Human birth tissue products as a non-opioid medicine to inhibit post-surgical pain
Figures

Intra-paw injections of FLO inhibited heat nociception in naive wild-type (WT) mice and attenuated both heat and mechanical hyperalgesia after the plantar-incision.
(A) Paw withdrawal latency (PWL) to heat stimulation in naive WT mice before and after injection of FLO (0.5 mg, 20 μl) or the vehicle (saline, 20 μl) into the dorsum of the hind paw. Ipsilateral: injected side; Contralateral: un-injected side. N = 10/group. (B) The PWL ipsilateral to the side of the plantar-incision was measured before and 1, 2, and 4 hr after intra-paw injections of FLO (0.1 mg, 0.5 mg, 20 μl) or the vehicle in WT mice during Days 2–4 post-injury. N = 7–13/group. (C) The mechanical PWT to noxious pinch applied to the side of plantar-incision was measured before and 1 hr after an intra-paw injection of FLO (0.5 mg, 20 μl) or vehicle with the Randall–Selitto test during Days 2–4 post-injury. N = 10–11/group. (D) Schematic of the Catwalk gait analysis (left) and the representative paw print images (right). (E) Quantification of print area and maximum contact area in Catwalk test before and 1 hr after an intra-paw injection of FLO (0.5 mg, 20 μl) or vehicle on Day 2 post-injury. The left hind paw (LH) received the incision and drug treatment, and data were normalized to the right side (RH). N = 9–10/group. (F) Locomotor function and exploration were assessed in the open field test (30 min duration). The number of total, central, and peripheral beam breaks were measured before and at 1 hr after an intra-paw injection of FLO (0.5 mg, 20 μl) or vehicle during Days 2–4 post-injury. N = 10/group. Data are mean ± SEM. Two-way mixed model analysis of variance (ANOVA) followed by Bonferroni post hoc test. (A–C) *p < 0.05, **p <0.01, ***p <0.001 versus vehicle; #p < 0.05, ##p < 0.01, ###p < 0.001 versus baseline (A) or pre-drug (B, C). (E, F) *p < 0.05, **p < 0.01 versus pre-drug; ###p < 0.001, ####p < 0.0001 versus baseline. ns = not significant.
-
Figure 1—source data 1
Numerical source data files for Figure 1.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig1-data1-v1.xlsx
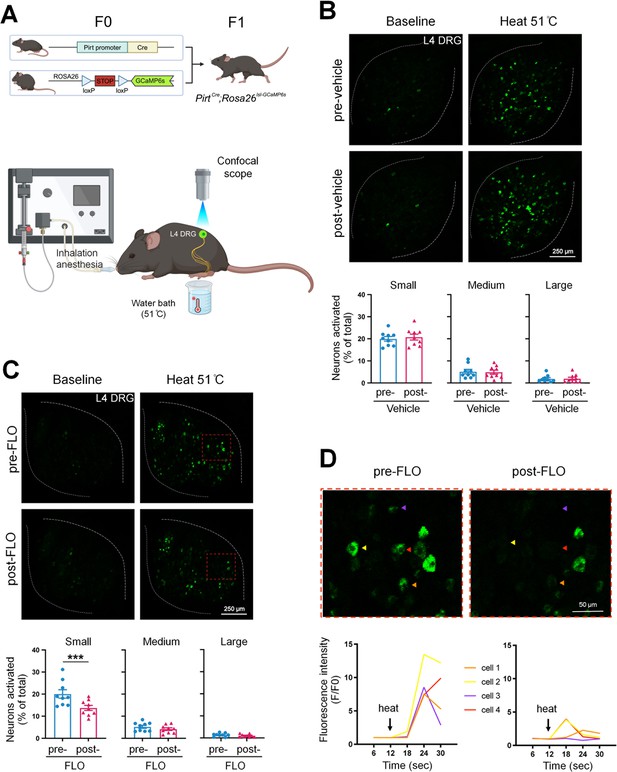
FLO acutely attenuated the responses of small dorsal root ganglion (DRG) neurons to noxious heat stimulation.
(A) Upper: Strategy for generating PirtCre;Rosa26lsl-GCaMP6s mice. Lower: The schematic diagram illustrates the experimental setup for in vivo optical imaging of L4 DRG neurons and applying test stimulation. (B, C) Upper: Representative images of calcium transients in DRG neurons in response to noxious heat stimulation (51°C water bath) applied to the hind paw before and 1 hr after an intra-paw injection of vehicle (B, saline) or FLO (C, 0.5 mg, 20 μl) at Day 2 after plantar-incision. Lower: Percentages of small-, medium-, and large-size neurons that were activated (ΔF/F ≥ 30%) by heat stimulation before and after vehicle or FLO. ‘% of total’ represented the proportion of activated neurons relative to the total number of neurons counted from the same analyzed image. DRG neurons were categorized according to somal size as <450 μm2 (small), 450–700 μm2 (medium), and >700 μm2 (large). N = 9/group. (D) The higher-magnification representative images (upper) and calcium transient traces (lower) show increased fluorescence intensities in four DRG neurons (indicated by colored arrows) responding to heat stimulation, and decreased responses after FLO treatment. Data are mean ± SEM. (B, C) Paired t-test. ***p < 0.001 versus pre-drug.
-
Figure 2—source data 1
Numerical source data files for Figure 2.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig2-data1-v1.xlsx
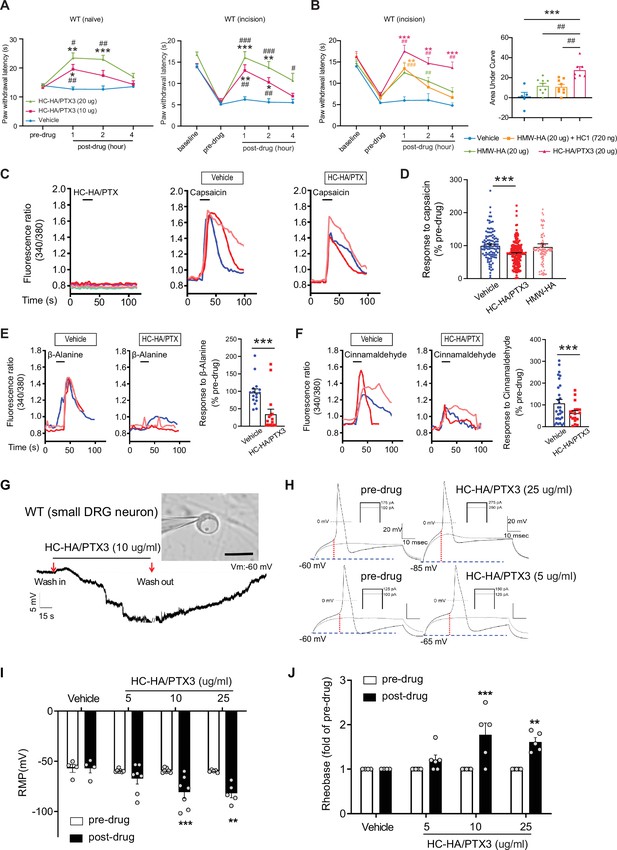
Heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3) inhibited heat hypersensitivity in wild-type (WT) mice after plantar-incision and attenuated dorsal root ganglion (DRG) neuron activation.
(A) Left: Intra-paw injection of HC-HA/PTX3 (10 μg or 20 μg, 20 μl), but not vehicle (saline), increased paw withdrawal latency (PWL) to heat stimulation in naive WT mice. N = 8–11/group. Right: Intra-paw injection of HC-HA/PTX3 (10 μg or 20 μg, 20 μl) dose-dependently attenuated the heat hypersensitivity during Days 2–4 after plantar-incision. N = 9–16/group. (B) Right: Intra-paw injection of HC-HA/PTX3 (20 μg, 20 μl) showed superior anti-hyperalgesic effect compared to high-molecular-weight hyaluronan (HMW-HA) (20 μg, 20 μl) alone and the mixture of HMW-HA (20 μg) and HC1 (720 ng) during Days 2–4 after plantar-incision. Left: Analyzing the area under the curve (AUC) to assess the anti-hyperalgesic effect of each group. N = 5–9/group. (C) HC-HA/PTX3 inhibited the calcium responses evoked by capsaicin (a TRPV1 agonist, 0.3 μM) in WT DRG neurons. HC-HA/PTX3 alone did not evoke [Ca2+]i elevation. Pretreatment (20 min) of HC-HA/PTX3 (15 μg/ml, bath application) reduced capsaicin-evoked [Ca2+]i rising. (D) The quantification of [Ca2+]i rising evoked by capsaicin in DRG neurons pretreated with the vehicle, HC-HA/PTX3 (15 μg/ml), or HMW-HA (15 μg/ml). N = 109–170 neurons/group. (E) Left: Traces show that the β-alanine (a MrgprD agonist, 1 mM) evoked an increase in [Ca2+]i, which was also inhibited by HC-HA/PTX3. Right: The quantification of evoke [Ca2+]i rising by β-alanine. N = 10–25 neurons/group. (F) Left: Traces show that cinnamaldehyde (a TRPA1 agonist, 1 mM) evoked an increase in [Ca2+]i, which was inhibited by HC-HA/PTX3. Right: The quantification of evoke [Ca2+]i rising by cinnamaldehyde. N = 15–35 neurons/group. (G) An example trace of membrane potential (Vm) which changed from resting level (−60 mV) toward a more hyperpolarized state after HC-HA/PTX3 (10 μg/ml) in a small DRG neuron (insert, scale bar: 25 μm). Vm returned to pre-drug level after washout. DRG neurons were categorized according to cell body diameter as <20 μm (small), 20–30 μm (medium), and >30 μm (large). (H) Example traces of action potentials (APs) evoked by injection of current in small DRG neurons 5 min after bath application of vehicle or HC-HA/PTX3 (5, 25 μg/ml). (I) HC-HA/PTX3 concentration-dependently altered the intrinsic membrane properties of small DRG neurons. Quantification of the resting membrane potential (RMP) before and at 5 min after bath application of vehicle or HC-HA/PTX3 (5, 10, and 25 μg/ml). N = 4–7/group. (J) Quantification of rheobase in small DRG neurons at 5 min after vehicle or HC-HA/PTX3. The rheobase after the drug was normalized to pre-drug value. N = 5–7/group. Data are mean ± SEM. (A, B: right) Two-way mixed model analysis of variance (ANOVA) followed by Bonferroni post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle; #p < 0.05, ##p < 0.01, ###p < 0.001 versus pre-drug. (B: left, C) One-way ANOVA followed by Bonferroni post hoc test. ***p < 0.001 versus vehicle; ##p < 0.01 versus other groups. (E, F) Paired t-test. ***p < 0.001 versus vehicle. (I, J) Two-way mixed model ANOVA followed by Bonferroni post hoc test. *p < 0.05, **p < 0.01 versus pre-drug.
-
Figure 3—source data 1
Numerical source data files for Figure 3.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig3-data1-v1.xlsx
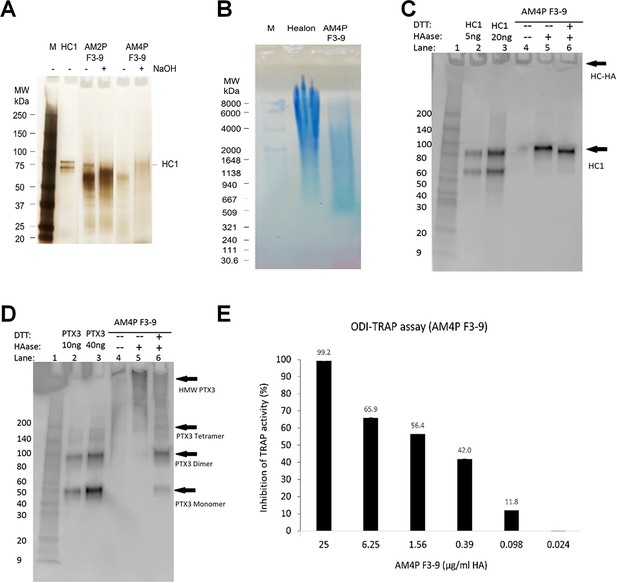
Purification and characterization of heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3).
(A) HC-HA/PTX3 was prepared from the human amniotic membrane by two runs (AM2P F3-9) or four runs (AM4P F3-9) of CsCl/4 M GnHCl ultracentrifugation and was then analyzed by silver staining. Each lane was loaded with 0.25 µg of HA without or with 100 mM NaOH treatment (25°C, 1 hr) to cleave the bond between HA and HC1. (B) HC-HA/PTX3 (AM4P F3-9) purified from the amniotic membrane was electrophoresed on 0.5% agarose gel and stained with All-stains dye. Healon as a high-molecular-weight (HMW) HA control and HC-HA/PTX3 were loaded at 10 µg HA/lane; M: HA molecular weight ladder. (C, D) HC1 and PTX3 in HC-HA/PTX3 were detected by western blot using respective antibodies without (−) or with (+) hyaluronidase (HAase) treatment to release HC1 or HMW-HA-PTX3, of which the latter can then be resolved into dimer or monomer without (−) or with (+) reduction with DTT. (E) Cloned murine RAW264.7 monocytes were seeded at 1 × 104 cells/cm2 in MEM α/10% fetal bovine serum (FBS) and differentiated into multi-nucleated osteoclasts with 25 ng/ml RANKL as the positive control and treated with HC-HA/PTX3 at different concentrations (0.024–25 µg/ml) for 3 days. The inhibition of TRAP activity in cell lysates was calculated as a percentage (%) of that of the positive control (shown on the top of each bar).
-
Figure 3—figure supplement 1—source data 1
PDF file containing original western blots for Figure 3—figure supplement 1, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig3-figsupp1-data1-v1.pdf
-
Figure 3—figure supplement 1—source data 2
Original files for western blot analysis displayed in Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig3-figsupp1-data2-v1.zip
-
Figure 3—figure supplement 1—source data 3
Numerical source data files for Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig3-figsupp1-data3-v1.xlsx
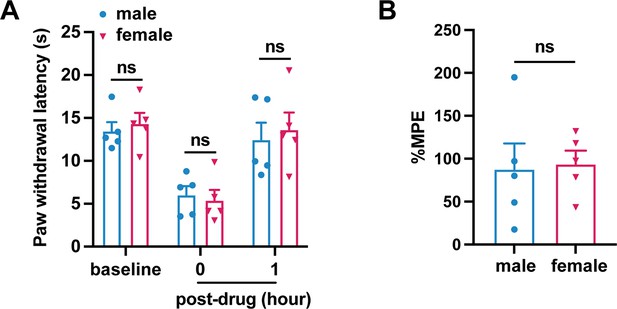
Heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3)-induced comparable inhibition of heat hyperalgesia in male and female mice after plantar-incision.
(A) Paw withdrawal latency at different time points of male and female mice is included in Figure 3B (N = 5/sex) (B) The percentage of maximal possible effects (%MPE) 1 hr post-drug were calculated for male and female mice included in Figure 3B. %MPE = 1 − (baseline − post-drug)/(baseline − pre-drug). Data are mean ± SEM. (A) Two-way mixed model analysis of variance (ANOVA) followed by Bonferroni post hoc test. *p < 0.05 versus male. (B) Unpaired t-test.
-
Figure 3—figure supplement 2—source data 1
Numerical source data files for Figure 3—figure supplement 2.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig3-figsupp2-data1-v1.xlsx
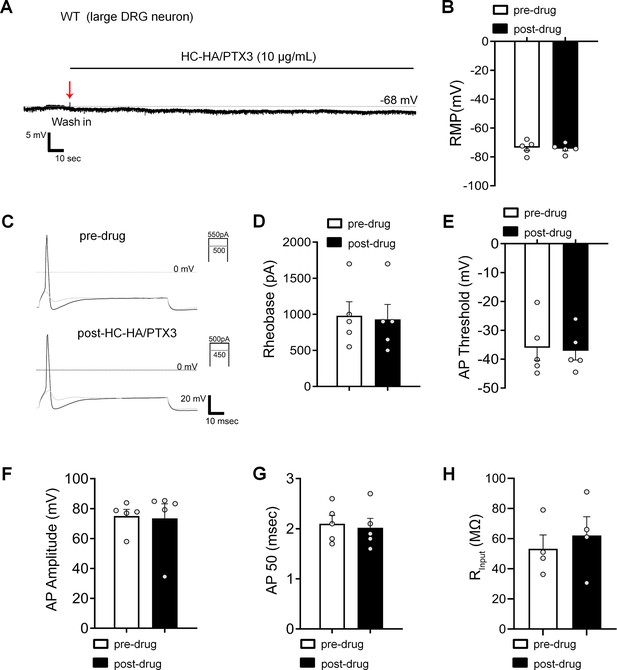
Heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3) did not affect the excitability of large dorsal root ganglion (DRG) neurons in wild-type (WT) mice after the plantar-incision.
(A) The representative trace of membrane potential was recorded under current-clamp conditions before and after HC-HA/PTX3 (15 µg/ml) treatment in a large DRG neuron of WT mice. Neurons were categorized according to cell body diameter as <20 μm (small), 20–30 μm (medium), and >30 μm (large). (B) The resting membrane potential (RMP) in large DRG neurons was not significantly changed at 5 min after HC-HA/PTX3 (10 μg/ml) treatment, compared to pre-drug (p = 0.41). N = 5. (C) Representative traces of rheobase measurements before and after HC-HA/PTX3 (10 μg/ml). Quantification of the rheobase (D, p = 0.09), action potential (AP) threshold (E, p = 0.78), AP amplitude (F, p = 0.8), AP duration (G, p = 0.41), and the mean input resistance (Rinput, H, p = 0.17) before and at 5 min after HC-HA/PTX3 (10 μg/ml) treatment. N = 5. Data are presented as mean ± SEM. (B, D–H) Paired t-test.
-
Figure 3—figure supplement 3—source data 1
Numerical source data files for Figure 3—figure supplement 3.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig3-figsupp3-data1-v1.xlsx
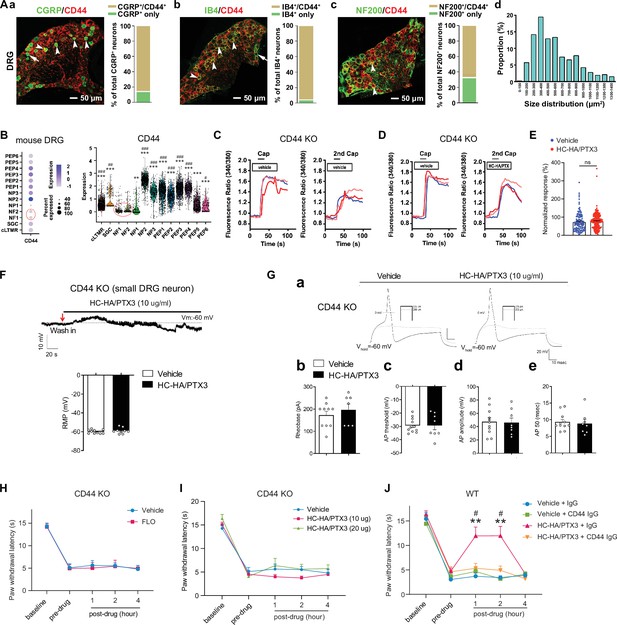
FLO and heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3) inhibited pain via CD44-dependent mechanisms.
(A) The expression of CD44 in the dorsal root ganglion (DRG) of wild-type (WT) mice. Left: Colocalization of CD44 and CGRP (a), IB4 (b), and NF200 (c) immunoreactivity (IR). Right: The quantification of CD44-expressing neurons (as % of total neurons in each subpopulation, IB4+: 96%; CGRP+: 82%; NF200+: 68%, N = 4). (d) The size distribution of CD44-expressing neurons. (B) Left: Dot plot of CD44 gene expression in different clusters [SGC (1), NF (2), NP (3), PEP (6), cLTMR (1)] of DRG cells from WT mice in single-cell RNA-sequencing study. The dot size represents the percentage of cells expressing CD44, and the color scale indicates the average normalized expression level. The NF1 and NF2 clusters were indicated with a red circle. Right: Violin plot shows the CD44 expression levels in each cluster. SGC: satellite glial cells; NF, Aβ or Aδ low-threshold mechanoreceptors or proprioceptors; NP, non-peptidergic nociceptors or pruriceptors; PEP, peptidergic nociceptors; C-LTMR, C-fiber low-threshold mechanoreceptors. One-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. **p < 0.01, ***p < 0.001 versus NF1; #p < 0.05, ##p < 0.01, ###p < 0.001 versus NF2. (C) Traces show that the capsaicin (0.3 μM) evoked an increase of [Ca2+]i in a small neuron from a CD44 KO mouse. Compared to [Ca2+]i rising evoked by the first capsaicin application, there was a reduction of [Ca2+]i rising to the second treatment, indicating TRPV1 desensitization. DRG neurons were categorized according to cell body diameter as <20 μm (small), 20–30 μm (medium), and >30 μm (large). (D) Capsaicin-evoked increases of [Ca2+]i before and after treatment (20 min) with HC-HA/PTX3 (10 μg/ml) in small DRG neurons from CD44 KO mice.(E) The quantification of evoked [Ca2+]i rising by capsaicin. HC-HA/PTX3 pretreatment did not reduce capsaicin-evoked [Ca2+]i rising in CD44 KO neurons. N = 100–120 neurons/group. (F) HC-HA/PTX3 did not change the intrinsic membrane property of small DRG neurons from CD44 KO mice. Upper: An example trace of membrane potential (Vm) which remained around resting level (−60 mV) after HC-HA/PTX3 (10 μg/ml). Lower: Quantification of the resting membrane potential (RMP) at 5 min after vehicle (saline) and HC-HA/PTX3 (p = 0.48). (G) Upper: Examples of traces of action potentials (APs) and rheobase evoked by injection of current in a small CD44 KO DRG neuron at 5 min after vehicle or HC-HA/PTX3 (10 μg/ml). Lower: Quantification of the rheobase levels (p = 0.2), AP threshold (p = 0.87), AP amplitude (p = 0.75), and duration (p = 0.82) in small DRG neurons from CD44 KO mice. N = 7–11/group. (H) Paw withdrawal latency (PWL) that was ipsilateral to the side of plantar-incision before and after an intra-paw injection of FLO (0.5 mg, 20 μl) or vehicle (saline, 20 μl) in CD44 KO mice (H, N = 8–9/group) after plantar-incision. (I) The ipsilateral PWL before and after an intra-paw injection of HC-HA/PTX3 (10 μg or 20 μg, 20 μl) or vehicle in CD44 KO mice after plantar-incision. N = 7–9/group. (J) The ipsilateral PWL before and after intra-paw injection of vehicle + control IgG, vehicle + CD44 IgG, HC-HA/PTX3 (10 μg) + control IgG, or HC-HA/PTX3 (10 μg) + CD44 IgG (all IgG at 10 μg, 10 μl) in WT mice after plantar-incision. N = 8–11/group. Data are mean ± SEM. (E) One-way ANOVA followed by Bonferroni post hoc test. ns = not significant. (F, G) Student’s t-test. (H–K) Two-way mixed model ANOVA followed by Bonferroni post hoc test. **p < 0.01 versus vehicle or saline + IgG; #p < 0.05 versus pre-drug.
-
Figure 4—source data 1
Numerical source data files for Figure 4.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig4-data1-v1.xlsx
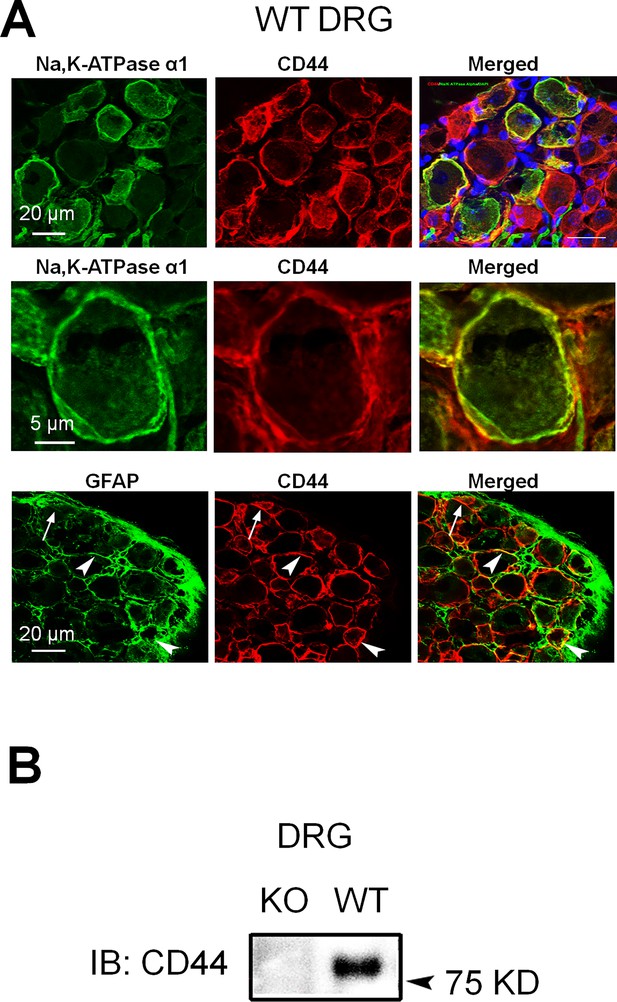
The expression of CD44 in mouse dorsal root ganglion (DRG).
(A) Upper: Colocalization of CD44 and Na, K-ATPase alpha1 (a neuronal marker) immunoreactivity (IR) in wild-type (WT) mouse DRG. Blue: 4′,6-diamidino-2-phenylindole (DAPI). Middle: A higher power view of CD44 and Na, K-ATPase alpha1 colocalization. Lower: Colocalization of CD44 and GFAP (a satellite glial cell marker) in DRG. Arrow: single-labeled cell; arrowhead: double-labeled cell. (B) The specific CD44 band was not observed in the protein extracts derived from DRG tissues of CD44 knockout (KO) mice in the western blot study.
-
Figure 4—figure supplement 1—source data 1
PDF file containing original western blots for Figure 4—figure supplement 1, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig4-figsupp1-data1-v1.pdf
-
Figure 4—figure supplement 1—source data 2
Original files for western blot analysis displayed in Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig4-figsupp1-data2-v1.zip
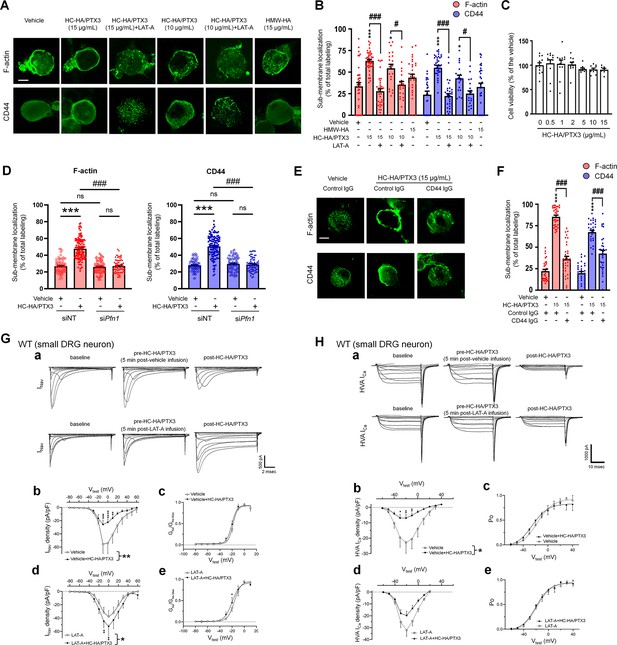
Heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3) induced cytoskeletal rearrangement which contributed to its inhibition of INav and HVA-ICa.
(A) Example images show the distribution of F-actin and CD44 staining in small dorsal root ganglion (DRG) neurons of wild-type (WT) mice. Neurons were treated with bath application of vehicle (saline), high-molecular-weight hyaluronan (HMW-HA) (15 μg/ml), HC-HA/PTX3 (10, 15 μg/ml), or HC-HA/PTX3 (10, 15 μg/ml) combined with Latrunculin A (LAT-A, 1 μM) for 45 min. Scale bar: 5 μm. DRG neurons were categorized according to cell body diameter as <20 μm (small), 20–30 μm (medium), and >30 μm (large). (B) Quantification of sub-membranous F-actin polymerization and translocation of CD44 in small WT DRG neurons after drug treatment. N = 30–80/group. (C) Proliferation MTT assay showed a lack of neuronal toxicity from 0.5, 1, 2, 5, 10, and 15 μg/ml HC-HA/PTX3, compared to vehicle (100% viable cells). N = 6–12 repetitions/group. (D) Quantification of sub-membranous F-actin polymerization and translocation of CD44 in small DRG neurons. DRG neurons were electroporated with siRNA targeting Pfn1 (siPfn1) or non-targeting siRNA (siNT, control). Neurons were treated with vehicle (saline) or HC-HA/PTX3 (10 μg/ml) for 45 min. N = 70–111/group. (E) Changes in the sub-membrane distribution of F-actin and CD44 in WT DRG neurons treated with vehicle + control IgG (2 µg/ml), HC-HA/PTX3 (15 μg/ml) + control IgG (2 µg/ml), or HC-HA/PTX3 (15 μg/ml) + CD44 IgG (2 µg/ml) for 45 min. Scale bar: 5 μm. (F) Quantification of the sub-membrane F-actin and CD44 labeling in each group. (G) Infusion of LAT-A attenuated the inhibition of INav by HC-HA/PTX3 in WT DRG neurons. (a) Representative traces of INav after 5 min infusions of vehicle (top row) or LAT-A (bottom row, 0.5 nM) through the recording electrode, followed by bath application of HC-HA/PTX3 (10 µg/ml). Lumbar DRG neurons were harvested on Days 2–3 after plantar-incision. (b) There was a significant interaction between the variation produced by HC-HA/PTX3 (10 µg/ml) and test voltages (VTest) applied in vehicle-infused neurons, resulting in an overall INav inhibition (F(14,90) = 3.29, ***p < 0.001), and significantly decreased INav density (pA/pF) from VTest = −10 to +10 mV, as compared to pre-HC-HA/PTX3 treatment. N = 7/group. (c) HC-HA/PTX3 did not alter GNa/GNa max across the test voltages (F(9,60) = 0.44, p = 0.9) in vehicle-infused neurons. N = 7/group. (d) There was a significant interaction between the variation produced by HC-HA/PTX3 (10 µg/ml) and VTest applied in LAT-A-infused neurons, resulting in overall INav increase (F(14,120) = 1.87, *p < 0.05) and increased INav density (pA/pF) from VTest = −10 to 0 mV, as compared to pre-HC-HA/PTX3. N = 9/group. (e) HC-HA/PTX3 significantly increased the GNa/GNa max at VTest = −20 mV in LAT-A-infused neurons (*p < 0.05, N = 9/group). (H) LAT-A attenuated the inhibition of HVA-ICa by HC-HA/PTX3 in WT DRG neurons. (a) Representative traces of HVA-ICa in small WT DRG neurons after 5 min infusions of vehicle (top row) or LAT-A (bottom row, 0.5 nM), followed by bath application of HC-HA/PTX3 (10 µg/ml). (b) In vehicle-infused neurons, HC-HA/PTX3 (10 µg/ml) significantly decreased HVA-ICa (F(1,12) = 6.52, *p = 0.02) and HVA-ICa conductance (I/Imax) from VTest = −40 to +10 mV, as compared to pre-HC-HA/PTX3. N = 7. (c) HC-HA/PTX3 did not alter the channel open probability (Po) in vehicle-infused neurons (p = 0.82, N = 7). (d) In LAT-A-infused neurons, HC-HA/PTX3 only modestly reduced HVA-ICa conductance across test voltages applied (F(1,12) = 0.27, p = 0.6, N = 8). (e) HC-HA/PTX3 did not alter Po in LAT-A-infused neurons (p = 0.94, N = 8). Data are mean ± SEM. (B–D, F) One-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. *p < 0.05, ***p < 0.001 versus vehicle; #p < 0.05, ###p < 0.001 versus indicated group. (G, H) Two-way repeated measures ANOVA with Holm–Sidak post-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 versus vehicle infusion or LAT-A infusion group.
-
Figure 5—source data 1
Numerical source data files for Figure 5.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig5-data1-v1.xlsx
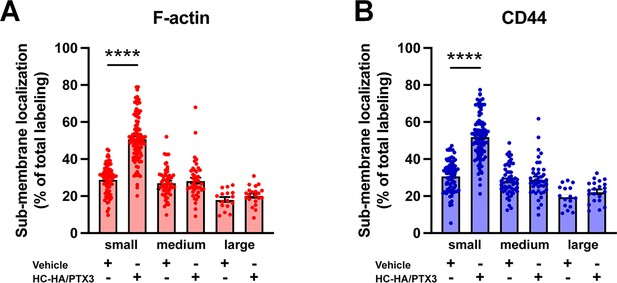
Differential effects of heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3) on sub-membranous F-actin polymerization and translocation of CD44 in small, medium, and large wild-type (WT) dorsal root ganglion (DRG) neurons.
(A) Quantification of sub-membranous F-actin polymerization and (B) translocation of CD44 in different sizes of WT DRG neurons after HC-HA/PTX3 (10 μg/ml) or the vehicle (saline) treatment. DRG neurons were categorized according to cell body diameter as <20 μm (small), 20–30 μm (medium), and >30 μm (large). HC-HA/PTX3 increased sub-membranous F-actin polymerization and translocation of CD44 exclusively in small neurons. N = 16–91/group. Data are mean ± SEM. One-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. ****p < 0.0001 versus vehicle.
-
Figure 5—figure supplement 1—source data 1
Numerical source data files for Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig5-figsupp1-data1-v1.xlsx
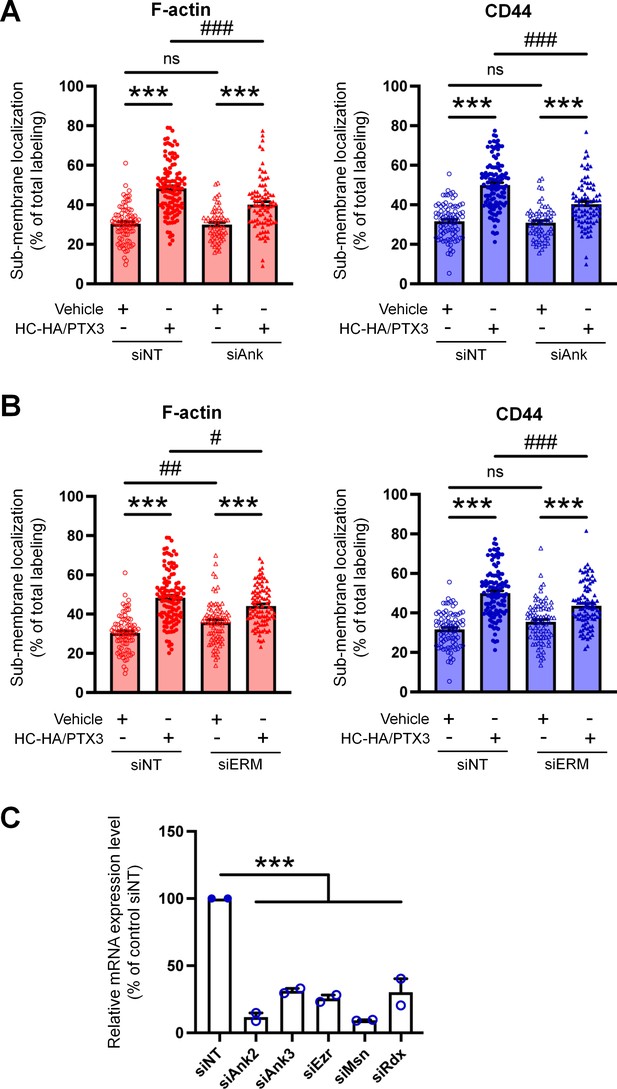
Quantification of sub-membranous F-actin polymerization and translocation of CD44 in small wild-type (WT) dorsal root ganglion (DRG) neurons in each group.
(A) DRG neurons were electroporated with siRNAs specifically targeting Ank2 and Ank3 (siAnk), (B) and those targeting Ezr, Rdx, and Msn (siERM) complex. Neurons were treated with a bath application of vehicle (saline) or heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3) (10 μg/ml) for 45 min. N = 59–114/group. (C) The mRNA expression of Ank2, Ank3, Ezr, Msn, and Rdx in DRG neurons electroporated with specific siRNAs were assayed by qPCR. N = 2. Data are mean ± SEM. One-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. ***p < 0.001 versus vehicle; #p < 0.05, ##p < 0.01, ###p < 0.001 versus indicated group.
-
Figure 5—figure supplement 2—source data 1
Numerical source data files for Figure 5—figure supplement 2.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig5-figsupp2-data1-v1.xlsx
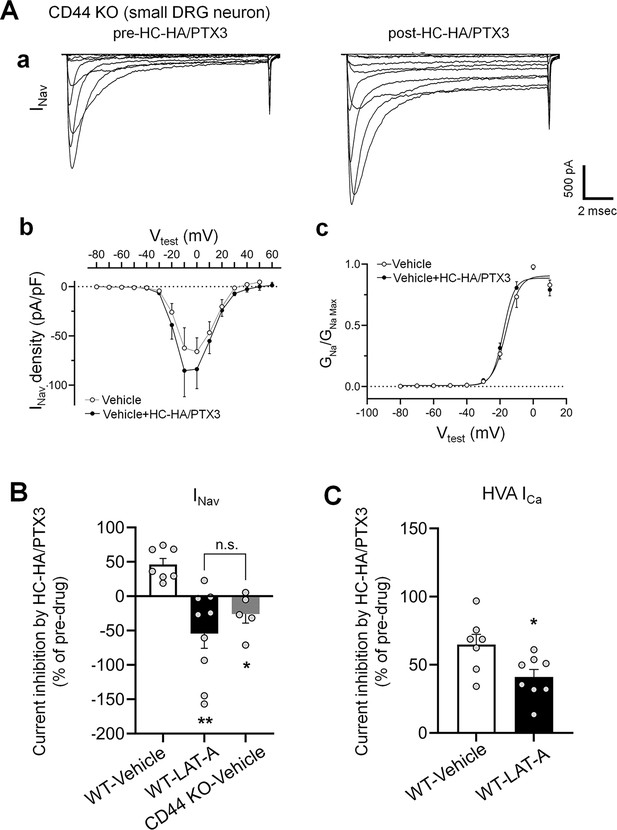
The inhibitions of INav by heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3) in small dorsal root ganglion (DRG) neurons were diminished in CD44 KO mice and were inhibited by a pretreatment of LAT-A in neurons from WT mice.
(A) HC-HA/PTX3 (10 µg/ml) did not inhibit INav in small DRG neurons from CD44 KO mice. (a) Representative traces of INav in a CD44 KO neuron before and at 5 min after bath application of HC-HA/PTX3 (10 µg/ml). (b) There was no significant change in INav density (pA/pF) before and after HC-HA/PTX3 (10 µg/ml) treatment in CD44 KO neurons. (c) HC-HA/PTX3 did not alter GNa/GNa max across the test voltages in CD44 KO neurons. N = 5. (B) Changes of INav after bath application of HC-HA/PTX3 (10 µg/ml) in vehicle-infused (N = 7) and LAT-A-infused small WT DRG neurons (p < 0.01, N = 9), and in vehicle-infused CD44 KO neurons (N = 5). DRG neurons were infused with the vehicle or LAT-A (0.5 nM) through the recording electrode, followed by bath application of HC-HA/PTX3 (10 µg/ml) 5 min later. The lumbar DRG neurons were harvested on Days 2–3 after the plantar-incision. DRG neurons were categorized according to cell body diameter as <20 μm (small), 20–30 μm (medium), and >30 μm (large). Data are mean ± SEM. One-way analysis of variance (ANOVA) with Holm–Sidak post-test. *p < 0.05, **p < 0.01 versus WT-vehicle pretreatment group. (C) The inhibition of HVA-ICa by HC-HA/PTX3 (10 µg/ml) in vehicle-infused (N = 7) and LAT-A-infused small WT DRG neurons (p = 0.02, N = 8). Data are mean ± SEM. Unpaired Student’s t-test.
-
Figure 5—figure supplement 3—source data 1
Numerical source data files for Figure 5—figure supplement 3.
- https://cdn.elifesciences.org/articles/101269/elife-101269-fig5-figsupp3-data1-v1.xlsx
Intracellular infusion of LAT-A did not change the gross morphology of dorsal root ganglion (DRG) neurons in patch-clamp recordings.
Example images show a small DRG neuron after infusion vehicle or Latrunculin A (LAT-A, 0.5 nM) through the recording electrode, followed by bath application of heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3) (15 μg/ml). Scale bar: 25 µm. DRG neurons were categorized according to cell body diameter as <20 μm (small), 20–30 μm (medium), and >30 μm (large).
Additional files
-
Supplementary file 1
The measures of intrinsic membrane properties of small-diameter dorsal root ganglion (DRG) neurons in wild-type (WT) and CD44 knockout (KO mice).
Knocking out of CD44 did not significantly alter the intrinsic membrane property of DRG neurons, as compared to that in WT mice.
- https://cdn.elifesciences.org/articles/101269/elife-101269-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/101269/elife-101269-mdarchecklist1-v1.docx





