of 6 μM ascr#3 and the indicated amounts of heat-killed (blue circles) or live (red circles) OP50. Each filled circle represents one assay; n > 65 animals per assay, five independent experiments. Line represents best fit to the data. Dashed line indicates the curve for wild-type animals from Figure 1B shown for comparison. *, **, and *** indicate different from values using 160 μg of heat-killed bacteria at p < 0.05, 0.01, and 0.001, respectively. (G) Dauers formed by the indicated strains on 80 μg live OP50 and 6 μM ascr#3. For each assay: n > 65 animals; four independent experiments. Errors are SEM. Except where indicated, *, **, and *** indicate different from wild-type at p < 0.05, 0.01, and 0.001, respectively; #, ##, and ### indicate different from cmk-1(oy21) at p < 0.05, 0.01, and 0.001, respectively (ANOVA and Games-Howell post-hoc test).
-
Figure 5—source data 1
Dauer assay data for individual trials in Figure 5.
- https://doi.org/10.7554/eLife.10110.017
-
Figure 5—source data 2
Dauer assay data for individua reliably quantify the effects of lower concentrations of live bactl trials in Figure 5—figure supplement 2.
- https://doi.org/10.7554/eLife.10110.018
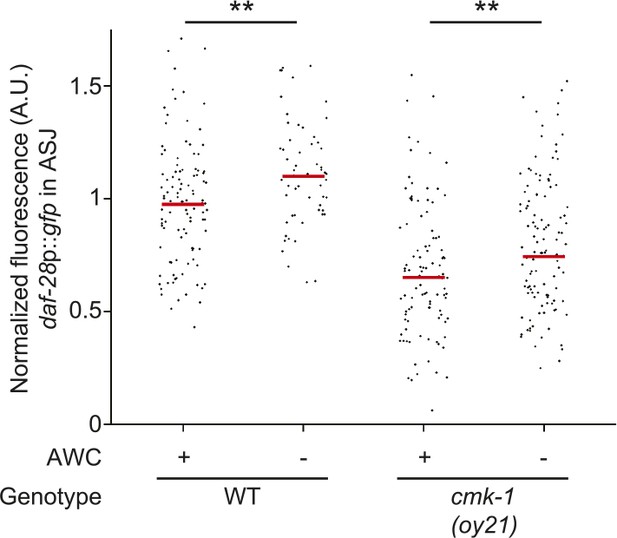
Scatter plot of fluorescence intensity of daf-28p::gfp expression in ASJ.
Median is indicated by red horizontal line. Animals were grown on live OP50 in the absence of exogenous pheromone. Each dot is the fluorescence intensity of a single neuron in a given experiment; n > 50 neurons each, at least three independent experiments. ** indicates different between indicated values at p < 0.01 (Student's t-test).
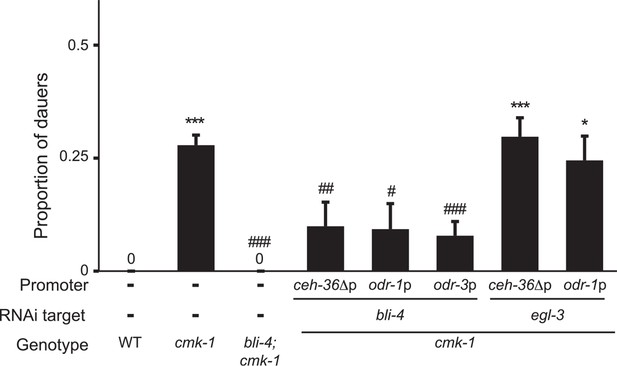
CMK-1 regulates a BLI-4-dependent pro-dauer signal from AWC.
Dauers formed by the indicated strains on 6 μM ascr#3 and 80 μg live OP50. Alleles used were: cmk-1(oy21) and bli-4(e937). bli-4 and egl-3 sense and antisense (SAS) constructs were expressed in AWC under the indicated promoters in cmk-1 mutants. Numbers shown are from two transgenic lines each with the exception of odr-1p::egl-3(SAS). * and *** indicate different from wild-type at p < 0.05 and 0.001, respectively; #, ##, and ### indicate different from cmk-1 at p < 0.05, 0.01, and 0.001, respectively (ANOVA and Games-Howell post-hoc test). n > 65 animals each; ≥3 independent experiments.
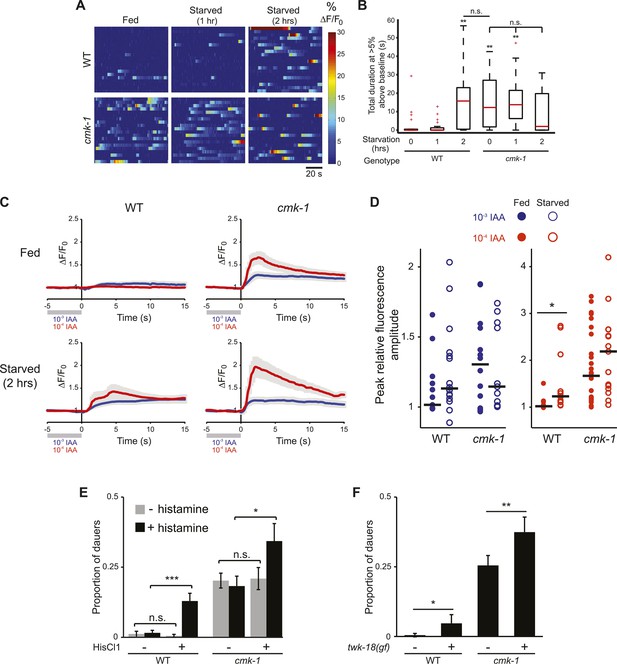
The AWC neurons exhibit increased basal activity in fed cmk-1, and starved wild-type animals.
(A) Heat maps showing the fluorescence intensity (∆F/F0) in the soma of AWC neurons in fed or starved wild-type and cmk-1(oy21) L2 larvae expressing GCaMP 3.0 in AWC under the ceh-36∆ promoter. Animals were cultured at 20°C, starved for 0, 1, or 2 hr and imaged at 20°C. Each horizontal line shows calcium dynamics in a single AWC neuron; n = 20 neurons each. (B) Box-and-whisker plots quantifying total duration of calcium responses >5% above baseline for each genotype and condition shown in A. Median is indicated by a red line. Tops and bottoms of boxes indicate the 75th and 25th percentiles, respectively; whiskers represent fifth and 95th percentiles. Outliers are indicated by + signs. ** indicates different from wild-type at 0 hr at p < 0.01 (Kruskal–Wallis test). n.s.—not significant. (C) Average calcium responses in AWC neurons of fed and starved animals expressing GCaMP 3.0 under the ceh-36∆ promoter in the presence, or upon removal of the odorant isoamyl alcohol (IAA), diluted to 10−3 (blue) or 10−4 (red). Error bars are SEM and are represented by light gray shading. n ≥ 10 for each genotype and condition shown. (D) Scatter plot of the peak fluorescence amplitudes of individual neuron responses following odorant removal for the indicated conditions and genotypes. Horizontal black bars represent the median. * represents different between the indicated values at p < 0.05 (Student's t-test). (E) Dauer formation in the presence of 80 μg live OP50 and 6 μM ascr#3 by non-transgenic and transgenic animals expressing the Drosophila histidine-gated chloride channel 1 (HisCl1) in the presence (black) or absence (gray) of 10 mM histidine. Error bars are SEM. n.s.—not significant; * and *** indicate different between indicated values at p < 0.05 and 0.001, respectively (Student's t-test). (F) Dauer formation in the presence of 80 μg live OP50 and 6 μM ascr#3 by non-transgenic and transgenic animals expressing the constitutively active potassium channel TWK-18(gf). Error bars are SEM. * and ** indicate different between indicated values at p < 0.05 and 0.01, respectively (Student's t-test).
-
Figure 6—source data 1
Dauer assay data for individual trials in Figure 6.
- https://doi.org/10.7554/eLife.10110.022
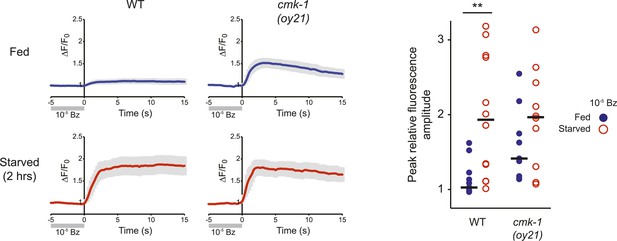
AWC neurons exhibit increased responses to odorant removal in fed cmk-1 and starved wild-type animals.
(Left) Average calcium responses in AWC neurons of fed (blue) or starved (red) animals expressing GCaMP3.0 under the ceh-36Δ promoter in the presence, or upon removal of, the odorant benzaldehyde (Bz). Error bars are SEM and are represented by light gray shading. n ≥ 10 for each genotype and condition shown. (Right) Scatter plot of the peak fluorescence amplitudes of individual neuron responses following odorant removal for the indicated conditions and genotypes. Horizontal black bars are the median. ** indicates different between indicated values at p < 0.01 (Student's t-test).
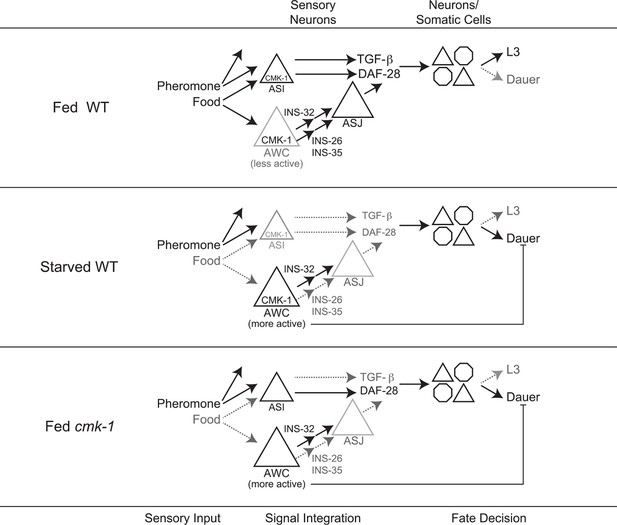
Model for the role of AWC and CMK-1 in the regulation of the dauer decision as a function of feeding state.
CMK-1 acts in AWC to drive expression of the anti-dauer ins-26 and ins-35 ILP genes. CMK-1 may also regulate expression of the ins-32 (or other) pro-dauer signal in AWC; alternatively, the pro-dauer signal could be present tonically regardless of environmental state. Under fed conditions, CMK-1-regulated anti-dauer signals from AWC predominate and drive expression of the daf-28 ILP gene in ASJ. CMK-1 also acts cell-autonomously to regulate expression of daf-7 TGF-β in ASI. Together, daf-7 TGF-β and daf-28 ILP signals promote reproductive development. When starved, anti-dauer signals from AWC are downregulated resulting in decreased expression of daf-28 in ASJ. daf-7 and daf-28 expression in ASI are also downregulated upon starvation. The shifted balance towards pro-dauer signals promotes dauer formation in the presence of pheromone. In cmk-1 mutants, loss of the anti-dauer signals from AWC downregulates daf-28 expression in ASJ under fed conditions. daf-7 expression in ASI is also downregulated in cmk-1 mutants. Consequently, the inappropriate predominance of pro-dauer signals in cmk-1 mutants promotes dauer formation in the presence of plentiful food and pheromone. Feeding conditions also modulate AWC basal neuronal activity; increased activity upon starvation or in cmk-1 mutants may limit dauer formation via a parallel pathway.
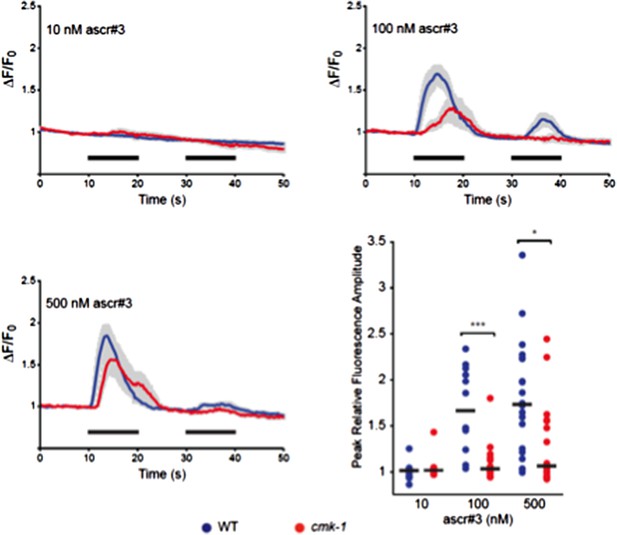
Responses of ADL neurons in WT and cmk-1(oy21) mutants to ascr#3. Panels show average calcium responses of ADL to the indicated concentrations of ascr#3. Errors are SEM. Also shown is a scatter plot of peak fluorescence amplitudes of individual neuron responses in WT and cmk-1 mutants to ascr#3. Horizontal black bars are the median. * and *** indicate P<0.05 and 0.001, respectively.
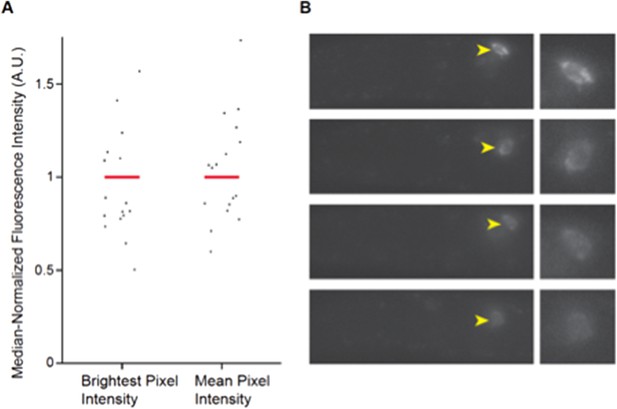
Expression of daf-7 in ASI analyzed via smFISH. A) Maximum and mean pixel intensity of daf-7 expression in ASI neurons. Median is indicated by red bars. B) Representative images showing the range of daf-7 smFISH signals in ASI. Yellow arrowheads point to ASI cell bodies which are magnified in panels at right. Anterior is at left.
Wild-type L1 larvae were fixed and hybridized with a daf-7 probe as described previously (8). Fluorescence intensities were quantified from maximum projection images and background subtracted. n=19 neurons; 10 animals.
Additional files
-
Supplementary file 1
List of strains used in this work.
- https://doi.org/10.7554/eLife.10110.025
-
Source code 1
MATLAB script to quantify GCaMP intensity in ROI (spontaneous).
- https://doi.org/10.7554/eLife.10110.026
-
Source code 2
MATLAB script for baseline correction.
- https://doi.org/10.7554/eLife.10110.027
-
Source code 3
MATLAB script for peak response identification and quantification.
- https://doi.org/10.7554/eLife.10110.028
-
Source code 4
MATLAB script for data compilation.
- https://doi.org/10.7554/eLife.10110.029
-
Source code 5
MATLAB script to quantify GCaMP intensity in ROI (evoked).
- https://doi.org/10.7554/eLife.10110.030
-
Source code 6
MATLAB subscript for TIFF image quantification.
- https://doi.org/10.7554/eLife.10110.031





