Image-based identification and isolation of micronucleated cells to dissect cellular consequences
Figures
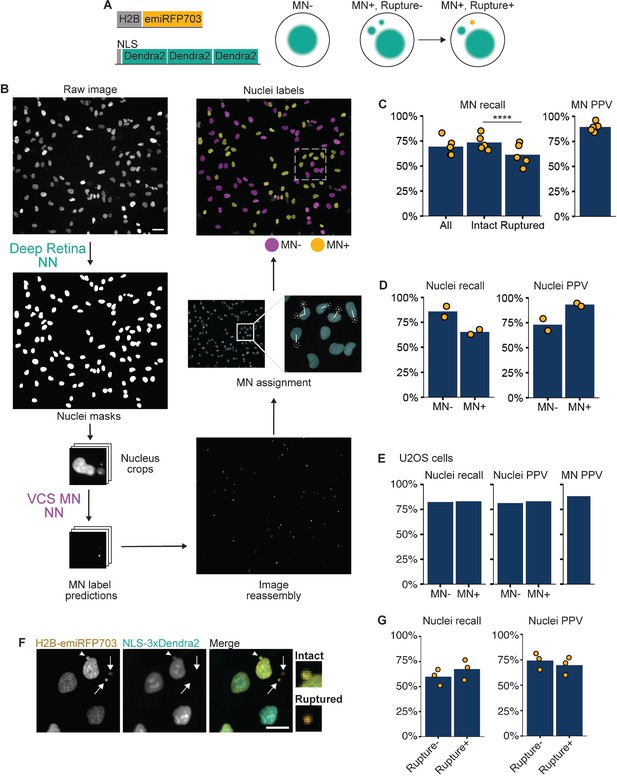
VCS MN neural net module identifies micronucleated cells.
(a) Diagrams of the two constructs transduced into hTERT-RPE1 cells to make the RFP703/Dendra cell line and how the proteins localize in micronucleated (MN+) cells before and after MN rupture. (b) Diagram of VCS MN image analysis pipeline on RFP703/Dendra cells incubated in Mps1i. Raw image is of H2B-emiRFP703 taken on a 20 x widefield objective. Nuclei are first segmented using the Deep Retina segmenter and these masks are used to generate image crops centered on each nucleus. Image crops are then resized to fit the ResNet18 UNet model (see Figure 1—figure supplement 1) and analyzed by VCS MN to generate MN label predictions, which are mapped back to the raw image. After image reconstruction, MN are assigned to the nearest nucleus based on proximity. Nucleus masks from the Deep Retina segmenter are then classified as MN +or MN- to generate the nuclei labels. Scale bar = 40 µm. (c) Recall and positive predictive value (PPV) of MN identification within the image crops. MN were manually labeled as intact or ruptured using an image of NLS-3xDendra2 acquired at the same time. N=5, n=264, 158, 365, 283, 249. (d) Recall and PPV of MN- and MN +labeled nuclei. N=2, n=328, 186. (e) Same as for (d) except images were of U2OS cells expressing H2B-emiRFP703 and 3xDendra2-NLS. N=1, n=85, 95. (f) RFP703/Dendra cells with ruptured (arrows) and intact (arrowhead) MN showing a loss of NLS-3xDendra2 signal in ruptured MN. Scale bar = 10 µm. (g) Recall and PPV for MN rupture-based nucleus classification. N=3, n=120, 91, 82.
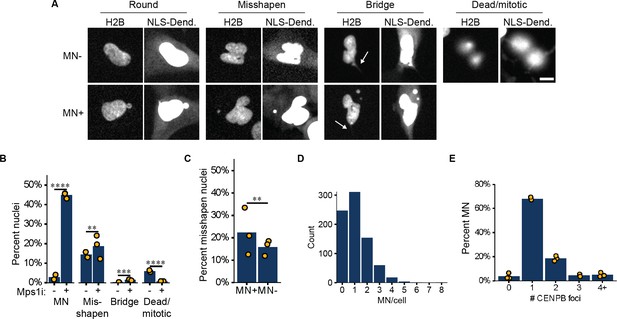
Analysis of nuclear and MN characteristics after Mps1i addition to RPE1 RFP703/Dendra cells.
(a) Representative images of nucleus morphology classes in RFP703/Dendra cells after 24 hr Mps1i treatment. The NLS-3xDendra2 channel (NLS-Dend.) is blown out to highlight the cytoplasmic signal. H2B=H2B-emiRFP703 channel. Arrows indicate chromatin bridges. Scale bar=10 µm. (b) Nuclei were manually scored for indicated phenotypes. Rare multinucleated cells were included in the misshapen nuclei class. Nuclei clearly in late telophase based on the cup shape of the chromatin were scored as normal, not misshapen. Msp1i- (DMSO): N=2, n=531, 833. Mps1i +: N=3, n=647, 597, 627 p: **≤0.01, ***≤0.001, ****≤0.0001 by Barnard’s test. (c) Quantification of misshapen nuclei in cells manually labeled as MN +and MN- from (b). N=3, n=839, 1032. p: **≤0.01, by Barnard’s test. (d) Histogram of number of MN per cell in Mps1i treated RFP703/Dendra cells. N=5, n=1323. (e) Quantification of MN chromosome content in Mps1i treated RFP703/Dendra cells. Chromosomes enumerated using a CENPB PNA probe. N=3, n=86, 91, 82. Single chromosome MN are highly enriched in this population and MN containing chromatin fragments (0 foci) are rare.
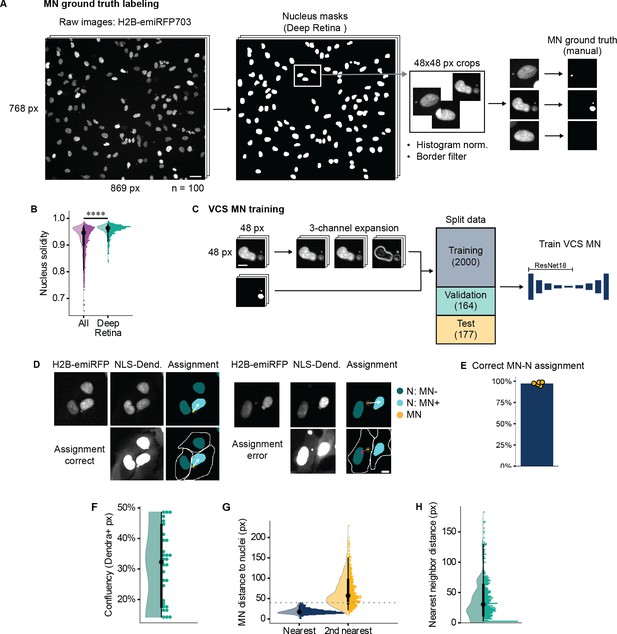
Description of VCS MN classifier training and assessment of MN assignment accuracy.
(a) Graphic depiction of pipeline for generating MN ground truth labels for VCS MN training and testing datasets. Two-channel images of Mps1i treated RPE1 RFP703/Dendra cells were acquired using 20 x widefield epifluorescence. Nuclei were segmented on the H2B-emiRFP703 channel using the Deep Retina segmenter. These labels were used to generate 48x48 pixel crops centered on each nucleus. Crops underwent histogram normalization and filtering out of nuclei touching the image border. MN pixels were then manually annotated on each crop by an expert user. 2341 crops from 100 images (90 treated with Mps1i, 10 untreated) were annotated. Scale bar = 40 µm. (b) Quantification of nucleus solidity in all nuclei versus nuclei labeled by the Deep Retina segmenter in RFP703/Dendra cells after Mps1i treatment. Highly lobulated nuclei (low solidity) were routinely missed by the Deep Retina segmenter. N=1, n=1357, 1492. p: ****≤0.0001 by KS test. (c) Graphic depiction of pipeline for training the VCS MN classifier. VCS MN was trained on the image crops and MN ground truth labels generated in (a). To accommodate Torchvision’s ResNet18’s expectation of a 3-channel 96x96 px image, each crop was resized and then expanded as follows: the first 2 channels are duplicates of the H2B-emiRFP703 image and the final channel is the result of Sobel edge detection. This image set was split into three parts for training, validation, and testing, as indicated, and fed into the VCS MN classifier. (d) Representative images of MN assignment pipeline results. Automatic assignment depicted in top row (arrow) and manual assignment based on the NLS-3xDendra signal (NLS-Dend.) (arrow) in bottom row. Examples of correct and incorrect proximity-based assignment are shown. Scale bar=10 µm. (e) Quantification of proportion correctly assigned MN by proximity. Pooled proportion=97%. N=5 experiments, n=1319 MN. (f) Quantification of cell confluency in training images. Proportion of field covered by cells was defined by thresholding on the total NLS-3xDendra2 signal. Median=33.2%. n=30 images from three experiments. (g) Distance between MN, nearest nucleus, and second nearest nucleus, centroid to centroid, in pixels. Median distances are 17.1 px (nearest) and 57.1 px (second nearest). n=981 MN from images from five experiments. (h) Distance between nuclei, border to border, in pixels in training images. Median = 30 px. N=11 images, n=507 nuclei.
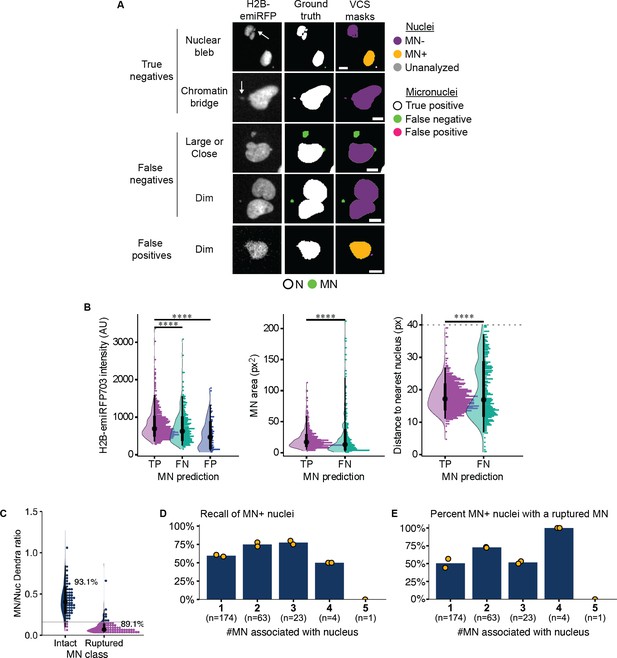
Analysis of VCS MN classification accuracy.
(a) Representative images of VCS MN analysis of RFP703/Dendra cells after Mps1i addition. Arrows on H2B-emiRFP703 channel indicate nuclear feature on left. Ground truth masks were annotated manually for nuclei (N) and micronuclei (MN). VCS masks and labels were generated automatically. In the VCS masks column, true positive MN are white, missed MN are green, and false positive MN are magenta. Nucleus blebs and chromatin bridges were rarely identified as MN by VCS MN. Instead, false positives were frequently associated with dim H2B signal (bottom). Examples of false negative MN show enrichment for large MN, MN overlapping the nucleus, and MN with dim H2B signal compared to nearby nuclei. Scale bars = 10 µm. (b) Quantification of H2B-emiRFP703 intensity, MN area, and distance to nucleus for MN that were properly classified by VCS MN (true positives, TP), missed (false negatives, FN), or misclassified (false positives, FP). N=5, 5, 1. n=654, 327, 80. p: ****≤0.0001 by KS test with Bonferroni multiple test correction. (c) MN:nucleus NLS-3xDendra2 intensity ratios for manually classified intact and ruptured MN. Solid gray line=set VCS MN threshold. N=3, n=179, 113. (d, e) Recall and rupture frequency for MN +nuclei by # MN. MN + cells were manually classified. N=2, n values on graph. Data show that nuclei associated with more than 1 MN are more likely to be correctly identified as MN +by VCS MN (d) and that the presence of multiple MN increases the likelihood that at least 1 MN will be ruptured (e).
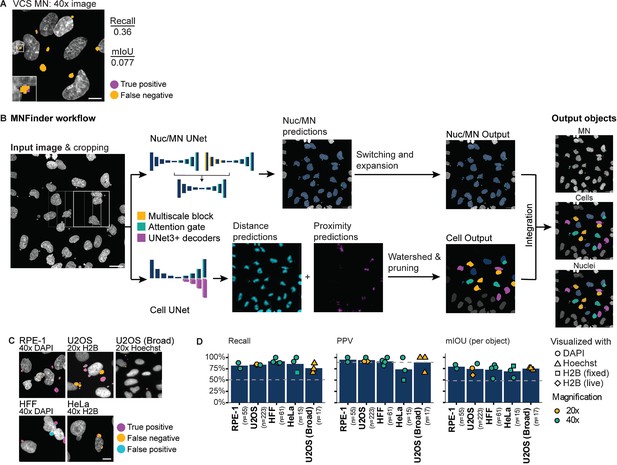
MNFinder robustly segments MN across cell types and imaging conditions.
(a) Representative image showing undersegmentation and low recall by the VCS MN neural net on DAPI-labeled RPE1 cells imaged at ×40 magnification after Mps1i incubation. Images were scaled down prior to VCS MN analysis to match ×20 pixel size. Mean intersection-over-union (mIoU) and recall quantified from N=2, n=33, 31 images. Scale bar = 10 µm. (b) Overview of MNFinder module for classifying and segmenting MN and nuclei. Raw images of chromatin are first tiled by a sliding window and then processed by two groups of neural nets: one classifies pixels as either nuclei or MN (Nuc/MN) and one is an instance classifier for cells, with cells being defined as the smallest object that encloses a nucleus and its associated MN. Nuc/MN classifier results are post-processed to reassign MN that were misclassified as small nuclei and to expand MN masks. The cell instance classifier outputs gradient maps, which are used to define cells through watershed-based post-processing (see Figure 2—figure supplement 1). Nuc/MN results are integrated with cell results to produce final labels of individual MN, nuclei, and their associations. Image crops are reassembled onto the final image by linear blending. Scale bar = 40 µm. (c–d) Example images and MN pixel predictions using MNFinder on multiple cell types (RPE1 H2B-emiRFP703, U2OS, U2OS H2B-emiRFP703, HeLa H2B-GFP, and HFF), chromatin labels (DAPI, H2B-FP), and magnifications (×20, ×40). Scale bar = 10 µm. MN recall, PPV, and mIOU were quantified and averaged per image for each condition. Dotted line=performance of the VCS neural net on RPE1 H2B-emiRFP703 ×20 images. MNFinder performance is similar across conditions except images of H2B-GFP in fixed HeLa cells. N=1. n (on graph)=cells.
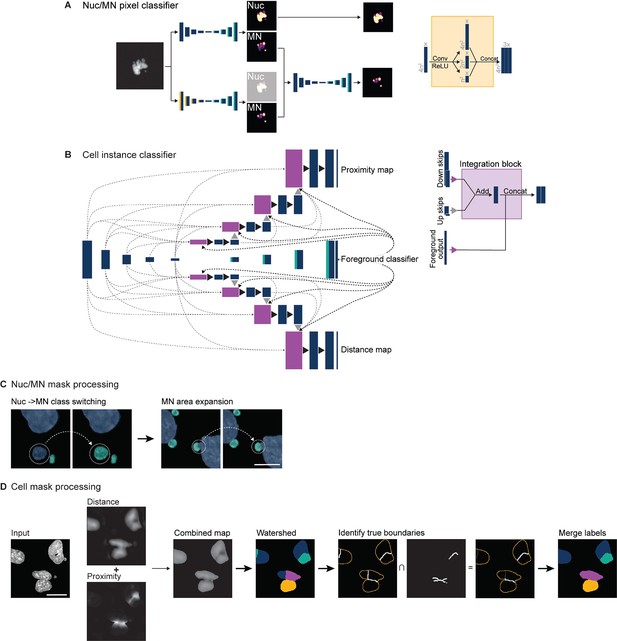
Details of UNet architectures and object processing by the MNFinder module.
(a) The nucleus and MN (Nuc/MN) pixel classifier takes as input a cropped single channel image of chromatin and feeds it into two parallel, attention-gated UNets, one of which also has multiscale downsamplers (yellow). The nucleus weights from the basic UNet (top) are retained and MN weights from both UNets are fed to a third UNet for ensembling to produce the final MN predictions. (b) Diagram of the triple decoder cell instance classifier. Two of the decoders have a UNet3+-like architecture with multiple skip connections and deep supervision during training. Feature depths are kept constant and most concatenation/max-pooling operations are replaced with addition to reduce training time. One decoder generates distance maps of a concave hull containing each nucleus and any associated MN (a ‘cell’) and the other generates a proximity map of each cell’s distance to all others. A third decoder uses a standard UNet with attention gates (cyan) to classify foreground pixels (nuclei and MN) and is used as input into every level of the distance- and proximity-map decoders via an integration block (magenta). (c) Results from the nucleus/MN pixel classifier and cell instance classifier are further processed to improve accuracy. To limit misclassification of large MN as small nuclei, nuclei under a user-set area threshold are reclassified as MN. To limit MN undersegmentation, MN pixel groups are expanded by transforming each object to its convex hull. Scale bar = 5 µm. (d) Distance and proximity maps from the cell instance classifier are combined to generate seeds for watershed segmentation. To correct for oversegmentation, only labels with boundaries that intersect a skeletonized version of the proximity map or background pixels are retained. Scale bar = 10 µm.
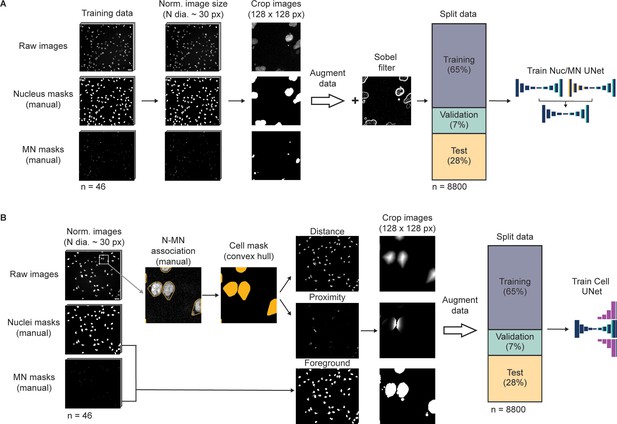
Schematic of training data generation and use in MNFinder classifiers.
(a) Graphic depiction of pipeline to generate training data for the MNFinder MN and nucleus pixel classifier. A collection of single channel fluorescence images from several cell lines, chromatin labels, and microscope settings were manually annotated for nuclei and MN. Training, validating, and testing data were generated from these images by first normalizing image dimensions so that nuclei were approximately 30 px across their shortest diameter, generating random 128 by 128 px crops from all images, augmenting the data with a series of transformations, and then adding a second channel from a Sobel filter. This yielded a dataset of ~8800 images, which was split for training, validation, and testing as indicated. (b) Graphic depiction of pipeline to generate training data for MNFinder cell instance classifier, where cell is a defined as a nucleus and its associated MN. The same starting image set was used as in (a). Ground truth associated nuclei and MN groups were manually labeled using the NLS-3xDendra2 cytoplasm signal. Grouped objects were then transformed into cells by drawing the convex hulls. These hulls were then transformed into distance and proximity maps using Euclidian distance formulas. These maps were combined with a mask of the foreground pixels to generate a three-channel image. These images were randomly cropped and transformed to generate a training dataset of ~8800 images. This dataset was then split for training, validating, and testing of the cell instance classifier as indicated. Scale bars = 40 µm.
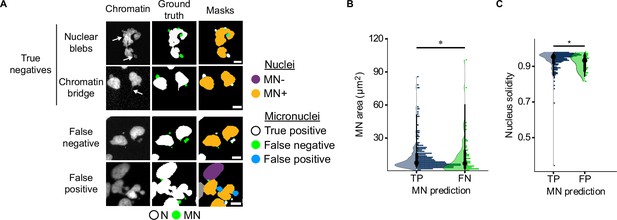
Analysis of MNFinder errors.
(a) Representative images of MNFinder analysis from human fetal fibroblasts (DAPI, top), RPE1 RFP703/Dendra (H2B-emiRFP703, second) or U2OS RFP703/Dendra (H2B-emiRFP703, bottom two) cells. Arrows on the chromatin images point to the nuclear feature listed at left. Ground truth annotations for nuclei (N) and micronuclei (MN) were generated manually. Nucleus and MN masks were automatically defined using MNFinder. In the mask channel, true positive MN are white, missed MN are green, and false positive MN are blue. Neither nuclear blebs nor chromatin bridges were frequently miscategorized as MN. False negatives were enriched in small MN, and false positives were frequently nuclear lobes. Scale bars=10 µm. (b) Quantification of false negative (FN) MN area. MN missed by MNFinder were enriched in small MN across the testing dataset. TP=true positives, N=12 images, n=329, 60 MN. p: *≤0.05 by KS test. (c) Quantification of nucleus solidity in nuclei associated with false positive (FP) MN. False positives were frequently associated with highly lobulated nuclei with low solidity. N=12, n=329, 21. p: *≤0.05 by KS test.
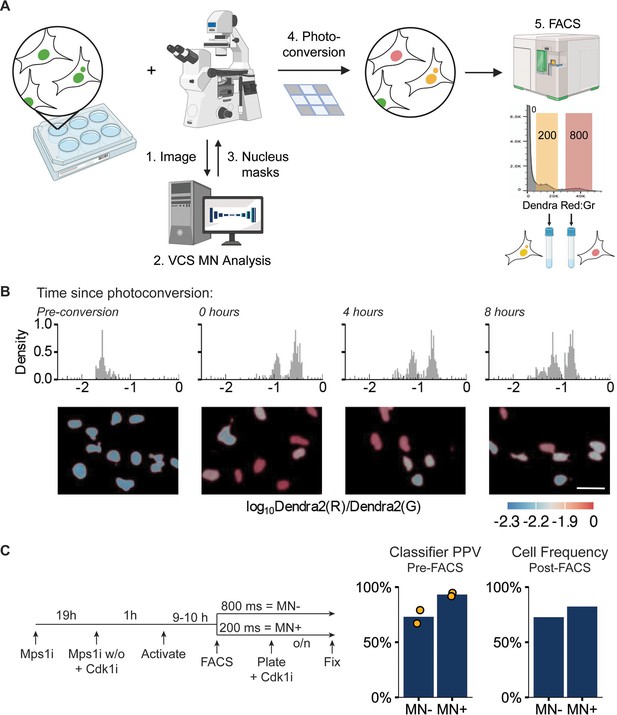
Visual cell sorting can isolate RPE1 RFP703/Dendra micronucleated cells.
(a) Overview of visual cell sorting protocol. Cells are plated in multi-well plates, cellular phenotypes are quantified on-demand during imaging by VCS MN, and labeled nuclei (e.g. MN +and MN-) are photoconverted for either 200 or 800ms, yielding two different ratios of red:green fluorescence (red and yellow nuclei). These differences are quantified by FACs and gated on the red:green ratios for cell sorting. Graphic created with BioRender.com. (b) Histogram of nuclear red:green ratio quantification measured on repeated imaging of the same fields 0, 4, and 8 hr after photoconversion. Representative images are pseudo-colored by log10 Dendra red:green ratio (below). N=1, n=82, 353, 285, 313. Scale bar = 40 µm. (c) Design of MN cell isolation validation experiment. VCS MN classifier PPV calculated on images acquired during activation and frequency of MN- or MN + cells manually quantified in cells plated and fixed post-FACs sorting. Pre-FACS: N=2, n=251, 263. post-FACS N=1, n=338, 353.
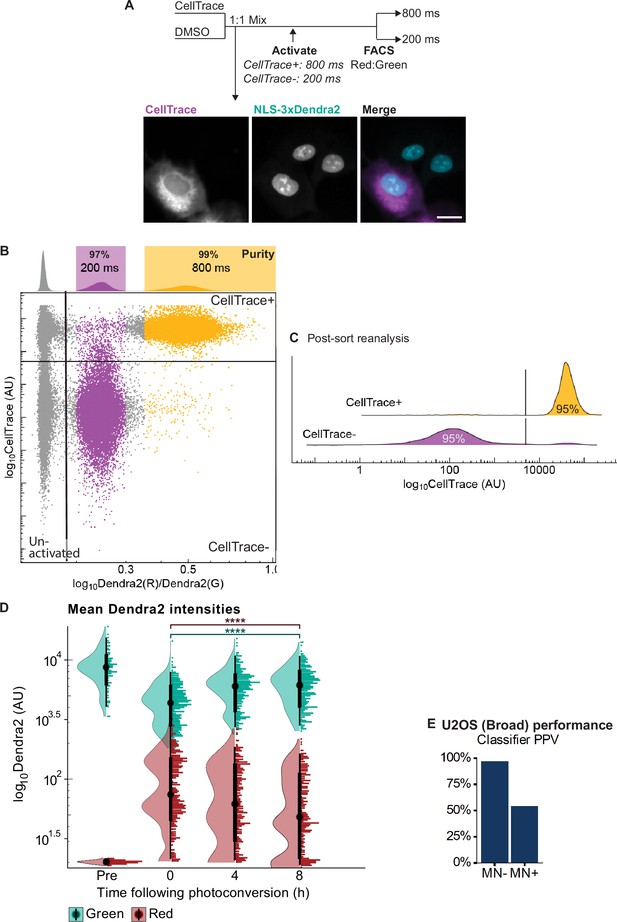
Controls for VCS MN isolation experiments.
(a) Outline of RFP703/Dendra visual cell sorting validation experiment using CellTrace labeling as the activation trigger. Cells were incubated with CellTrace far-red and mixed with unlabeled cells at a 1:1 ratio. Nuclei were labeled based on CellTrace fluorescence intensity and converted with either an 800ms (CellTrace+) or 200ms (CellTrace-) UV pulse. The well was only partially converted prior to FACs analysis and sorting. Representative image of the mixed population prior to photoconversion is shown. Scale bar=10 µm. (b) FACS plot of Dendra2 red:green ratio versus CellTrace fluorescence. Colored bars represent gates. As expected, CellTrace + cells generally have a high red:green ratio, CellTrace- cells a middle red:green ratio, and unanalyzed cells from both classes a low red:green ratio. Percentages on graph are the percentage of CellTrace- and CellTrace + cells in their expected gate, i.e. the population purity. (c) Histogram of CellTrace fluorescence in cells sorted on the Dendra2 red:green ratio in (b) after re-analysis by FACs. Values show a slight drop in cell purity for both populations after sorting. (d) Data from Figure 3b replotted with NLS-3xDendra2 red and green fluorescence intensity values plotted separately. Two peaks of red fluorescence intensity are visible immediately after photoconversion (0 hr) and maintained over time. The increased population of nuclei at 4 and 8 hr with only background levels of red fluorescence (Dendra2:Red <101.5) likely represents unconverted nuclei moving into the imaging frame. The downward shift in red fluorescence intensity over time likely represents turnover of converted red fluorophores within the cells. N=1, n=82, 353, 285, 313. p: ****≤0.0001 by KS test. (e) Predicted classifier PPV (population purity) for low MN frequency U2OS cells (U2OS Broad). We observe a lower, but still substantial, enrichment of micronucleated cells in the MN +population compared to a highly micronucleated population (e.g. Figure 3c). N=1, n=17 cells.
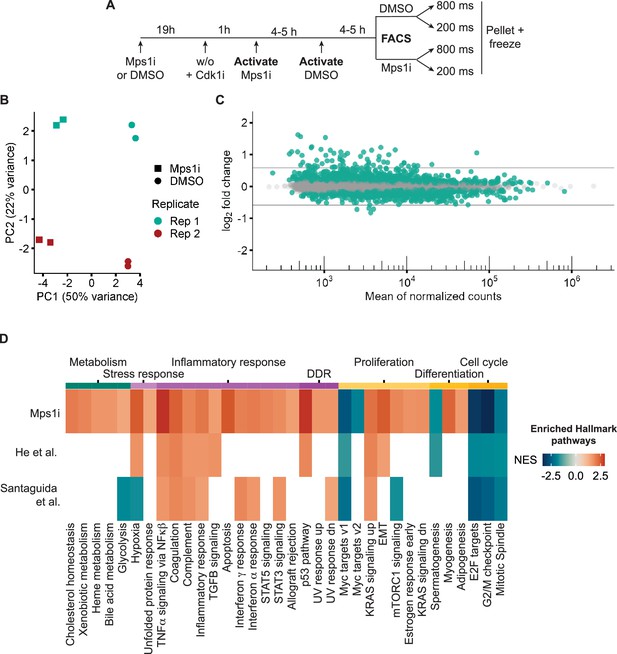
Visual cell sorting pipeline identifies Mps1i transcriptional response.
(a) Outline of experiment. Nuclei identified by the Deep Retina segmenter were randomly activated for 800 or 200ms. (b) PCA plot showing clustering of Mps1i-treated and DMSO-treated cells by treatment (major) and by replicate (minor). Each experimental replicate represents two technical replicates. (c) MA plot of genes identified by RNASeq. Differentially expressed genes (FDR adjusted p-value <0.05) are in green. Gray lines represent 1.5-fold-change in expression. (d) Heatmap of Hallmark pathway enrichment between visual cell sorting data and data from Santaguida et al., 2017 and He et al., 2019 analyses of RPE1 cells after chromosome missegregation. Hallmark pathways (bottom) were clustered based on manually annotated categories (top).
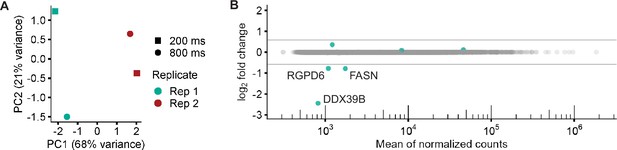
Differential UV pulses do not induce substantial transcriptional changes.
(a) PCA plot of cells treated with DMSO and exposed to 800ms or 200ms UV. (b) MA plot of the data in (a). Only six differentially expressed genes were identified in cells exposed to 800ms vs 200ms UV pulses and only three were downregulated over 1.5-fold: DDX39B, FASN, RGPD6.
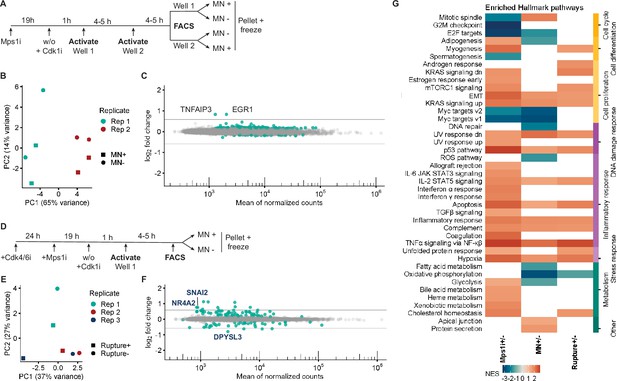
Micronucleation and rupture result in few transcriptional changes.
(a) Outline of experiment for MN +and MN- cell isolation from Mps1i treated RFP703/Dendra cells. (b) PCA plot showing clustering of MN + and MN- cells by replicate (major) and condition (minor). (c) MA plot of genes identified in MN +/-RNASeq. Of the identified DEGs, only two have fold-changes larger than 1.5. Both, TNFAIP3 and EGR1, are also significantly upregulated in Mps1i-treated cells. (d) Outline of experiment for MN rupture + and rupture- cell isolation. (e) PCA plot showing clustering of intact MN and ruptured MN cells by condition and replicate. (f) MA plot of genes identified in MN rupture +/-RNASeq. The three DEGs with a fold-change higher than 1.5 and unique to this dataset are labeled on the plot. (g) Heatmap of Hallmark pathway enrichment in datasets of DMSO vs Mps1i, Mps1i-treated cells with and without MN, and synchronized, Mps1i-treated, MN + cells with and without MN rupture. All DEGs, defined as an FDR less than 0.05, are included. Pathways are grouped based on manual annotation (right). 6/43 pathways are unique to the MN +/- profile compared to Mps1i+/-, and 2/43 pathways are unique to MN rupture +/-compared to Mps1i+/-.
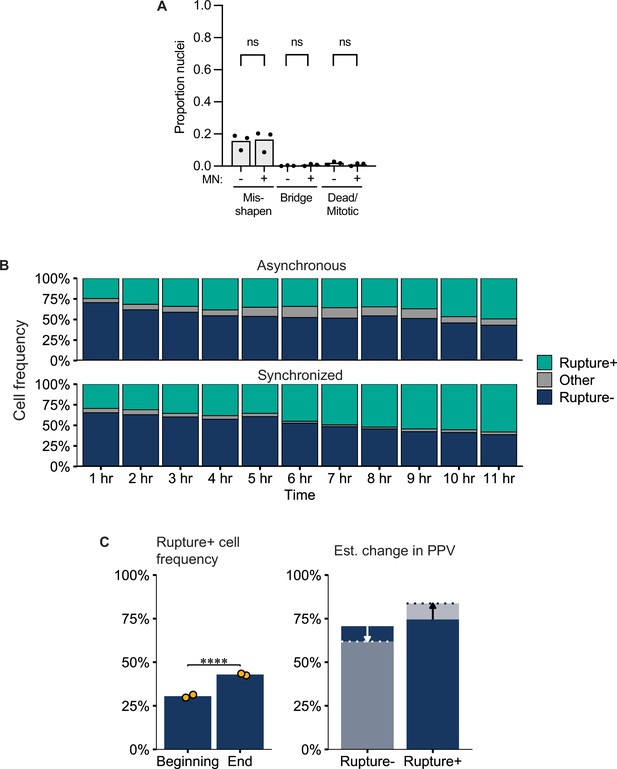
Analysis of visual cell sorting MN images.
(a) Manual analysis of nuclei features in cells labeled as MN + or MN- by VCS MN in RNASeq experiments. Categories defined as in Figure 1—figure supplement 1. N=3, n=875 MN +nuclei and 1375 MN- nuclei. p: ns >0.05 by Barnard’s test. (b) Change in MN rupture frequency over time in asynchronous and synchronized cells treated with Cdk1i. Other=mitotic, MN-, or Dendra2- cells. N=1, n=~200 cells per time point. (c) Change in MN rupture frequency between the start and end of a visual cell sorting experiment (4 hr) and predicted change in classifier PPV due to ongoing rupture of intact MN based on values in (b).
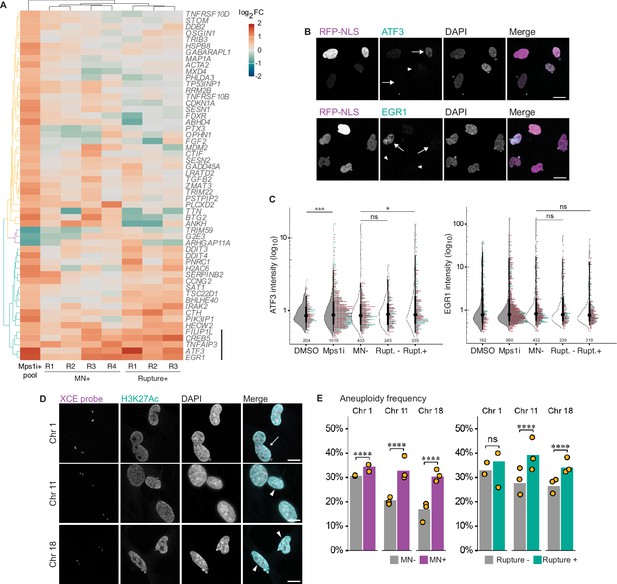
Micronucleation and rupture do not significantly alter the aneuploidy transcription response.
(a) Heatmap of Mps1i+DEGs (cutoff: absolute fold-change ≥1.5) compared to MN +and rupture +replicates. Euclidean distances calculated for features and samples and clustered by complete-linkage. Genes lacking values for one more experiment types were excluded. Line=gene cluster upregulated in MN rupture + cells. (b) Representative images and quantification of ATF3 and EGR1 labeling in RPE1 2xRFP-NLS cells after Mps1i incubation. Arrows = ruptured MN, arrowheads=intact MN. Scale bar=20 µm. (c) Quantification of normalized ATF3 and EGR1 mean nuclear intensity in manually classified cells. N=2 (graph colors), n=on graph, p: ns >0.05, *≤0.05, ***≤0.001 by GEE. (d) Representative images of DNA FISH for chromosomes 1, 11, and 18 and H3K27Ac identification of intact MN. Arrows=ruptured MN, arrowheads=intact MN. (e) Quantification of aneuploidy frequency (foci do not equal 2) per chromosome. Cells manually classified as MN- or MN+, and MN rupture- or rupture+. MN: Chr 1: N=2, n=429, 158; Chr 11: N=3, n=406, 313, 160; Chr 18: N=3, n=425, 202, 230. Rupture: Chr 1: N=2, n=187, 74; Chr 11: N=3, n=190, 108, 71; Chr 18: N=3, n=186, 102, 101.
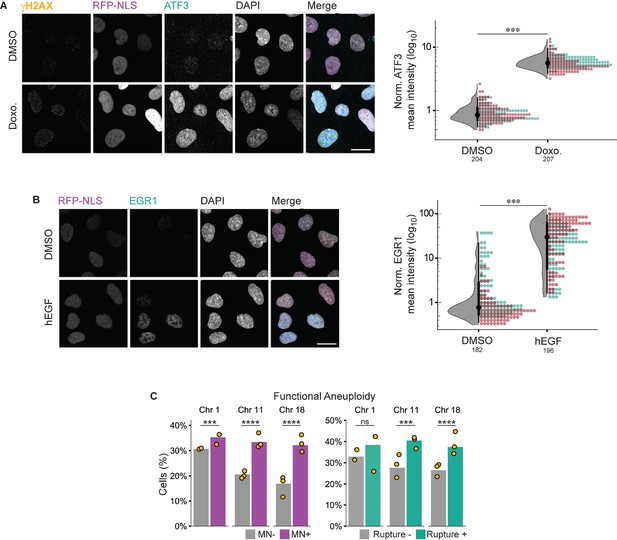
Controls related to Figure 6.
(a) Representative images and quantification of ATF3 nuclear mean fluorescence intensity in cells treated with DMSO or doxorubicin (Doxo.). N=2 (colors on graph), n=on graph. Scale bar = 20 µm. (b) Representative images and quantification of EGR1 nuclear mean fluorescence intensity in cells treated with DMSO or hEGF. N=2 (colors on graph), n=on graph. For a-b: p: ***≤0.001, by GEE. Scale bar=20 µm. (c) Same analysis as Figure 6b, but with chromosomes in ruptured MN excluded from the foci count. Similar levels of aneuploidy were observed between groups as in Figure 6b.
Tables
Comparison of neural net model metrics for MN segmentation across cell lines.
| Model | PPV (%) | Recall (%) | mIoU, per object (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell type | RPE-1 | U2OS | HeLa | HFF | U2OS (Broad) | RPE-1 | U2OS | HeLa | HFF | U2OS (Broad) | RPE-1 | U2OS | HeLa | HFF | U2OS (Broad) |
| UNet | 89.3 (±4.5) | 88.2 | N/A | N/A | 44.8 | 50.8 (±6) | 52 | N/A | N/A | 59.1 | 48.1 (±1.7) | 40.8 | N/A | N/A | 33.7 |
| Attention | 100.0 (±0) | 92.6 (±6.5) | 63.9 (±12.7) | 90.8 (±7.3) | 88.9 (±19.2) | 85.8 (±7.3) | 82.8 (±2.5) | 85.6 (±17.1) | 92.7 (±5.4) | 76.4 (±10.5) | 78.7 (±6.5) | 75.0 (±13.6) | 69.0 (±13.7) | 74.4 (±13.3) | 75.5 (±2.8) |
| MSAttention | 94.8 (±7.3) | 92.2 (±6.8) | 68.8 (±27.2) | 87.6 (±6.6) | 87.9 (±21.0) | 84.4 (±0.7) | 83.2 (±2.75) | 74.4 (±13.5) | 92.3 (±6.4) | 80.6 (±12.0) | 75.2 (±0.4) | 73.6 (±9.2) | 74.9 (±8.4) | 71.3 (±13.4) | 75.6 (±4.0) |
| Ensemble | 100.0 (±0) | 93.8 (±5.4) | 75.0 (±25.0) | 89.9 (±8.74) | 88.9 (±19.2) | 82.6 (±7.4) | 82.8 (±2.5) | 85.6 (±17.1) | 92.7 (±5.4) | 76.4 (±10.5) | 79.3 (±6.0) | 74.2 (±12.8) | 68.8 (±13.1) | 72.4 (±13.4) | 74.8 (±2.3) |
List of image sets used for training, validating, and testing MNFinder.
| Cell_type | DNA_Label | Integrity_label | Max_Int_Proj | Objective mag. | Microscope | Initial image resolution (px/um) | Scaled_image_resolution | Number_images | Training | Validation | Test |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HeLa | H2B-GFP | None | Y | 40 | Confocal_LSM | 5.6 | 2.8 | 1 | 1 | ||
| HeLa | DAPI | None | Y | 40 | Confocal_LSM | 5.6 | 2.8 | 6 | 3 | 1 | 2 |
| HeLa | DAPI | None | N | 20 | Confocal_LSM | 2.8 | 2.8 | 4 | 4 | ||
| hTERT-HFF | DAPI | H3K27-acetyl | Y | 40 | Confocal_Spinning_Disk | 3.9 | 1.95 | 4 | 4 | ||
| hTERT-RPE1 | DAPI | 2xRFP-NLS | Y | 40 | Confocal_LSM | 5.6 | 2.8 | 2 | 2 | ||
| hTERT-RPE1 | H2B-emiRFP703 | NLS-3xDendra2 | N | 20 | Widefield | 1.55 | 1.55 | 16 | 14 | 1 | 1 |
| MCC13 | DAPI | None | N | 20 | Confocal_LSM | 2.8 | 2.8 | 3 | 3 | ||
| U2OS | DAPI | H3K27-acetyl | Y | 20 | Confocal_LSM | 2.8 | 2.8 | 1 | 1 | ||
| U2OS | DAPI | H3K27-acetyl | Y | 40 | Confocal_LSM | 5.6 | 2.8 | 1 | 1 | ||
| U2OS | H2B-emiRFP703 | NLS-3xDendra2 | N | 20 | Widefield | 1.55 | 1.55 | 8 | 6 | 1 | 1 |
| Total: | 46 | 30 | 3 | 13 |
Quantification of MN frequency (propotion of cells with at least 1 MN) in images used to evaluate MNFinder.
| RPE-1 | U2OS | HeLa | HFF | U2OS (Broad) |
|---|---|---|---|---|
| 0.706 | 0.623 | 0.286 | 0.5 | 0.078 |
Comparison of MNFinder to existing programs that quantify MN frequency in adherent cell images.
| Study | Cell lines tested | Multiple imaging conditions assessed? | PPV | Recall | Only MN identified? | mIoU |
|---|---|---|---|---|---|---|
| Pons and Mauvezin, 2024; QATS | U2OS and HeLa cells | No | 99% | 60% | Yes | ND |
| Ibarra-Arellano et al., 2024; micronuclAI | Multiple human and mouse cell lines | Yes | 93.20% | 98.70% | No, nuclear buds classified as MN | ND |
| This study; MNFinder | Multiple human cell lines | Yes | 75.0–100% | 83.2–92.7% | Yes | 74.4–79.3% |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo sapiens) | HEK293T (transformed, kidney) | ATCC | CRL-3216, RRID:CVCL_0063 | |
| Cell line (Homo sapiens) | U2OS (transformed, osteosarcoma) | ATCC | HTB-96. RRID:CVCL_0042 | |
| Cell line (Homo sapiens) | HeLa (transformed, cervical carcinoma) | ATCC | CRM-CCL-2, RRID:CVCL_0030 | |
| Cell line (Homo sapiens) | hTERT RPE-1; RPE1 (immortalized, epithelial) | ATCC | CRL-4000, RRID:CVCL_4388 | |
| Cell line (Homo sapiens) | hTERT HFF; HFF (immortalized, fibroblast) | PMID:9817205 | Cell line maintained in D. Galloway lab. | |
| Cell line (Homo sapiens) | MCC13 (transformed, Merkel cell carcinoma) | ECACC | 10092302, RRID:CVCL_2583 | Cell line maintained in P. Nghiem lab |
| Cell line (Homo sapiens) | HeLa H2B-GFP | Millipore | SCC117, RRID:CVCL_ZM02 | Cell line maintained in D. Avgousti lab |
| Cell line (Homo sapiens) | hTERT RPE-1 NLS-3xDendra2, H2B-emiRFP703; RFP703/Dendra | This study | RPE1 cells used for VCS MN experiments | |
| Cell line (Homo sapiens) | U2OS NLS-3xDendra2, H3B-emiRFP703 | This study | U2OS cells used for MNFinder training | |
| Cell line (Homo sapiens) | hTERT RPE-1 NLS-3xDendra2-P2A-H2B-miRFP703 | This study | RPE1 cells used for VCS MN training | |
| Antibody | Anti-gH2AX phospho (Ser139) (mouse monoclonal) | Biolegend | Cat# 613401, RRID:AB_315794 | IF (1:500) |
| Antibody | Anti-ATF3 (rabbit monoclonal) | CST | Cat# 18665, RRID:AB_2827506 | IF (1:400) |
| Antibody | Anti-EGR1 (rabbit monoclonal) | CST | Cat# 4154, RRID:AB_2097035 | IF (1:1600) |
| Antibody | Anti-H3K27Ac (rabbit polyclonal) | Abcam | Cat# ab4729, RRID:AB_2118291 | IF (1:500) |
| Antibody | AF647 goat-anti-mouse (polyclonal, secondary) | Life Technologies | Cat# A21236 | IF (1:1000) |
| Antibody | AF488 goat anti-rabbit (polyclonal, secondary) | Life Technologies | Cat# A11034 | IF (1:2000) |
| Recombinant DNA reagent | pH2B-emiRP703 | Addgene | Cat# 136567, RRID:Addgene_136567 | Verkhusha lab |
| Recombinant DNA reagent | pLVX-EF1a-NLS-3xDendra2-blast (plasmid) | This study | Lentiviral plasmid based on pLVX-puro backbone (Clontech) to express NLS-3xDendra2 | |
| Recombinant DNA reagent | pLVX-EF1a-H2B-emiRFP703-neo (plasmid) | This study | Lentiviral plasmid based on pLVX-puro backbone (Clontech) to express H2B-emiRFP703 | |
| Recombinant DNA reagent | pLenti-CMV-NLS-Dendra2x3-P2A-H2B-miRFP | PMCID:PMC7273721 | Lentiviral plasmid to co-express H2B-miRFP703 and NLS-Dendra2 | |
| Commercial assay or kit | RNAqueous micro | Thermo Fisher | AM1931 | |
| Commercial assay or kit | SMART-Seq v4 ultra-low input RNA | Takara | 634894 | |
| Commercial assay or kit | Nextera XT DNA library preparation | Illumina | FC-131–1024 | |
| Chemical compound, drug | DAPI | Life Technologies | D1306 | (1 µg/mL) |
| Chemical compound, drug | Vectashield | Vector Labs | H-1000 | |
| Chemical compound, drug | RO-3306; Cdk1i | Sigma-Aldrich | SML0569 | (10 µM) |
| Chemical compound, drug | BAY1217389; Mps1i | Fisher Scientific | 501872752 | Msp1 inhibitor (100 nM) |
| Chemical compound, drug | PD-0332991; Cdk4/6i | Sigma-Aldrich | PZ0199 | Cdk4/6 inhibitor (1 µM) |
| Chemical compound, drug | doxorubicin hydrochloride | Fisher Scientific | BP25165 | (2 µg/mL) |
| Chemical compound, drug | hEGF | Peprotech | AF-100–15- | (5 ng/mL) |
| Chemical compound, drug | Phenol-red free DMEM/F12 | GIBCO | 21041025 | Used during VCS imaging |
| Chemical compound, drug | CellTrace Far Red | Thermo Fisher | C34572 | Cell label dye used in VCS validation |
| Software, algorithm | Metamorph (v7.10.1.161) | Molecular Devices | RRID:SCR_002368 | |
| Software, algorithm | Leica Application Suite X | Leica | RRID:SCR_013673 | |
| Software, algorithm | VCS MN | This study | Available at https://github.com/hatch-lab/fast-mn | |
| Software, algorithm | MNFinder | This study | Available at https://github.com/hatch-lab/mnfinder | |
| Other | Chromosome 1 XCE – orange (Homo sapiens) | MetaSystems | D-0801–050-OR | DNA-FISH probe |
| Other | Chromosome 11 XCE – orange (Homo sapiens) | MetaSystems | D-0811–050-OR | DNA-FISH probe |
| Other | Chromosome 18 XCE – orange (Homo sapiens) | MetaSystems | D-0818–050-OR | DNA-FISH probe |
| Other | PNA CENPB-Cy5 | PNA Bio | F3005 | DNA-FISH probe |
| Other | Leica DMi8 with Adaptive Focus | Leica | Microscope for VCS | |
| Other | Mosaic 3 Digital Micromirror Device | Andor | Microscope component for VCS | |
| Other | Leica DMi8 laser scanning confocal microscope | Leica | Confocal microscope | |
| Other | CSU spinning disk unit | Yokagawa | Confocal microscope component | |
| other | FACS Aria II | BD Biosciences | RRID:SCR_018934 | Cell sorter |
Additional files
-
Supplementary file 1
Additional tables containing transcriptomics data.
(a) DMSO 800 vs 200 DEG. List of differentially expressed genes (FDR ≤0.05) in DMSO treated RPE1 cells isolated after an 800ms or 200ms UV pulse. For all DEG analyses: padj = false-discovery rate adjusted p-values. (b) Table 2: Msp1i DEG. List of differentially expressed genes (FDR ≤0.05) in Mps1i versus DMSO RPE1 cells. (c) Msp1i DEG log2FC. List of differentially expressed genes in Mps1i versus DMSO RPE1 cells filtered for log2 fold change above 0.58 or below –0.58. (d) He et al DEG. List of differentially expressed genes (FDR ≤0.05) in RPE1 cells treated with nocodazole for 8 h versus control, initially reported in He et al., 2018. Overlap with Mps1i DEG list noted in last column. (e) Santaguida et al DEG. List of differentially expressed genes (FDR ≤0.05) in RPE1 cells that were treated with the Mps1i molecule reversine for 12 h, released, and ceased to divide, compared to control cells. Results initially reported in Santaguida et al., 2017. Overlap with Mps1i DEG list noted in last column. (f) Hallmark Mps1i. List of MSigDB Hallmark gene sets that are significantly enriched (FDR ≤0.05) in Mps1i treated RPE1 cells. For all Hallmark lists, NES=normalized enrichment score, padj = false discovery rate adjusted p-values, size = number of genes in gene set, leading_edge = , sig_genes_in_geneset=the gene names of set genes that were significantly upregulated. (g) Hallmark He et al. List of MSigDB Hallmark gene sets that are significantly enriched in nocodazole treated RPE1 cells from He et al., 2018. (h) Hallmark Santaguida et al. List of MSigDB Hallmark gene sets that are significantly enriched in reversine treated RPE1 cells from Santaguida et al., 2017. (i) MN +DEG. List of differentially expressed genes (FDR ≤0.05) in Mps1i treated RPE1 cells with MN versus without MN. (j) MN +DEG log2FC. List of differentially expressed genes in Mps1i treated RPE1 cells with MN versus without MN filtered for log2 fold change above 0.58 or below –0.58. Overlap with Mps1i DEG list noted in last column. (k) Ruptured +DEG. List of differentially expressed genes (FDR ≤0.05) in Mps1i treated micronucleated RPE1 cells with only intact MN versus at least 1 ruptured MN. (l) Ruptured DEG +log2 FC. List of differentially expressed genes in Mps1i treated micronucleated RPE1 cells with only intact MN versus at least 1 ruptured MN MN filtered for absolute log2 fold change ≥0.58. Overlap with Mps1i DEG list noted in last column. (m) Hallmark MN+. List of MSigDB Hallmark gene sets that are significantly enriched in Mps1i treated RPE1 cells with MN. (n) Hallmark ruptured+. List of MSigDB Hallmark gene sets that are significantly enriched in Mps1i treated RPE1 cells with MN and at least 1 ruptured MN versus no ruptured MN. (o) Log2FC per replicate. List of log2 fold change values for Mps1i DEGs with absolute log2FC ≥0.58 broken out by MN +and rupture +replicate. Genes with all NA values for either all MN +or all rupture +replicates were excluded from analysis. enriched_in_rupture+_cluster=genes present in cluster enriched in increased expression over Mps1i DEGs.
- https://cdn.elifesciences.org/articles/101579/elife-101579-supp1-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/101579/elife-101579-mdarchecklist1-v1.docx





