Zinc finger homeobox-3 (ZFHX3) orchestrates genome-wide daily gene expression in the suprachiasmatic nucleus
Figures
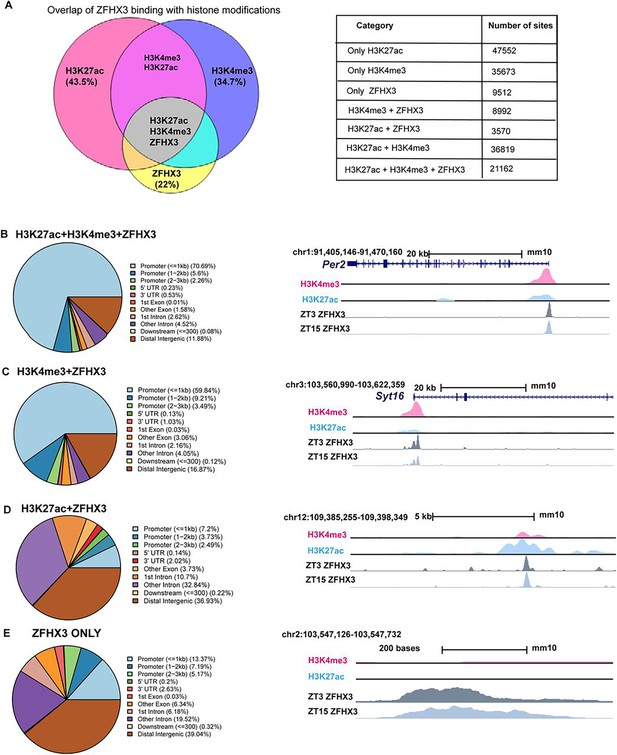
Genomic occupancy of ZFHX3 in the suprachiasmatic nucleus (SCN).
(A) Left: Venn diagram illustrating overlap of ZFHX3 peaks with histone marks, H3K4me3 and H3K27ac; right: table showing the number of sites co-occupied by ZFHX3 and histone marks. (B–E) Left: genomic feature distribution of ZFHX3 peaks with histone marks (ChIPSeeker); right: UCSC Genome Browser tracks showing histone modifications H3K4me3 (pink), H3K27ac (blue), and ZFHX3 (ZT3 = dark grey and ZT15 = light grey) normalized ChIP-seq read coverage at representative examples for each category. The chromosome location and scale (mm10 genome) indicated at the top.
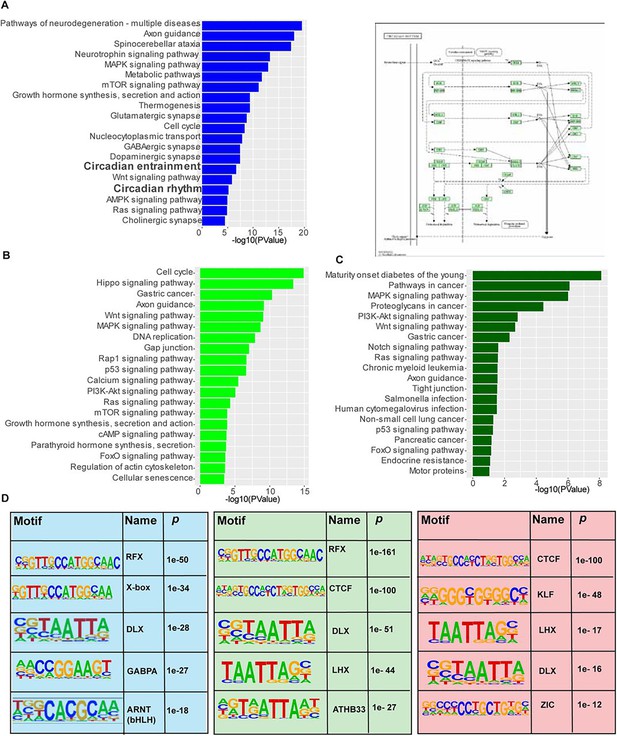
Gene ontology and motif analysis of ZFHX3 bound sites.
(A) Left: Functional annotation of the nearest neighbouring gene (TSS) to active promoter sites occupied by ZFHX3 and histone marks (H3K4me3 and H3K27ac) using KEGG pathway (DAVID); right: circadian rhythm pathway genes that showed co-occupancy of ZFHX3 and histone marks at TSS illustrated using KEGG pathway. (B, C) Functional annotation of the nearest neighbouring gene (TSS) to sites occupied by ZFHX3 + H3K4me3 (light green) and ZFHX3 alone (dark green). (D) Over-represented transcription factor binding motifs at ZFHX3 + H3K4me3+H3K27ac sites (shaded blue), ZFHX3 + H3K27ac sites (shaded green) and ZFHX3 + H3K4me3 (shaded red) by HOMER.
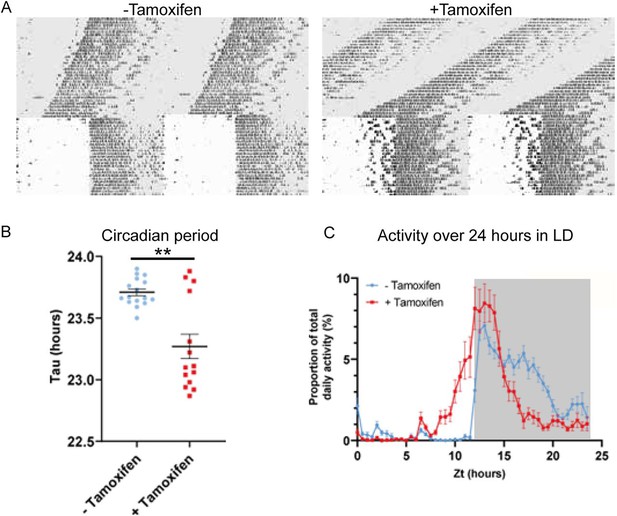
Circadian wheel running analysis of control and mutant animals.
(A) Double-plotted actograms showing wheel running activity of −Tamoxifen and +Tamoxifen animals through 32 days of constant darkness and 24 days under a 12 h light/dark cycle. Regions in grey denote periods in darkness. (B) +Tamoxifen animals show a reduced circadian period. (C) Graph showing the distribution of activity in −Tamoxifen and +Tamoxifen animals over 24 h in a 12 h light/dark cycle. Data is taken from the final 7 days the animals were in the light/dark cycle shown in (A). Grey denotes the dark period of the cycle.
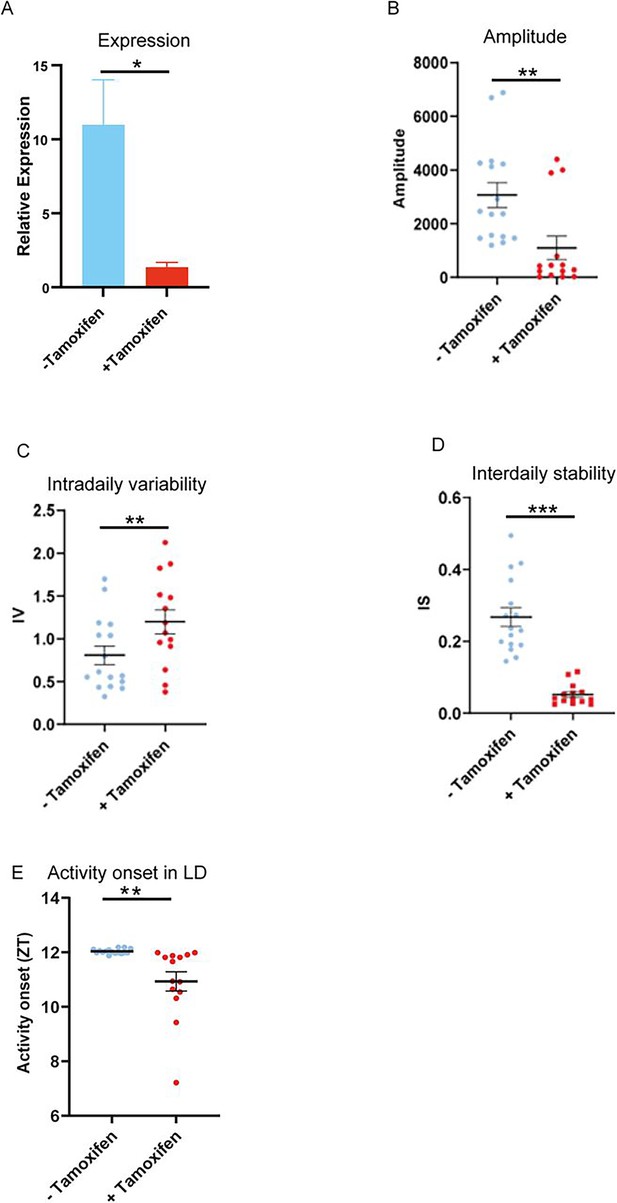
+Tamoxifen animals show changes in circadian wheel running parameters in conditions of constant darkness.
Compared to −Tamoxifen animals,+Tamoxifen show a significantly (A) reduced Zfhx3 gene expression (p<0.02, unpaired t-test) (B) reduced circadian amplitude, (C) increased intradaily variability (IV), (D) reduced interdaily stability (IS), and (E) advanced activity onset in LD.
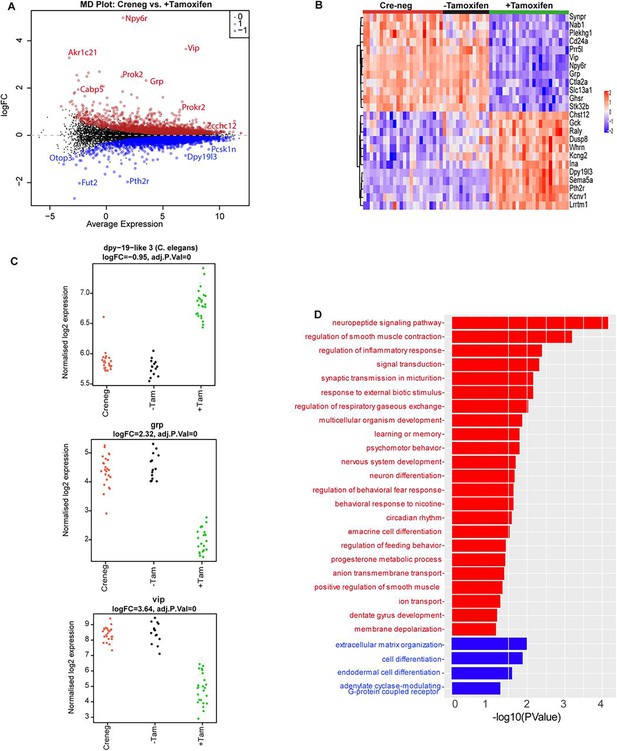
Effect of ZFHX3-KO on suprachiasmatic nucleus (SCN) transcriptome.
(A) Mean difference plot (MD-plot) showing downregulated (red) and upregulated (blue) gene expression after the loss of ZFHX3. (B) Heatmap showing normalized expression of top 25 (by adj pvalue) differential genes for the compared groups. (C) Stripcharts of differentially expressed genes (Dpy19l13, Grp, Vip). (D) Functional annotation of downregulated (red) and upregulated (blue) genes after ZFHX3-KO using the GO::BP (biological processes) terms by DAVID.
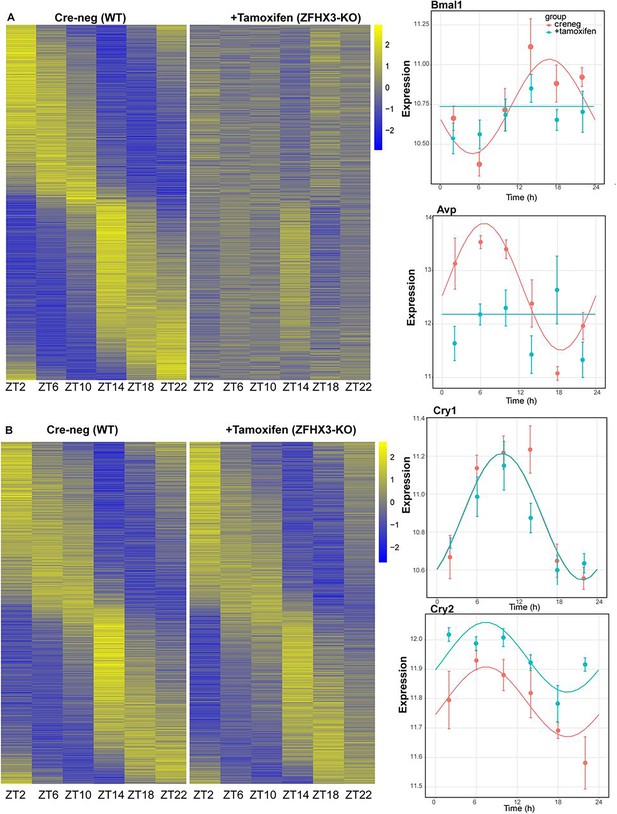
Effect of ZFHX3-KO on rhythmic gene expression.
(A) Left: heatmap showing loss of rhythmic gene expression after Zfhx3-KO (module 2) as computed by dryR statistical framework, Right: illustrative examples of daily abundance of module 2 genes, Bmal1 and Avp, in Cre-neg and +Tamoxifen conditions. (B) Left: heatmap showing no effect on rhythmic gene expression after Zfhx3-KO (module 4); right: illustrative examples of module 4 genes, Cry1 and Cry2, in Cre-neg and +Tamoxifen conditions.
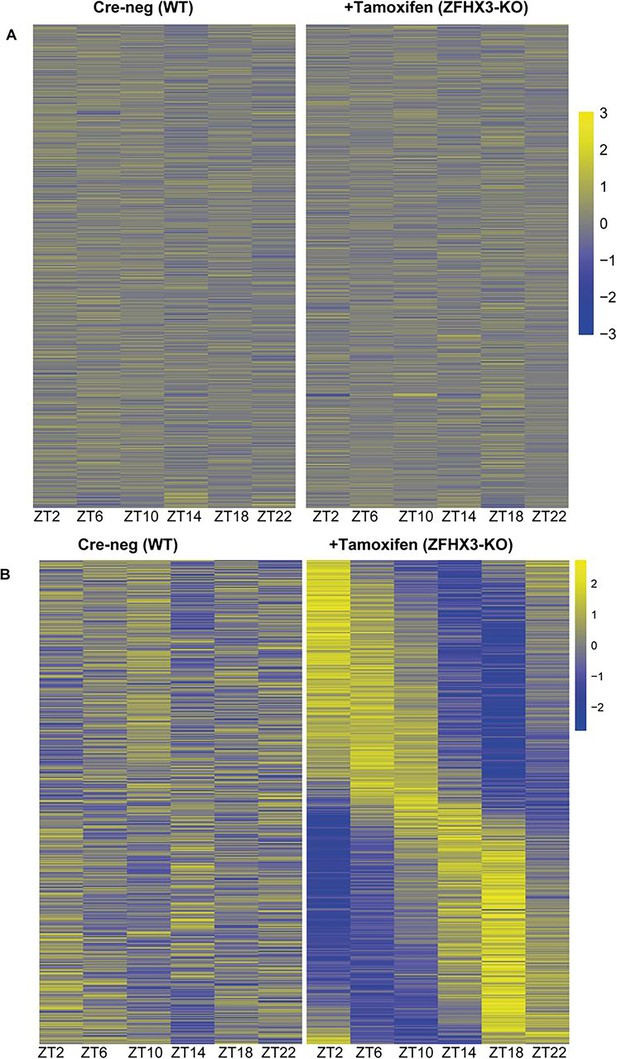
Effect of Zfhx3-KO on suprachiasmatic nucleus (SCN) transcriptome.
(A) Heatmap showing module 1 genes (n = 16,832) that show no rhythmic expression before and after Zfhx3-KO as computed by dryR statistical framework. (B) Heatmap showing module 3 genes (n = 403) that showed rhythmic expression after Zfhx3-KO.
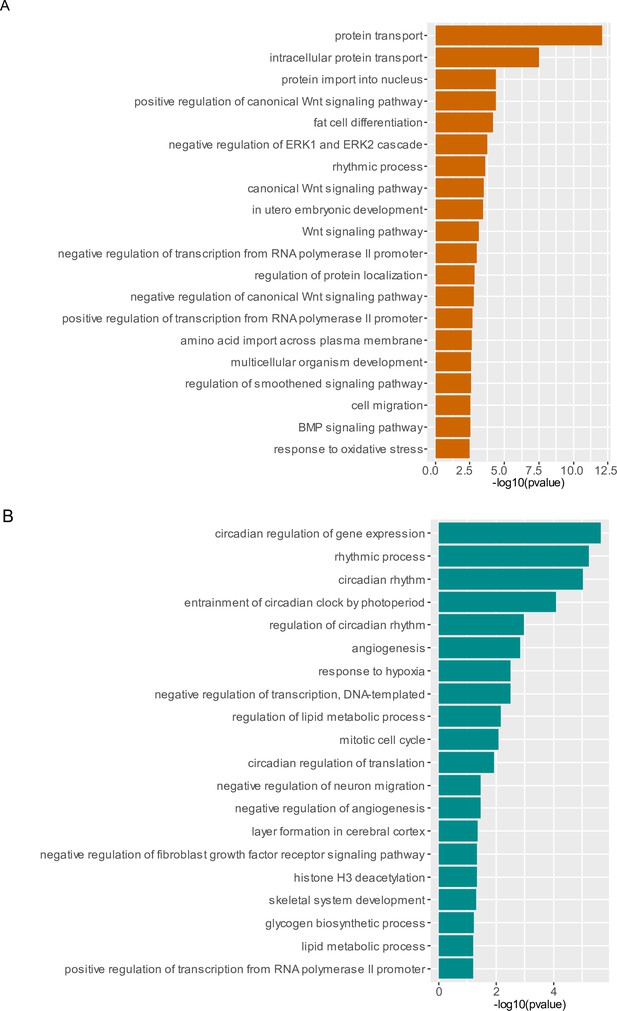
Functional annotation of rhythmic genes in the suprachiasmatic nucleus (SCN).
(A) Top 20 enriched functional pathways from genes that lost rhythmic transcription after gene deletion (module 2) and (B) genes that showed change in phase and/or amplitude (module 5) using the GO::BP (biological processes) terms by DAVID.
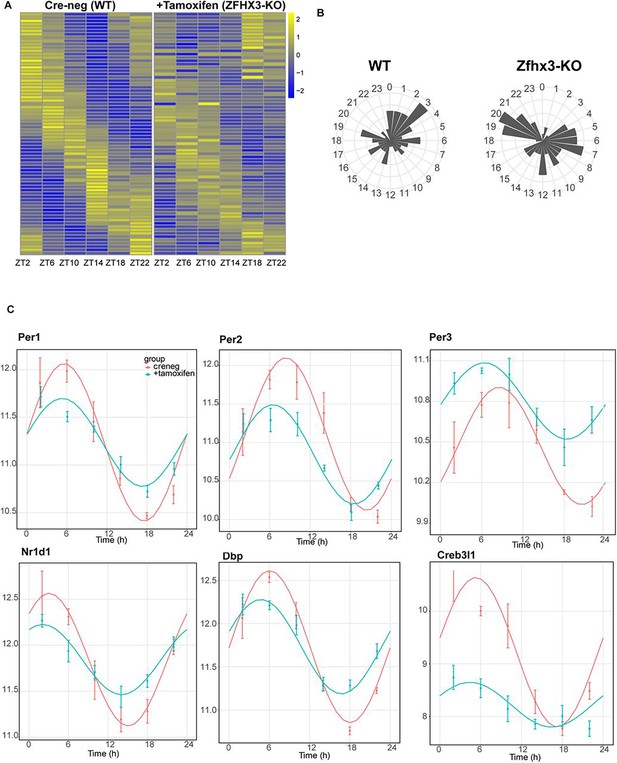
Effect of ZFHX3-KO on clock gene expression clustered in module 5.
(A) Heatmap showing change in rhythmic gene expression after Zfhx3-KO (module 5) as computed by dryR statistical framework. (B) 24 h radial plots showing the effect on phase (peak expression) of rhythmic genes in Cre-neg (WT) vs. +Tamoxifen (ZFHX3-KO) conditions. (C) Illustrative examples of daily abundance of module 5 genes, showing change in phase and amplitude in ZFHX3-KO condition (blue) compared to control (pink). The y-axis values represent normalized expression in the suprachiasmatic nucleus (SCN).
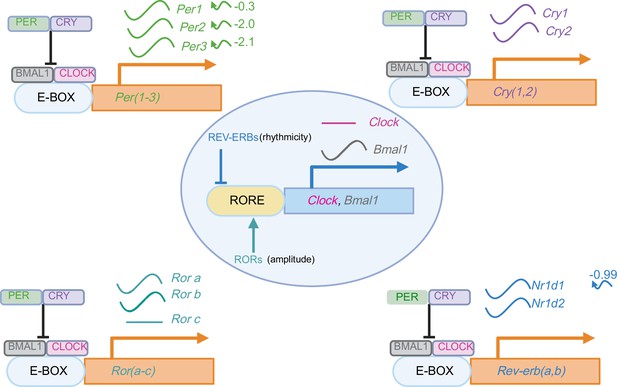
Effect of ZFHX3-KO on circadian clock.
The effect of loss of ZFHX3 on the primary and ancillary transcriptional-translational feedback loop (TTFL) in the suprachiasmatic nucleus (SCN). Blue and red arrows depict decreased/increased average gene expression in Zfhx3-deficient mice compared to the control, respectively. The waveform symbol next to the gene name depicts detected 24 h rhythmic expression in our study. The curly arrows (right) of the gene illustrate the change in phase of the rhythmic expression.
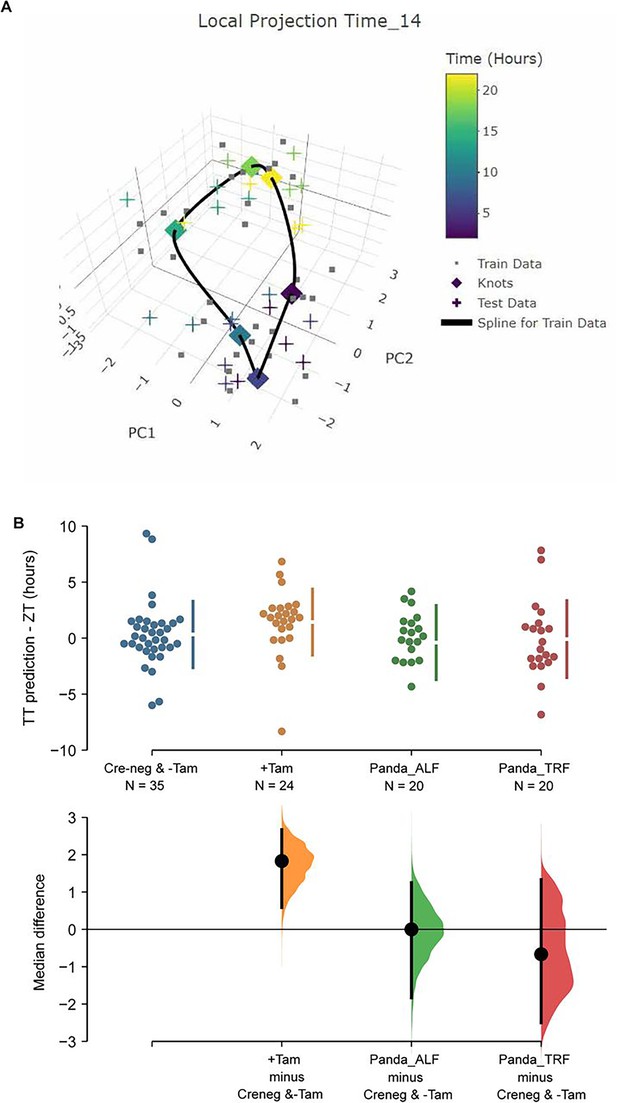
TimeTeller model suprachiasmatic nucleus (SCN) circadian clock in control and ZFHX3-KO mice.
(A) TimeTeller (TT) model build with the data of all 35 available SCN from Creneg and −Tam mice. Only the knots of the model at the collected ZTs, and predictions for test data of +Tam SCN according to when they were collected are shown. (B) Quantitation of SCN phases as difference between ZT to TT prediction for all genotypes of this study and previously published wild-type SCNs from ad libitum or night fed mice (Panda_ALF and Panda_TRF). Only the +Tam group is different from Creneg and −Tam control training data (p=0.0028).
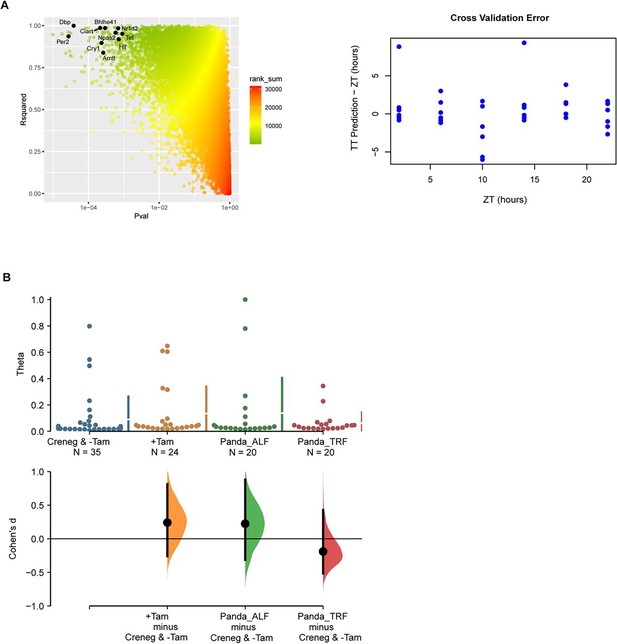
Suprachiasmatic nucleus (SCN) circadian clock using TimeTeller.
(A) Left: choosing genes for the TimeTeller model. Looking for good rhythmicity p-val and high R2 in population cosinor analysis leads to selection of a limited panel of well-behaved clock genes (named), that is, clock genes with adjusted pval<0.10 and R2 > 0.80. Right: results of the cross-validation of the TT model using the indicated genes in the left panel. (B) TimeTeller theta parameter is indistinguishable between the genotypes. Theta is a measure of certainty for phase prediction in TimeTeller, that is, how closely the test samples can be projected into the training model.
Tables
Effect of loss of ZFHX3 on core-clock genes in the suprachiasmatic nucleus.
| Gene | Rhythm | Phase | Amplitude |
|---|---|---|---|
| Per1 | Altered | –0.36 | 0.72 (low) |
| Per2 | Altered | –2.0 | 0.69 (low) |
| Per3 | Altered | –2.1 | 0.30 (low) |
| Cry1 | No change | - | - |
| Cry2 | No change | - | - |
| Bmal1 | Lost | - | - |
| Clock | No change | - | - |
| Nr1d1 | Altered | –0.99 | 0.67 (low) |
| Nr1d2 | No change | - | - |
| Rorb | Lost | - | - |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | C57BL/6J | Jackson Laboratories | RRID:IMSR_JAX:000664 | Maintained at MRC, Harwell |
| Strain, strain background (M. musculus) | UBC-cre (B6.Cg-Tg(UBC-cre/ERT2)1Ejb/J) | Jackson Laboratories | RRID:IMSR_JAX:007001 | |
| Strain, strain background (M. musculus) | Zfhx3Flox backcrossed to C57BL/6J | Wilcox et al., 2017 | MRC, Harwell | |
| Antibody | Anti-ZFHX3 (rabbit polyclonal) | Parsons et al., 2015 | 12 μl | |
| Software, algorithm | ClockLab | https://actimetrics.com/products/clocklab/clocklab-analysis-version-6/ | RRID:SCR_014309 | |
| Software, algorithm | MACS | https://taoliu.github.io/MACS/ | RRID:SCR_013291 | v2.1.0 |
| Software, algorithm | Diffbind | http://bioconductor.org/packages/release/bioc/vignettes/DiffBind/inst/doc/DiffBind.pdf | RRID:SCR_012918 | v2.10.0 |
| Commercial assay or kit | RNeasy Micro Kit | Qiagen | QIAGEN, Cat 74004 | |
| Commercial assay or kit | PrecisionPLUS OneStep RT-qPCR Master Mix with ROX | PrimerDesign | https://www.primerdesign.co.uk/media/downloads/df6ea8f4-e857-11ee-8882-fa163e309ced.pdf | |
| Chemical compound, drug | Tamoxifen | Cambridge Bioscience | CAY13258-1g | |
| Software, algorithm | dryR | Weger et al., 2021 | https://github.com/naef-lab/dryR | |
| Software, algorithm | TimeTeller | Vlachou et al., 2024 | https://github.com/VadimVasilyev1994/TimeTeller |
Additional files
-
Supplementary file 1
List of ZFHX3-binding sites in the SCN.
- https://cdn.elifesciences.org/articles/102019/elife-102019-supp1-v1.xlsx
-
Supplementary file 2
Circadian wheel running analysis comparing Zfhx3Flox/Flox;UBC animals dosed with Tamoxifen (+Tamoxifen) to those without Tamoxifen dosing (-Tamoxifen).
- https://cdn.elifesciences.org/articles/102019/elife-102019-supp2-v1.docx
-
Supplementary file 3
Gene expression changes after the loss of ZFHX3.
- https://cdn.elifesciences.org/articles/102019/elife-102019-supp3-v1.xlsx
-
Supplementary file 4
Effect of ZFHX3-KO on rhythmic gene expression in the SCN.
- https://cdn.elifesciences.org/articles/102019/elife-102019-supp4-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/102019/elife-102019-mdarchecklist1-v1.pdf





