Autologous P63+ lung progenitor cell transplantation in idiopathic pulmonary fibrosis: a phase 1 clinical trial
Figures
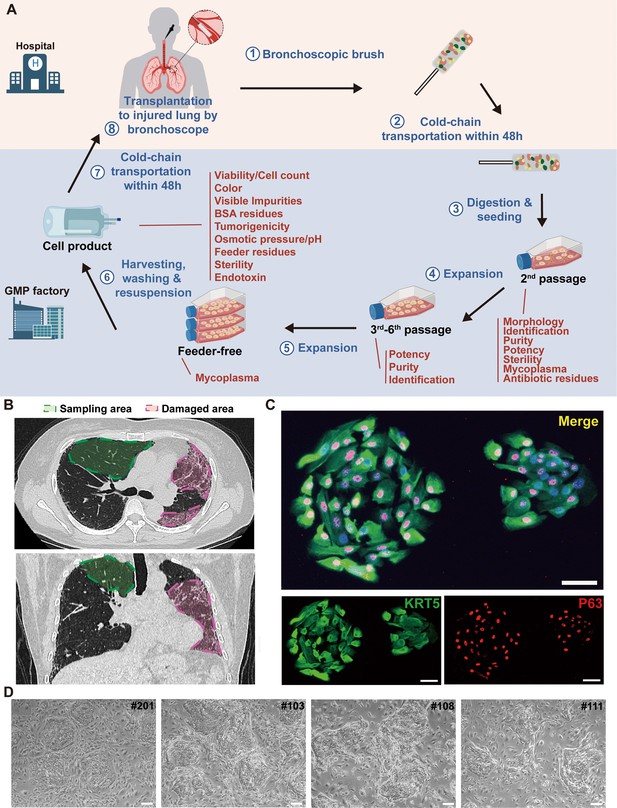
Cloning and characterization of P63+ lung progenitor cells isolated from IPF patients.
(A) A schematic diagram illustrating the manufacturing, quality control, and clinical administration of autologous P63+ lung progenitor cell product REGEND001. (B) Representative lung CT images of sampling and damaged areas of the IPF patients. Green circle: sampling area; red circle: damaged area. (C) Immunostaining of clonogenic cells with basal cell markers KRT5 (green) and P63 (red). Scale bar, 30 μm. (D) Representative images of cell clones showing distinct morphology from different individual patients (indicated by patient numbers). Scale bar, 80 μm.
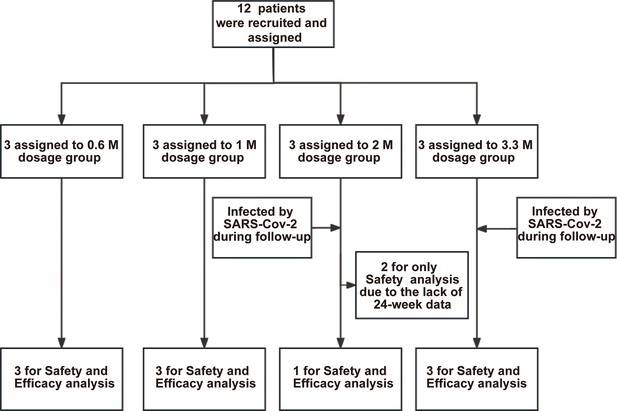
Profile of current clinical trial.
In total 12 patients were recruited for the trial and assigned to 4 dose groups. All patients in the 2 M and 3.3 M groups were infected by SARS-CoV-2 during the follow-up period. Two patients (#108 and #203) in the 2 M group were excluded from the efficacy analysis because they missed the 24-week visit due to the lockdown policy during COVID-19 pandemic. The remaining 10 patients were subjected to both safety and efficacy analysis.
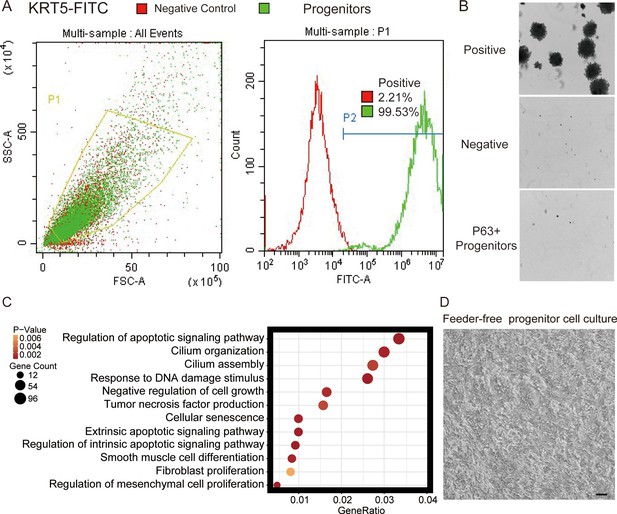
Quality control of cultured lung progenitor cells.
(A) FACS gating strategy for cell identity and purity test. KRT5 was immunostained as a marker of lung progenitor cells. SSC, side-scatter, FSC, forward scatter. (B) Soft agar assay, also known as a tumorigenicity test, showing the lack of tumor formation by P63+ lung progenitor cells. Human melanoma cells were used as a positive control, while growth-arrested 3T3 cells served as a negative control. (C) Gene ontology (GO) terms that were significantly enriched in basal cells isolated from distal lungs of IPF patients. (D) Representative image of feeder-free cultured lung progenitor cells before being harvested for transplantation. Scale bar, 80 μm.
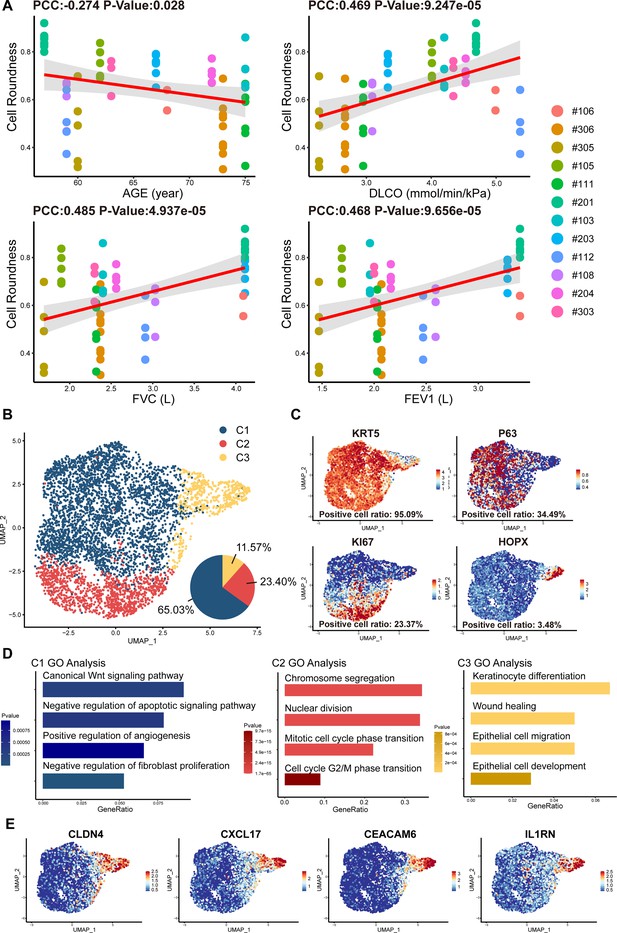
Cell morphology and single-cell RNA sequencing analysis of P63+ lung progenitor cells isolated from IPF patients.
(A) Correlation analysis of cell colony roundness with patient age and lung functions. Each point represents one single cell colony, and each color represents an individual patient. (B) Uniform manifold approximation and projection (UMAP) plot showing three subpopulations of cells cloned from a patient. (C) Feature plots of representative cell markers. (D) Gene ontology enrichment analysis of the differentially expressed genes identified in three subpopulations. (E) Feature plots of representative variant progenitor cell markers.
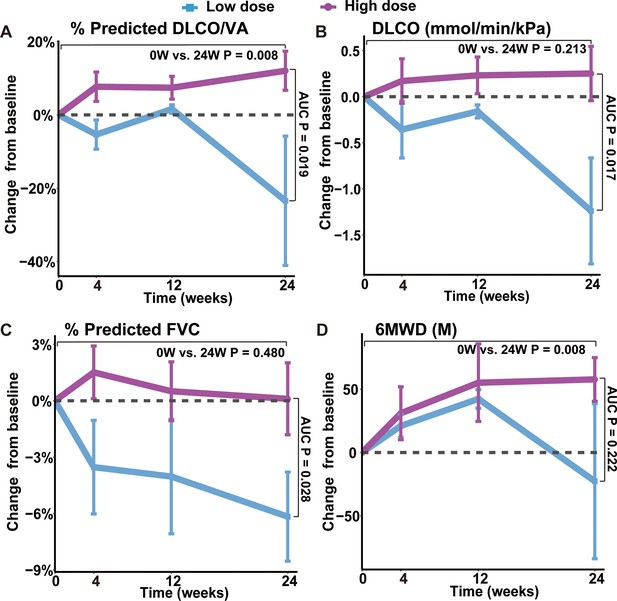
Changes of lung functions and 6MWD at different time points after REGEND001 treatment.
(A–D) The plots indicated mean (S.E.M.) changes from baseline in absolute value of DLCO/VA percentage to predicted value, DLCO absolute value, FVC percentage to predicted value and 6MWD. The low dose group included the 3 patients in the 0.6 M group, while the high dose groups included the 7 patients in the 1 M, 2 M, and 3.3 M dose groups. p Values between two groups were calculated according to the area under curve (AUC) and are labeled as ‘AUC P’. p Values for the change from baseline to 24 weeks within high dose group are labeled as ‘0 W vs. 24 W P’.
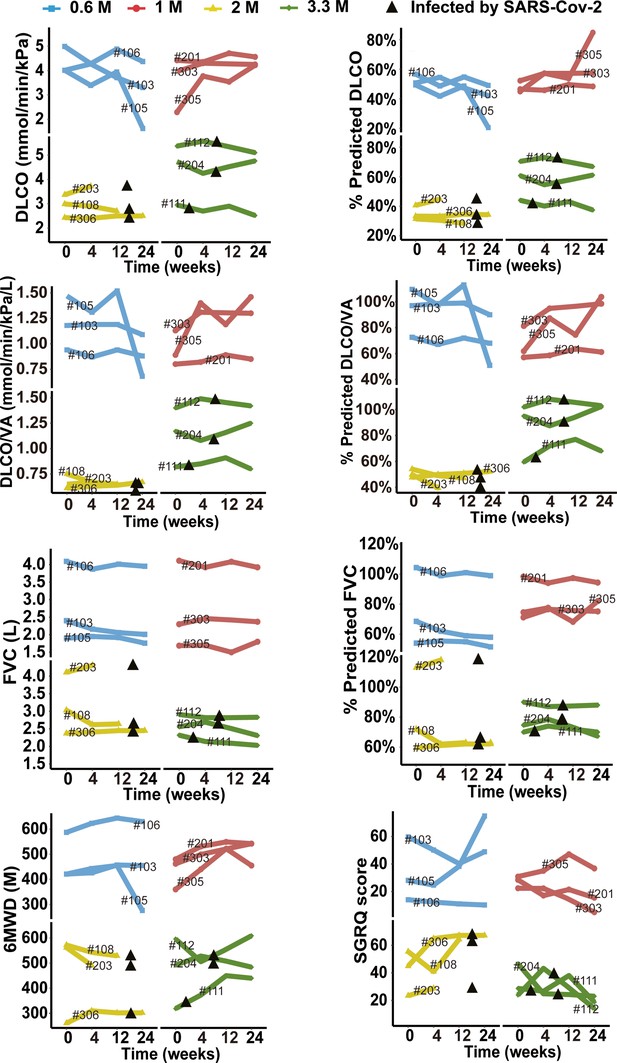
Changes of predicted DLCO%, DLCO, predicted DLCO/VA%, DLCO/VA, predicted FVC%, FVC, 6MWD and SGRQ score at different time points after cell therapy.
The line plot displays the changes of various indicators for patients after cell therapy. Each line represents a patient, and the patient number is labeled along the line. The black triangles indicate the time when the patients were infected by SARS-CoV-2.
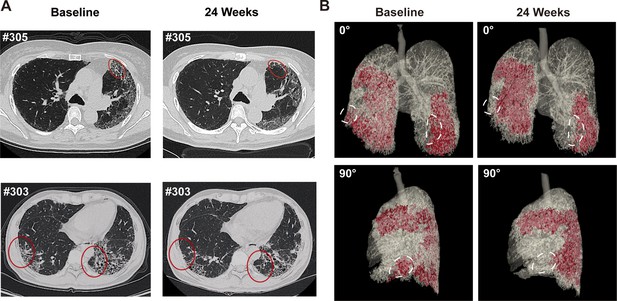
Representative lung CT image before and after REGEND001 treatment.
(A) Representative lung CT images of the Patient #305 and #303 at baseline and 24 weeks after REGEND001 treatment. Red circle indicated resolution of honeycomb lesion. (B) Three-dimensional visualization of consecutive CT images of the Patient #305. The red zone indicated the lung damaged areas (reticulation and honeycomb) before and after cell therapy. The white circle indicated resolution of the lesion in lower lobes.
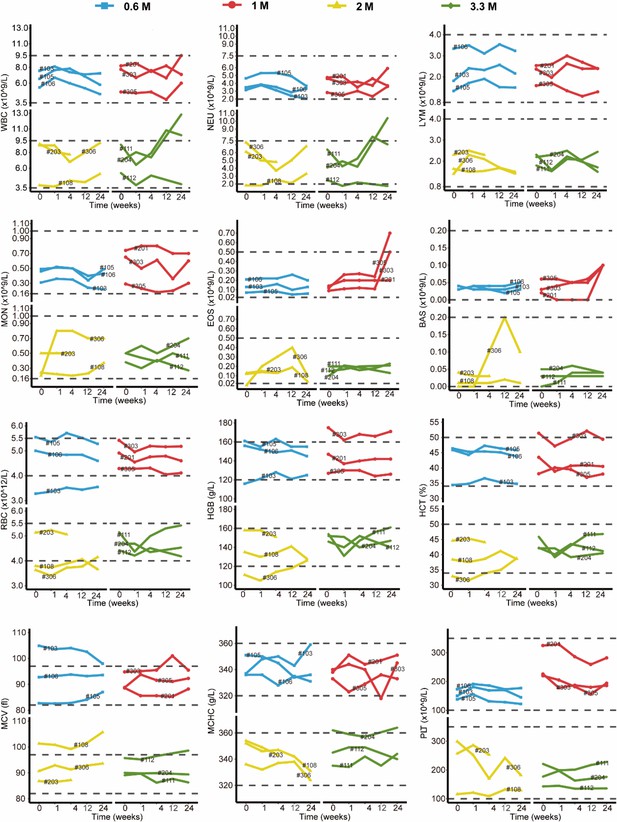
Changes in clinical laboratory evaluations of blood routine following cell therapy.
The line plot displays the changes of various indicators of blood routine for patients over time, with each line representing one patient, and the patient number is marked alongside the line. The horizontal dashed line indicates the normal reference range.
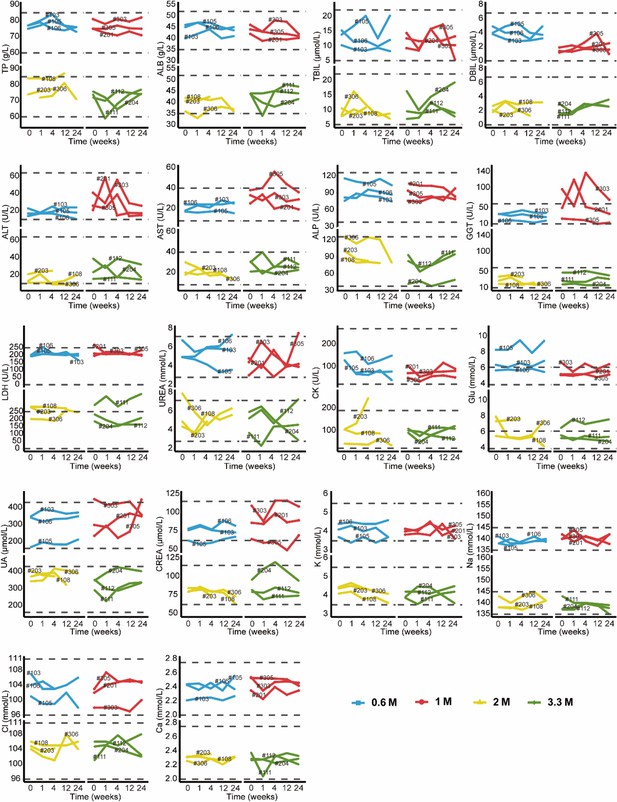
Changes in clinical laboratory evaluations of blood biochemistry following cell therapy.
The line plot displays the changes of various blood biochemistry indicators over time, with each line representing a patient, and the patient number is labeled alongside the line. The horizontal dashed line indicates the normal reference range.
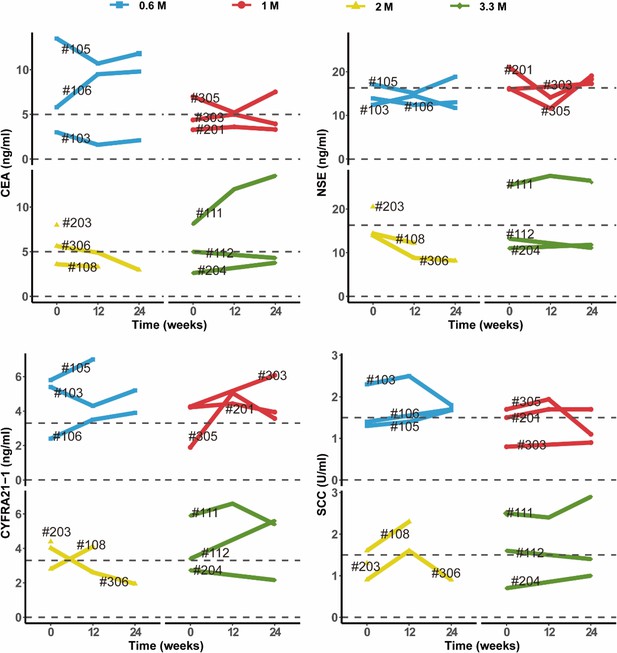
Changes in clinical laboratory evaluations of serum tumor markers following cell therapy.
The line plot displays the changes of various tumor markers in blood sample of patients over time, with each line representing one patient, and the patient number is labeled alongside the line. The horizontal dashed line indicates the normal reference range for that indicator.
Tables
Baseline demographic and clinical characteristics.
| Dose | 0.6 M | 1 M | 2 M | 3.3 M | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | #103 | #105 | #106 | #201 | #303 | #305 | #108 | #203 | #306 | #111 | #112 | #204 | |
| Demographics | |||||||||||||
| Age (years) | 75 | 62 | 68 | 57 | 63 | 60 | 59 | 67 | 73 | 75 | 59 | 72 | 0.6916 |
| Sex | Male | Male | Male | Male | Male | Female | Male | Male | Male | Male | Male | Male | 1.0000 |
| Body mass index (kg/m2) | 22.91 | 26.04 | 23.07 | 23.93 | 22.27 | 21.49 | 20.69 | 28.39 | 24.42 | 26.93 | 26.44 | 24.15 | 0.2334 |
| Cell characteristics | |||||||||||||
| Viability Rate (%) | 94.00 | 91.00 | 95.50 | 97.00 | 97.00 | 98.00 | 99.00 | 99.00 | 97.00 | 98.00 | 96.00 | 97.00 | 0.0503 |
| Biological Efficacy (%) | 59.00 | 36.00 | 44.00 | 57.00 | 47.00 | 78.00 | 48.00 | 86.00 | 76.00 | 56.00 | 40.00 | 64.00 | 0.4858 |
| IPF characteristics | |||||||||||||
| DLCO (%predicted) | 50.70 | 49.32 | 56.64 | 46.48 | 52.17 | 44.90 | 31.41 | 40.60 | 33.29 | 44.03 | 70.38 | 60.72 | 0.8516 |
| DLCO/VA (%predicted) | 97.00 | 109.60 | 72.60 | 57.10 | 81.30 | 61.90 | 54.00 | 49.80 | 47.60 | 59.50 | 102.00 | 95.00 | 0.4315 |
| FVC (%predicted) | 68.60 | 54.10 | 104.40 | 98.37 | 71.20 | 74.60 | 72.00 | 112.91 | 59.50 | 70.00 | 90.00 | 74.69 | 0.8790 |
| Pre-study medication for IPF | |||||||||||||
| Pirfenidone | √ | - | √ | √ | √ | - | √ | √ | √ | √ | √ | - | 1.0000 |
| Nintedanib | - | - | - | - | - | - | - | - | √ | - | √ | - | 1.0000 |
| Acetylcysteine | - | - | - | √ | √ | - | √ | - | - | - | √ | √ | 0.5909 |
| Tiotropium Bromide | - | - | - | - | - | - | - | - | - | - | - | √ | 1.0000 |
| Concomitant medication for IPF | |||||||||||||
| Pirfenidone | √ | - | √ | √ | √ | - | √ | √ | √ | √ | √ | - | 1.0000 |
| Acetylcysteine | - | - | - | √ | √ | - | √ | - | - | - | √ | √ | 0.5909 |
Adverse events*.
| Events | No. (%) of Patients With Adverse Events | |||
|---|---|---|---|---|
| 0.6 M | 1M | 2 M | 3.3 M | |
| Any adverse event | 2 (16.67) | 3 (25.00) | 3 (25.00) | 3 (25.00) |
| Serious adverse events | 2 (16.67) | 0 | 1 (8.33) | 1 (8.33) |
| Fatal adverse events | 0 | 0 | 0 | 0 |
| Adverse events leading to discontinuation | 0 | 0 | 0 | 0 |
| Frequent adverse events:† | ||||
| Likely related to Bronchoscopy | ||||
| Hemoptysis | 1 (8.33) | 2 (16.67) | 1 (8.33) | 1 (8.33) |
| Fever | 0 | 1 (8.33) | 2 (16.67) | 1 (8.33) |
| White blood cell counts increased | 0 | 1 (8.33) | 0 | 1 (8.33) |
| Productive cough | 1 (8.33) | 1 (8.33) | 0 | 0 |
| Bronchitis | 1 (8.33) | 0 | 0 | 0 |
| Others | ||||
| COVID-19 | 0 | 0 | 3 (25.00) | 3 (25.00) |
| Bronchitis | 2 (16.67) | 0 | 0 | 1 (8.33) |
| Hypokalemia | 1 (8.33) | 0 | 1 (8.33) | 0 |
-
*
Shown are adverse events occurred in patients from baseline examination to the end of the study visit. Events are listed in descending order of frequency.
-
†
The frequent adverse events were defined as those with an incidence ≥2 patients, which were ordered by frequency of occurrence here.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/102451/elife-102451-mdarchecklist1-v1.docx
-
Supplementary file 1
CTR20210349 (English Version).
- https://cdn.elifesciences.org/articles/102451/elife-102451-supp1-v1.pdf
-
Supplementary file 2
Ethics Committee Approval (English Version).
- https://cdn.elifesciences.org/articles/102451/elife-102451-supp2-v1.pdf
-
Supplementary file 3
Informed consent forms (English Version).
- https://cdn.elifesciences.org/articles/102451/elife-102451-supp3-v1.pdf
-
Supplementary file 4
Protocol for IPF Clinical Trial.
- https://cdn.elifesciences.org/articles/102451/elife-102451-supp4-v1.pdf





