Affinity and dose of TCR engagement yield proportional enhancer and gene activity in CD4+ T cells
Figures
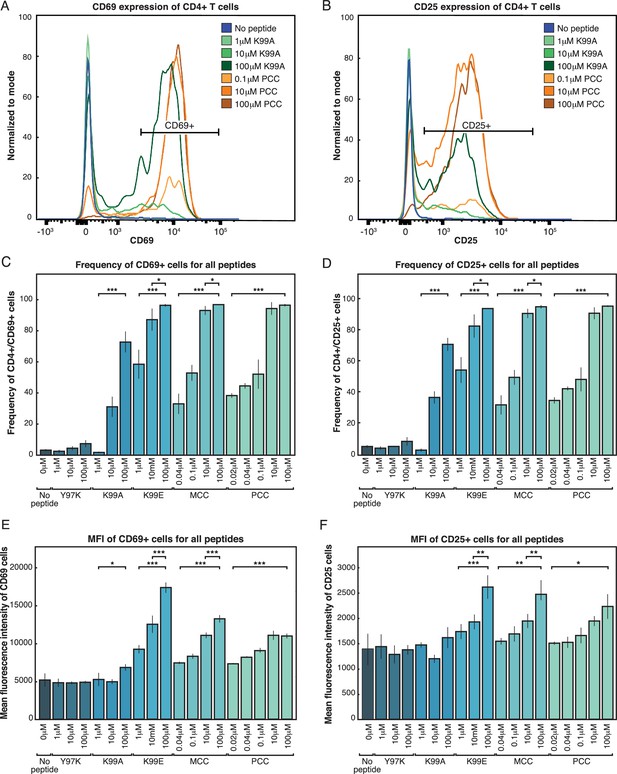
Both frequency of responding cells and per-cell activation levels increase with increasing signal strength.
(A) Purified AND T cells and CD11c+ APCs were co-cultured in the presence of indicated peptides at the indicated concentrations for 24 hr. Flow cytometry was then used to phenotype the CD4+ T cells. The histograms show CD69 expression of CD4+ T cells. The annotated bar indicates the gate used to identify CD69+ CD4 cells in subsequent figures. (B) Gating on CD4+ cells as in 1A, there is a bimodal distribution of CD25 expression resulting from activating levels of high-affinity (PCC) or low-affinity (K99A) peptides. The annotated bar indicates the gate used to identify CD25+ cells. (C) The percent of CD4+ cells that are CD69+ (using gate shown in 1A) varies with the peptide presented and concentration of the indicated peptide. (D) The percent of CD4+ cells that are positive for the activation marker CD25 varies with both peptide and dose. (E) Gating on CD4+ CD69+ cells (as shown in 1A), the geometric mean fluorescence intensity (MFI) of CD69 per cell in each condition varies. (F) The geometric MFI of CD25, gated on CD4+ CD25+ cells (as shown in 1C), varies with peptide and dose. (P-values based on Student’s t test; *p<0.05, **p<0.01, ***p<0.001.)
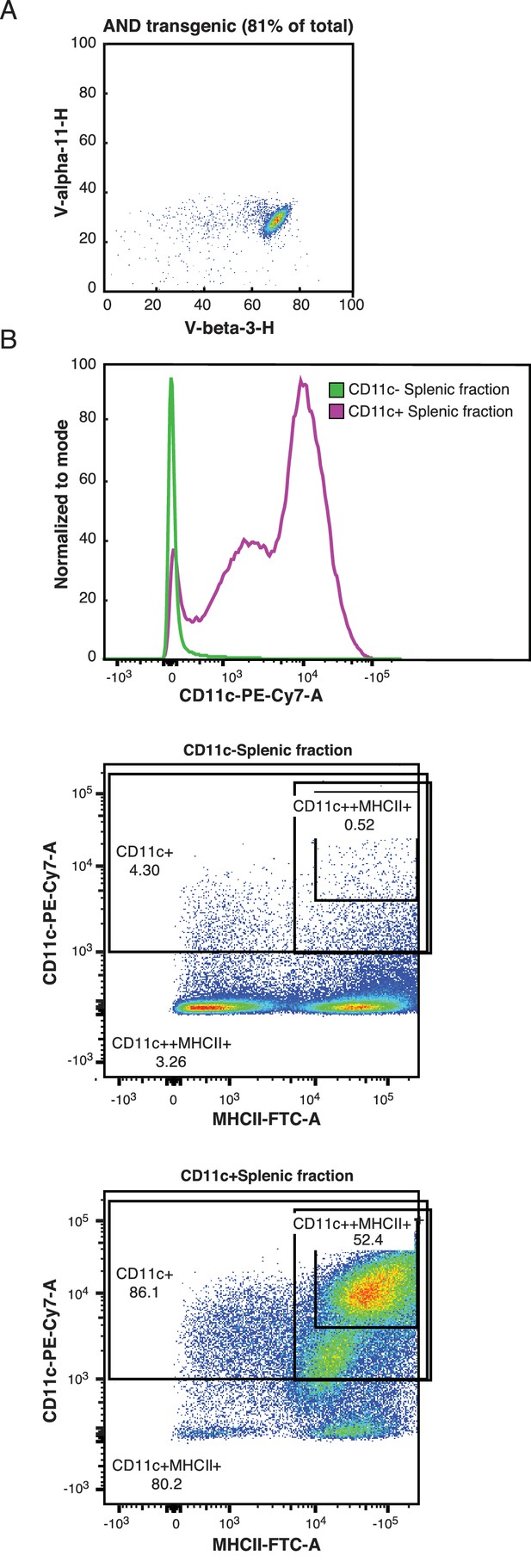
CD4+ T cells and APCs were purified from AND mice.
(A) The AND mouse TCR includes the V alpha 11 chain and the V beta 3 chain. The great majority of naïve CD4+ T cells extracted from AND mouse spleens expressed this pair of TCR chains. Naïve CD4+ T cells were isolated using negative selection with the Miltenyi MACS system, as described in the methods section. (B) APCs for peptide presentation were extracted from mouse spleens using positive selection for CD11c. The extracted cells were largely positive for both CD11c and MHC class II molecules.
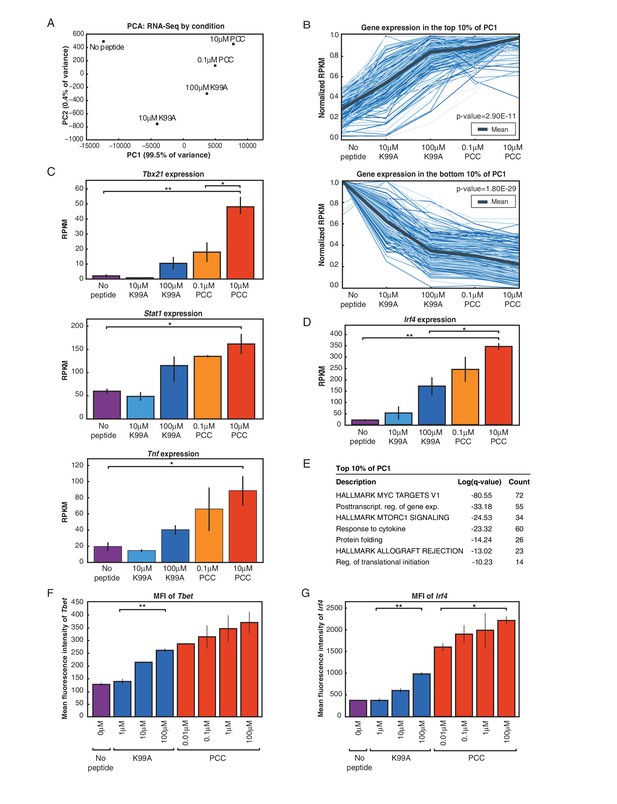
RNA-Sequencing reveals graded expression of activation signature genes.
(A) Principal component analysis (PCA) of the approximately 3,200 genes that changed between any two samples reveals that the primary axis of variation (PC1, shown along the x-axis) orders the five conditions by increasing TCR signal strength: No Peptide; low-dose, low-affinity (10 μM K99A); high-dose, low-affinity (100 μM K99A); low-dose, high-affinity (0.1 μM PCC); and high-dose, high-affinity (10 μM PCC). (B) After ordering the ~3200 genes used for PCA by their contribution to PC1, we extracted the top 10%—that is, the ~320 genes contributing most positively to a sample’s PC1 value—and the bottom 10%—that is, the ~320 genes contributing most negatively to a sample’s PC1 value. Each group displays a clear trend, with the top 10% increasing in expression as signal strength increases, and the bottom 10% decreasing in expression. Each blue line represents a gene, with reads per kilobase per million (RPKM) normalized from 0 to 1 across the five conditions. Significance was determined using permutation testing, where the mean difference between genes in the No Peptide sample as compared to 10 μM PCC was normally distributed over randomly generated groups of genes. This normal distribution was compared to the top 10% and bottom 10% genes to generate a p-value. (C) Genes in the top 10% of PC1, termed activation signature genes, include many genes previously identified as important to CD4+ T cell activation, such as Tbx21 (Tbet), Stat1, and Tnf. Reads per kilobase per million (RPKM) for each increases with increasing signaling strength. (D) Irf4, a transcription factor previously shown to be more highly expressed with increasing TCR affinity, shows graded expression across the five conditions. (E) Gene Ontology (GO) analysis of activation signature genes shows enrichment for protein biosynthesis and molecular chaperone genes. P-values shown are Benjamini-Hochberg adjusted p-values. (F) As measured by flow cytometry, the geometric MFI of Tbet in CD4+ cells increases on a per-cell basis with increasing signal strength. Note that MFI of the entire population of CD4+ cells is shown, as Tbet distribution is unimodal. (G) Similarly, per-cell protein levels of IRF4 increase with increasing signal strength when measured with flow cytometry. (p-values based on Student’s t test; *p<0.05, **p<0.01, ***p<0.001.)

RNA-Sequencing reveals graded expression of activation signature genes.
(A) 99.5% of the variance between the five conditions could be explained with a single principal component. (B) Eif3a, a protein biosynthesis gene, increased in expression with increasing TCR signaling strength. (C) Cct2, a molecular chaperone gene, increased in expression with increasing TCR signaling strength. (D) Not all gene expression changes are reflected in extracellular protein levels. Il2rb, an activation signature gene, does not significantly change when measured with extracellular flow cytometry. (E) Cd200, an activation signature gene, shows a graded gene expression pattern across the five conditions, and this is reflected by extracellular presentation of the CD200 molcule as measured by flow cytometry. This plot shows the geometric MFI of CD4+ CD200+ cells across the conditions. (F) Similarly, Ly6a increases in both population gene expression levels and single-cell protein levels as measured by flow cytometry. This plot shows the geometric MFI of CD4+ Ly6a+ cells across the conditions. (G) The receptor TNFSF11 (RANKL) shows a graded increase in gene expression that is reflected in the per-cell levels of extracellular expression of the protein. This plot shows the geometric MFI of CD4+ RANKL+ cells across the conditions. (p-values based on Student’s t test; *p<0.05, **p<0.01, ***p<0.001.)
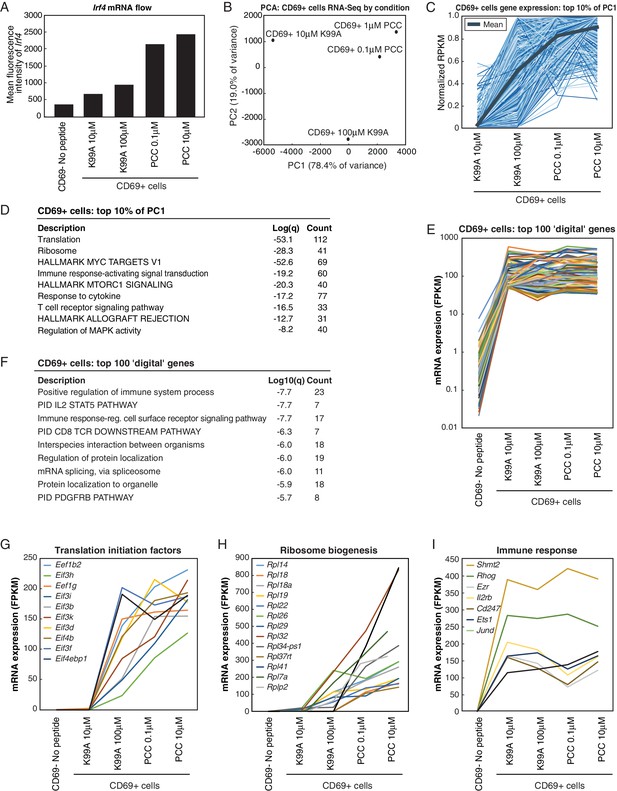
Single-cell RNA levels reflect whole-population levels for a subset of genes.
(A) RNA flow cytometry was used to determine the single-cell levels of RNA transcripts for Irf4 across the five peptide conditions. The mean fluorescence intensity of the Irf4 RNA probe increases across the five conditions assayed via RNA flow cytometry. Data is representative of two technical and two biological replicates. (B) Treated cells were sorted, and RNA-sequencing was performed on the CD69+ cells from each peptide condition such that the effect of responder frequency was controlled. PCA of the genes that changed between any two samples reveals that the primary axis of variation (PC1, shown along the x-axis) orders the four conditions that had any retrievable CD69+ cells by increasing TCR signal strength: low-dose, low-affinity (10 μM K99A); high-dose, low-affinity (100 μM K99A); low-dose, high-affinity (0.1 μM PCC); and high-dose, high-affinity (10 μM PCC). (C) As with the whole-population RNA-sequencing data, looking at the top 10% of genes along PC1 revealed an expression profile that reflects the analog signal seen with external activation markers such as CD25 and CD69. Each blue line represents a gene, with reads per kilobase per million (RPKM) normalized from 0 to 1 across the five conditions. (D) Gene Ontology (GO) analysis of the genes in the top 10% of PC1 shows enrichment for terms related to metabolic processes such as translation and RNA biosynthesis. (E) There was additionally a digital cluster of genes identified that showed threshholding behavior upon cell activation. For the digital cluster, expression levels were low in the control CD69- cells, and universally higher across all CD69+ samples. The peptide conditions are sorted along the x-axis, and the normalized RPKM for the 100 most highly induced/most consistently expressed genes in the cluster is shown along the y-axis. (F) GO analysis of the genes in the digital cluster shows an enrichment for terms related to immune signaling pathways. (G) RPKM levels for several genes classified as 'translation initiation factors' are shown. For most of the genes, expression is low for the CD69- control, and increases in an analog fashion across the CD69+ cells from each treated condition. The peptide conditions are sorted along the x-axis, and RPKM for each gene is shown along the y-axis. (H) RPKM levels for several genes in the 'ribosome biogenesis' GO category are shown. Expression is low for the CD69- control, and increases in an analog fashion across the CD69+ cells from each treated condition. The peptide conditions are sorted along the x-axis, and RPKM for each gene is shown along the y-axis. (I) RPKM levels for several genes indicative of the digital cluster are shown. Expression is low for the CD69- control, but relatively level across all CD69+ samples. The peptide conditions are sorted along the x-axis, and RPKM for each gene is shown along the y-axis.

RNA flow cytometry for Irf4 shows graded increases.
(A) RNA flow cytometry was used to determine the single-cell levels of RNA transcripts for Irf4 across the five peptide conditions. On the x-axis, the fluorescence intensity of CD69 protein is shown, and on the y-axis, the fluorescence intensity of an RNA-hybridizing probe targeted at Irf4 mRNA. Both the frequency and the per-cell intensity of Irf4 increase with increasing TCR signaling. Data is representative of two technical and two biological replicates. (B) RNA flow cumulative distribution plots for the Irf4 probe in CD69+ cells under the indicated treatment conditions. (C) RNA flow cumulative distribution plots for the beta actin probe in CD69+ cells under the indicated treatment conditions. (D) Mean Fluorescence Intensity of the beta actin probe in CD69+ cells under the indicated treatment conditions. (E) FPKM values for Rpl8, Rsp9 and Irf4 in CD69+ cells under the indicated treatment conditions. (F) FPKM values for Cd69, Tnf and Il2 in CD69+ cells under the indicated treatment conditions.
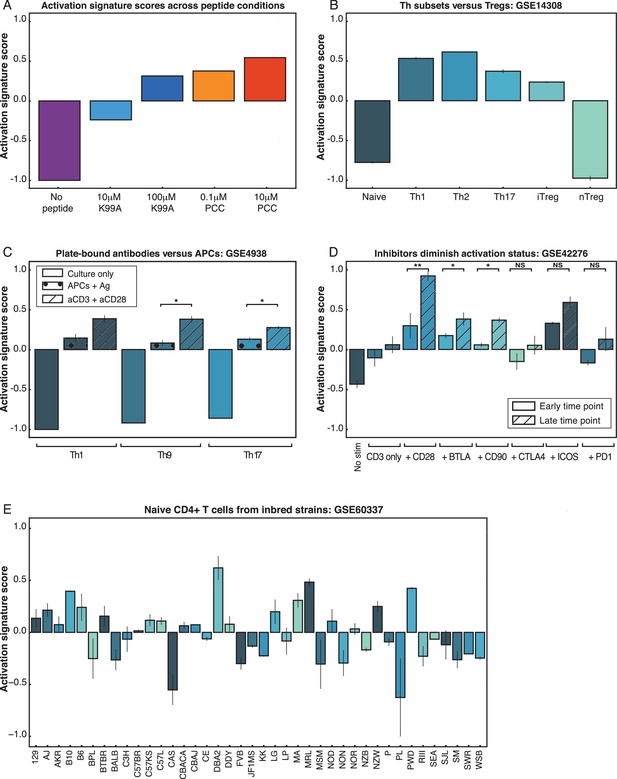
PC1 can be used to rank arbitrary CD4+ T cell data sets.
(A) An activation score derived from the top and bottom genes along PC1 ranks the five conditions according to TCR signaling strength. The score correctly captures that 100 μM K99A and 0.1 μM PCC are very similar in activation status. Note that the score ranks samples within an experiment, but is not an absolute metric for comparing across experiment groups. (B) The activation score can be used to compare arbitrary CD4+ T cell data sets. Here, activation scores were calculated for microarrays from helper T cell subsets, and they demonstrate that naïve cells and native regulatory T cells (nTregs) are less classically activated than Th1, Th2, and Th17 polarized subsets. (C) The activation score captures the fact that plate-bound anti-CD3 and anti-CD28 stimulation of helper T cell subsets results in stronger signaling than antigen as presented by APCs. (D) CD4+ T cells were subjected to a variety of stimulatory or inhibitory treatments: anti-CD3 alone, or anti-CD3 with anti-CD28, anti-BTLA, anti-CD80, anti-CTLA4, anti-ICOS, or anti-PD1. Gene expression profiles at early (1 hr and 4 hr) and late (20 hr and 48 hr) time points yield activation scores in line with the characterization of CD28, BTLA, CD80, and ICOS as co-stimulatory, and CTLA4 and PD1 as inhibitory. Although it might be expected that anti-CD80 would have an inhibitory effect, these results are in line with the conclusions from the originally published analysis. (E) Naïve CD4+ splenocytes were isolated from 39 mouse strains. Using the PC1-derived activation score, we can rank the CD4+ cells from each strain as either more or less activated under basal conditions. Using the activation score, we recapitulate the finding that C57Bl6 mice have more pro-inflammatory cells than BALB/c mice. The highest scoring strain, DBA/2, shows top-quartile expression of immune effectors as well as protein biosynthesis genes. (P-values based on Student’s t test; *p<0.05, **p<0.01, ***p<0.001.)
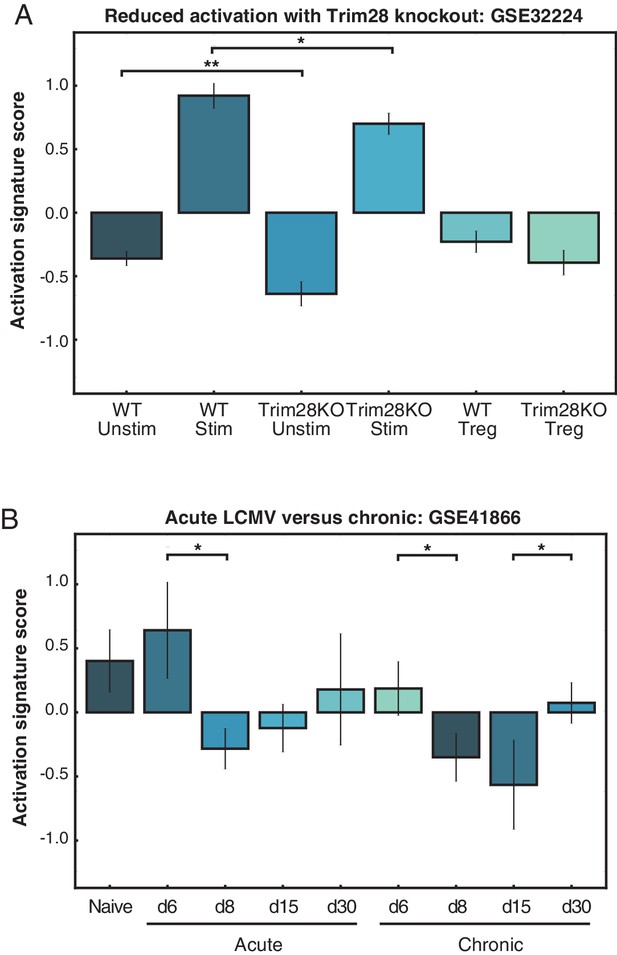
PC1 can be used to rank arbitrary CD4+ T cell data sets.
(A) The activation signature score was used to quantify CD4+ T cell activation status under Trim28 knockout conditions. Loss of Trim28 resulted in a lowering of the activation signature score, corroborating the previously reported results that the Trim28-deficient cells produced less IL2. (B) Acute LCMV infection results in a higher activation signature score for CD4+ T cells at early time-points, marking the peak of infection before cells begin to turn off activation programs. The activation signature score does not reach such a high level in a chronic infection model.
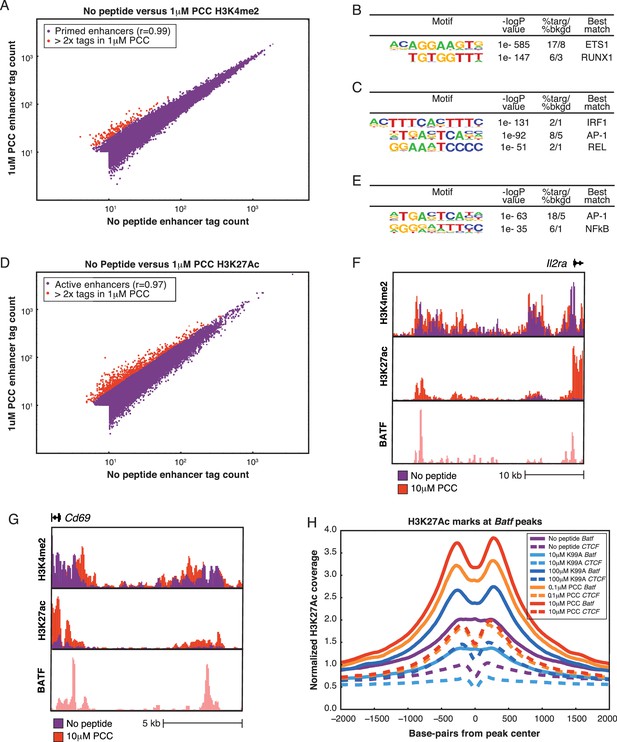
Primed enhancers are pre-existing, but gain activation markers with treatment.
(A) Comparing primed enhancers marked by H3K4me2 peaks reveals strong correlation between untreated and treated samples. Normalized tag counts in the No Peptide condition are plotted against those in a 1 μM PCC condition, with red dots coloring those that are more than two-fold up-regulated in the 1 μM PCC condition. The up-regulated enhancers are both few in number and low in tag count. (B) De novo motif finding identifies lineage-determining transcription factor (LDTF) motifs among primed enhancers shared by the five conditions. An ETS motif is most prominent, and a RUNX motif is likewise highly enriched over the randomly selected background. Both ETS and RUNX factors play important roles in T cell development. (C) Among primed enhancers shared by all five conditions, including the untreated condition, pro-inflammatory transcription factor motifs are enriched. An IRF family motif, AP-1 family motif (represented by BATF), and NF-κB motif (represented by REL) are all significantly enriched among shared enhancers marked by H3K4me2. (D). Comparing H3K27Ac tag counts at enhancers in No Peptide as compared to 1 μM PCC treatment reveals that many enhancers see increasing H3K27Ac deposition upon stimulation. Points in red indicate greater than two-fold increase in tags upon treatment. (E) Enhancers that are more active upon stimulation, as determined by greater than two-fold H3K27Ac tags in 1 μM PCC treatment as compared to No Peptide, are enriched for pro-inflammatory transcription factor motifs. BATF, an AP-1 family member, and NF-κB are most prominent. (F) Enhancers that are more active with stimulation are enriched near activation signature genes, as can be seen with this enhancer upstream of the activation signature gene Il2ra (CD25). (G) Enhancers upstream of the activation signature gene Cd69 show an increase in H3K27Ac deposition upon treatment with 1 μM PCC. (H) Genome-wide, deposition of H3K27Ac, a marker of transcription factor activity, reflects increasing TCR signal strength at the binding sites of AP-1 family members, including BATF.

Primed enhancers are pre-existing, but gain activation markers with treatment.
(A) Despite the similarity of enhancer profiles across conditions, the H3K4me2 mark 'spreads' from the transcription start site along the body of genes at a subset of genes. This subset is significantly enriched for activation signature genes such as Cd69, shown here. (B) Irf4, another activation signature gene, shows a similar spreading of dimethyl along the body of the gene after treatment. (C) Normalized tag counts along the first 4000 bp of all activation signature genes show that, overall, the No Peptide condition shows a narrow peak at the transcription start site, but this peak is smoothed out as the dimethyl mark spreads along the body of the gene in the treated samples. (D) The spread of dimethyl seen at activation signature genes is not present at genes in the bottom 10% of PC1, where all five conditions show a similar pattern of dimethyl deposition across the first 4000 bp of the genes. (E) The Interferon Response Family (IRF) motif is enriched in enhancers from many cells in the T cell lineage, with motif frequency peaking in thymocytes (DN1 through DP). The grey portion of the bars represents mean background enrichment of the motif, and the colored section of the bar shows the difference in enrichment between the cell type indicated and the background. From top to bottom, the cells indicated are: Embryonic stem cells; Lin−Sca-1+c-Kit+ (LSK) hematopoietic progenitor cells; fetal liver derived double negative 1 thymocytes; fetal liver derived double negative 2a thymocytes; fetal liver derived double negative 2b thymocytes; double negative 3 thymocytes; double positive thymocytes; Th1 polarized CD4+ T cells; Th2 polarized CD4+ T cells; Th1 polarized CD4+ T cells from a Stat1 knockout model; thioglycollate-elicited macrophages; and the adipocyte-derived 3T3L1 cell line. (F) De novo motif finding identifies lineage-determining transcription factor (LDTF) motifs among H3K27Ac-marked enhancers shared by the five conditions. As with the primed enhancers, the ETS family motif and a RUNX motif are highly enriched. (G) Among activated enhancers shared by all five conditions, pro-inflammatory transcription factor motifs are enriched. As in primed enhancers, IRF, NF-κB, and AP-1 motifs are all enriched in the shared H3K27Ac peaks. (H) As seen with BATF, deposition of H3K27Ac, a marker of transcription factor activity, reflects increasing TCR signal strength at the genome-wide binding sites of the AP-1 family member JunB. I. Similarly, deposition of H3K27Ac reflects increasing TCR signal strength at the genome-wide binding sites of the AP-1 family member cJun.
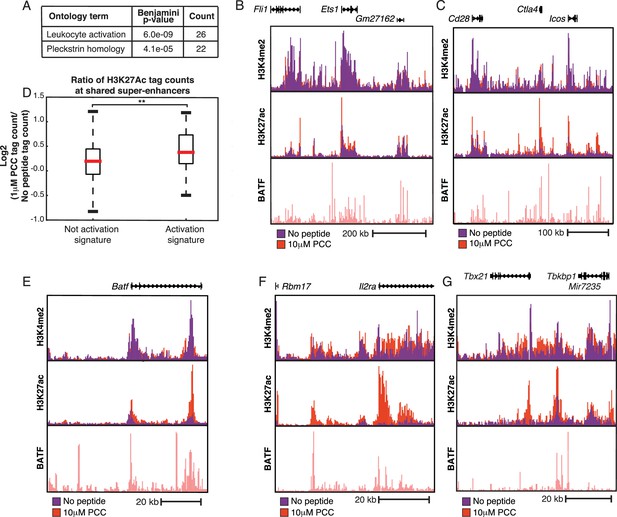
Super-enhancers prime T cell activation genes.
(A) Gene Ontology (GO) analysis of genes nearest to the ~450 super-enhancers shared by treated and untreated conditions show enrichment for T cell activation genes. P-values shown are Benjamini-Hochberg adjusted p-values. (B) H3K27Ac marks a large super-enhancer around the lineage-determining transcription factor Ets1 in both the No Peptide and 1 μM PCC conditions. The super-enhancer spans the ~600 kbp region shown. (C) The ~400 kbp super-enhancer region encompassing Cd28, Ctla4, and Icos is marked by H3K27Ac in both treated and untreated conditions. (D) Despite being heavily marked by H3K27Ac in both untreated and treated conditions, shared super-enhancers near activation signature genes show a significant gain in H3K27Ac tags in response to stimulation as compared to the shared super-enhancers not proximal to activation signature genes. In other words, basally primed super-enhancers near activation signature genes see significant increases in activity upon stimulation, correlating with increased gene expression at the activation signature genes. (E) Some regions of H3K27Ac deposition required TCR stimulation to pass the super-enhancer threshold, as can be seen here at the ~60 kbp region encompassing BATF, an AP-1 family member. While H3K27Ac is clearly present under basal conditions, there is a substantial increase in enhancer activity upon treatment with 10 μM PCC. (F) Il2ra (CD25) shows increased enhancer activity and formation of a super-enhancer in the treated condition. (G) Similarly, the region surrounding Tbx21 (Tbet) shows substantial increases in activity subsequent to stimulation, resulting in the formation of a super-enhancer. (p-values based on Student’s t test; *p<0.05, **p<0.01, ***p<0.001).

Super-enhancers prime T cell activation genes.
(A) A basally-primed super-enhancer encompasses the Runx1 locus. (B) Despite being up-regulated in response to treatment, the Irf4 locus has a super-enhancer even under untreated conditions. (C) Lag3, a negative regulator of T cell signaling that is up-regulated with treatment, sees increased enhancer activity and formation of a super-enhancer in treated conditions. (D) Stat5b, a transcription factor important for T cell signaling, shows increased enhancer activity and formation of a super-enhancer in the treated condition.
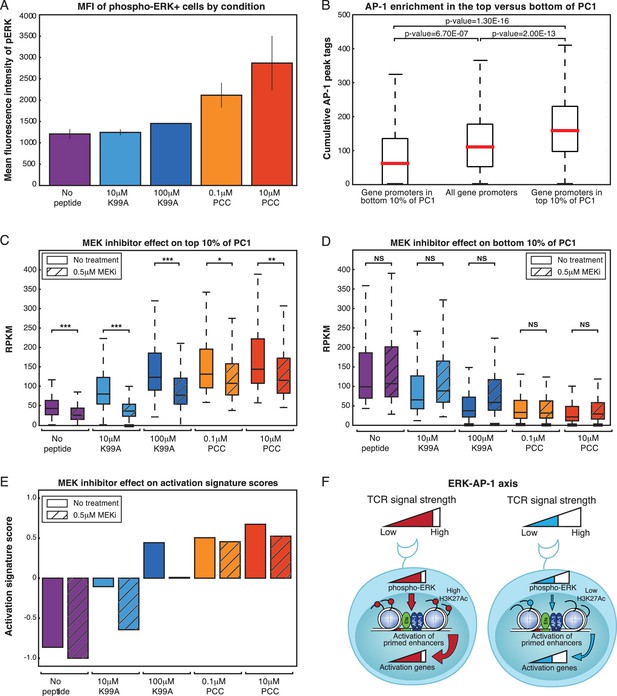
ERK signaling translates TCR signal strength into graded gene expression.
(A) ERK phosphorylation is a measure of ERK pathway activity. Flow cytometry for phospho-ERK after 3.5 hr of co-culturing shows that, on a per-cell basis, increasing signal strength yields increasing levels of phospho-ERK among CD4+ phospho-ERK+ cells. (B) ERK pathway activation is upstream of the transcription factor AP-1. ChIP-sequencing tags for four AP-1 family members (BATF, cJun, JunB, and JunD) in Th17 cells shows that there is an enrichment for AP-1 binding near the promoters (plus or minus 1,000 bp from the TSS) of activation signature genes (top 10% of PC1) as compared to all genes or the genes in the bottom 10% of PC1. (C) A MEK inhibitor that dampens signaling upstream of the ERK pathway preferentially diminishes expression of activation signature genes, as seen in the fact that the RPKM of genes in the top 10% of PC1 is significantly reduced with treatment. (D) The reduction of RPKM seen with the activation signature genes is not a general effect, as the RPKM of the genes in the bottom 10% of PC1 are not significantly affected by MEK inhibitor treatment. (E) We quantified the effect of MEK inhibitor treatment using the activation signature score. Treatment with the MEK inhibitor reduces the activation signature score for all samples. (F) Schematic of the ERK-AP-1 axis. See text for details. (p-values based on Student’s t test; *p<0.05, **p<0.01, ***p<0.001).
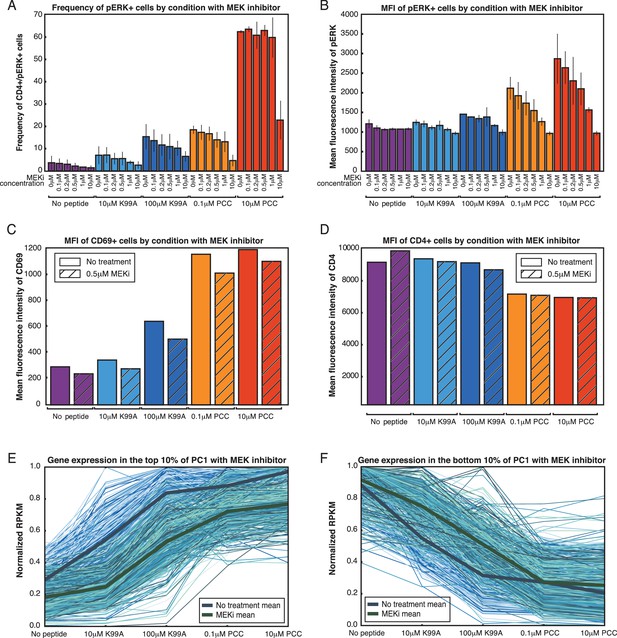
ERK signaling translates TCR signal strength into graded gene expression.
(A) ERK phosphorylation is a measure of ERK pathway activity. Flow cytometry for phospho-ERK after 3.5 hr of co-culturing shows that the frequency of pERK+ cells is dependent on both the peptide treatment and the dose of MEK inhibitor used, with increased strength of signaling and decreased MEKi leading to greater percentages of pERK+ cells. (B) Per-cell levels of phospho-ERK as measured by flow cytometry are analog with respect to the dosage of MEK inhibitor treatment. For each peptide condition, increasing the concentration of the MEK inhibitor gradually reduces the geometric MFI of phospho-ERK in the treated cells. (C) MEK inhibitor treatment at 0.5 μM (IC50) results in the preferential reduction of activation signature genes. CD69, one of the activation signature genes, reflects this decrease in expression level at the protein level, as measured by extracellular flow cytometry. (D) In contrast to CD69, CD4 does not show a change in expression level upon treatment with the MEK inhibitor. (E) For the genes in the top 10% of PC1, MEK inhibitor treatment (green lines) results in a decrease of expression as compared to the uninhibited samples (blue lines). Each line plots the normalized RPKM of one gene across the five samples, either with the MEK inhibitor (green) or without (blue). (F) In contrast to the top 10%, the genes in the bottom 10% of PC1 are not significantly affected by MEK inhibitor treatment, and, if anything, increase in expression level rather than decrease.
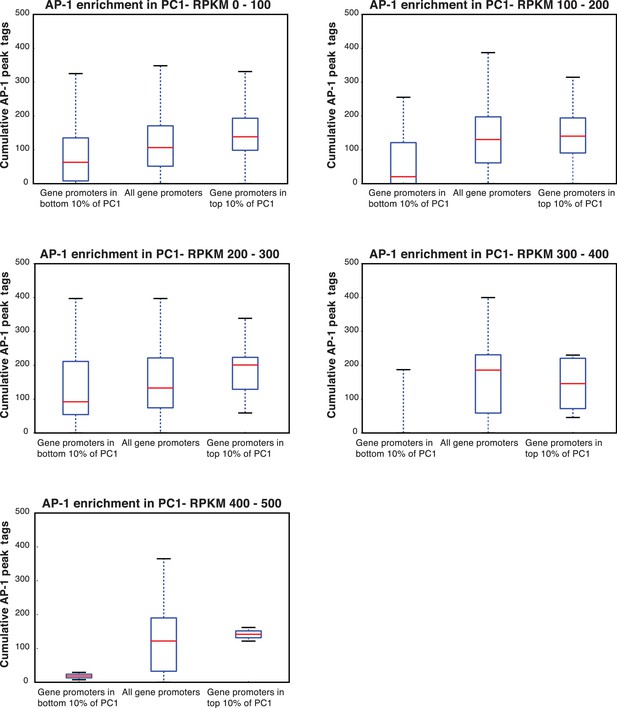
AP-1 enrichment is independent of expression level.
(A) The enrichment for AP-1 in promoters of genes in the top 10% of PC1 (shown in Figure 7B) is not dependent on the expression level of the genes. Here, we subset the genes in the top 10% of PC1 by RPKM, and the increasing levels of AP-1 across the different groups of genes holds.
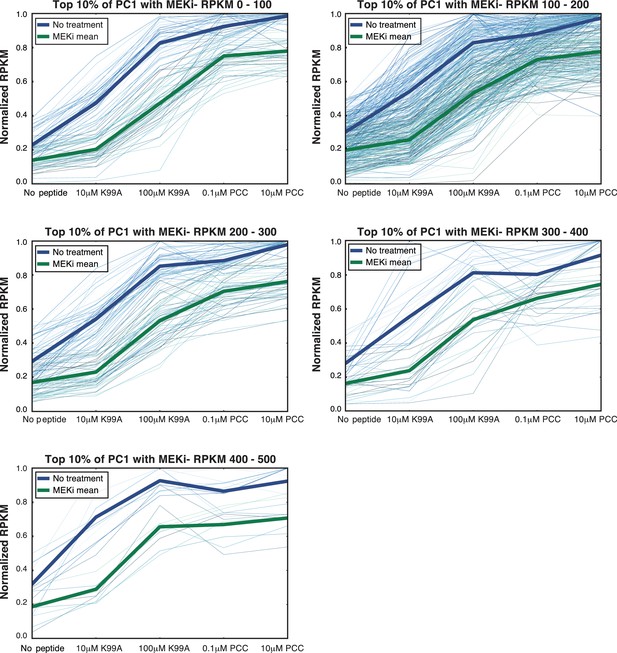
AP-1 enrichment is independent of expression level.
(A) The decrease in RPKM for MEKi treated samples for genes in the top 10% of PC1 (shown in Figure 7C) is not dependent on the expression level of the genes. Here, we subset the genes in the top 10% of PC1 by RPKM, and the affect of MEK inhibitor treatment is consistent.
Tables
Pairwise overlap between the H3K4me2 peaks. Each cell contains the count of overlapping peaks where each condition shown has at least 40 tags (normalized). In the diagonal is the total number of peaks with at least 40 tags for the given condition.
| No Peptide | 10 μM K99A | 100 μM K99A | 0.1 μM PCC | 10 μM PCC | |
|---|---|---|---|---|---|
| No Peptide | 16012 | 14951 | 12487 | 14783 | 13105 |
| 10 μM K99A | 15616 | 12549 | 14838 | 13192 | |
| 100 μM K99A | 12695 | 12603 | 12247 | ||
| 0.1 μM PCC | 15569 | 13281 | |||
| 10 μM PCC | 13444 |
Pairwise overlap between the H3K27Ac peaks. Each cell contains the count of overlapping peaks where each condition shown has at least 40 tags (normalized). In the diagonal is the total number of peaks with at least 40 tags for the given condition.
| No Peptide | 10 μM K99A | 100 μM K99A | 0.1 μM PCC | 10 μM PCC | |
|---|---|---|---|---|---|
| No Peptide | 6410 | 3591 | 5791 | 5988 | 5977 |
| 10 μM K99A | 3671 | 3557 | 3594 | 3584 | |
| 100 μM K99A | 8877 | 8699 | 8789 | ||
| 0.1 μM PCC | 9867 | 9368 | |||
| 10 μM PCC | 10021 |





