Preclinical systematic review of CCR5 antagonists as cerebroprotective and stroke recovery enhancing agents
Figures
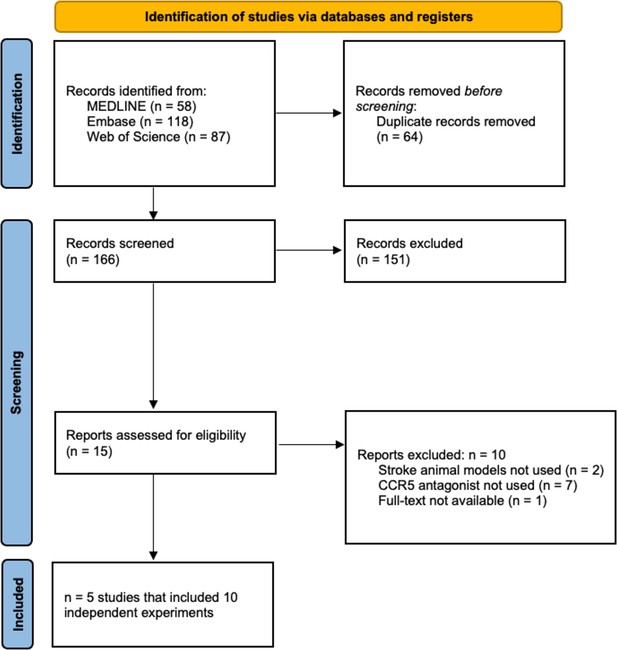
C-C chemokine receptor type 5 (CCR5) antagonists reduce infarct volume.
Data is presented as a forest plot with standardized mean differences and 95% confidence intervals. Effect sizes <0 favours drug treatment and >0 favours control. Data is stratified by timing of CCR5 antagonist administration (pre- or post-stroke induction). The ‘RE Model for All Studies’ represents a pooled estimate of the CCR5 antagonist drug effect on infarct volume from all studies combined. Separate pooled estimates are also reported for post- and pre-stroke CCR5.
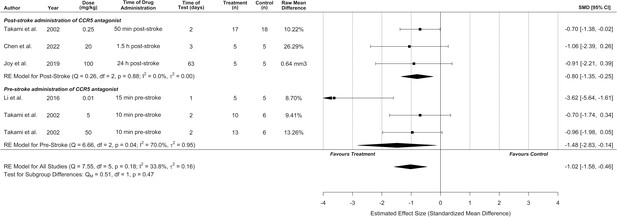
Sensitivity analysis for all included studies that reported infarct volume on a percentage scale.
Overall, both the original analysis using standardized mean differences (Figure 2) and the sensitivity analysis demonstrate a significant cerebroprotective effect of C-C chemokine receptor type 5 (CCR5) antagonists. Data is presented as a forest plot with mean differences and 95% confidence intervals. Effect sizes <0 favours CCR5 antagonist treatment and >0 favours control. Data is stratified by timing of CCR5 antagonist administration (pre- or post-stroke induction). The ‘RE Model for All Studies’ represents a pooled estimate of the CCR5 antagonist drug effect on infarct volume from all studies combined. Separate pooled estimates are also reported for post- and pre-stroke CCR5. T (n) = number of animals in the CCR5 treatment group. C (n) = number of animals in the control group.
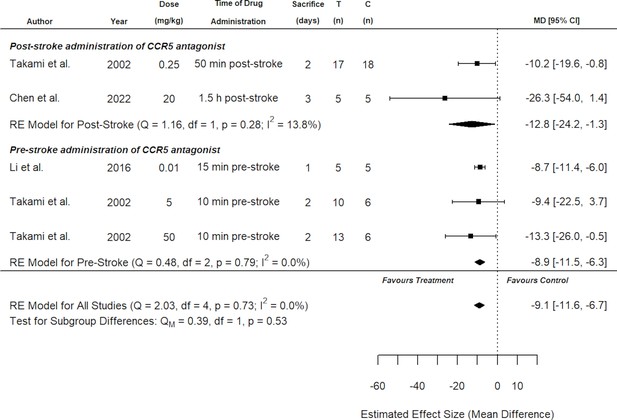
Subgroup analysis for all included studies of pre- and post-stroke C-C chemokine receptor type 5 (CCR5) antagonist administration that reported infarct volume.
Each row represents pooled estimate data from studies within that subgroup. Data is presented as a forest plot with a standardized mean difference and 95% confidence intervals. The I2 value represents the statistical heterogeneity within each subgroup. Effect sizes <0 favours drug treatment and >0 favours control. The ‘RE Model for All Studies’ represents a pooled estimate of the CCR5 antagonist drug effect on infarct volume from all studies combined. Considering all six experiments (irrespective of administration timing of CCR5 antagonist), subgroup analysis demonstrated no difference in effect size when considering route of administration, stroke model, species type, drug, dose, or whether behaviour tests were assessed.
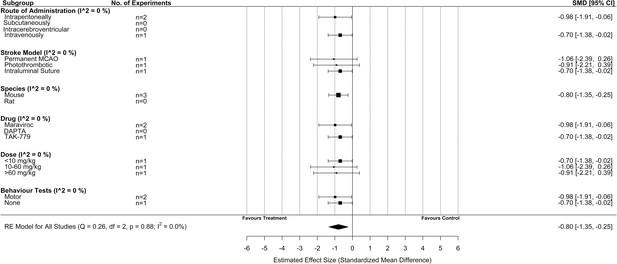
Subgroup analysis for all included studies of post-stroke drug administration of a C-C chemokine receptor type 5 (CCR5) antagonist that reported infarct volume.
Each row represents pooled estimate data from studies within that subgroup. Data is presented as a forest plot with a standardized mean difference and 95% confidence intervals. The I2 value represents the statistical heterogeneity within each subgroup. Effect sizes <0 favours drug treatment and >0 favours control. The ‘RE Model for All Studies’ represents a pooled estimate of the CCR5 antagonist drug effect on infarct volume from all post-stroke drug administration of a CCR5 antagonist studies combined. Subgroup analysis considering only studies that administered CCR5 antagonists post-stroke induction demonstrated no difference in effect size when considering any of the subgroups.
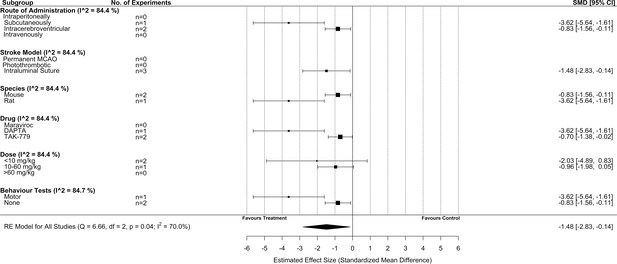
Subgroup analysis for all included studies of pre-stroke drug administration of a C-C chemokine receptor type 5 (CCR5) antagonist that reported infarct volume.
Each row represents pooled estimate data from studies within that subgroup. Data is presented as a forest plot with a standardized mean difference and 95% confidence intervals. The I2 value represents the statistical heterogeneity within each subgroup. Effect sizes <0 favours drug treatment and >0 favours control. The ‘RE Model for All Studies’ represents a pooled estimate of the CCR5 antagonist drug effect on infarct volume from all pre-stroke drug administration of a CCR5 antagonist studies combined. Subgroup analysis of studies that administered CCR5 pre-stroke induction suggested that infarct volume was reduced to a greater extent by the intraluminal suture stroke model versus other models (p = 0.04), in rats versus mice (p = 0.01), with DAPTA versus TAK-779 (p = 0.01), and when behaviour tests were performed versus not (p = 0.01).
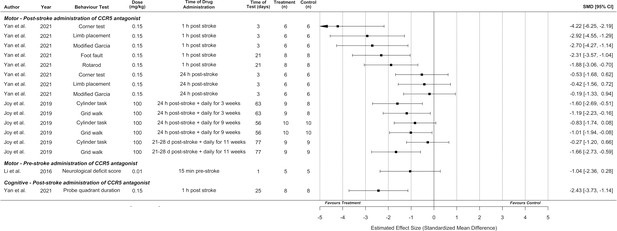
Synthesis without meta-analysis for all included preclinical C-C chemokine receptor type 5 (CCR5) antagonist studies that reported motor and/or cognitive behavioural outcomes.
Data is presented as a forest plot with a standardized mean difference and 95% confidence intervals. Effect sizes <0 favours drug treatment and >0 favours control.
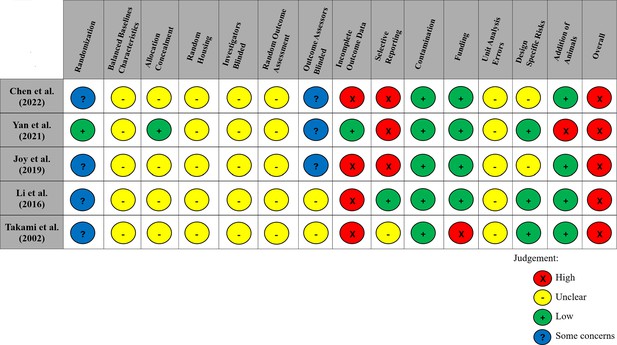
Modified risk of bias traffic light plot in accordance with the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) tool.
Yellow represents an unclear risk of bias, green represents a low risk of bias, and red represents a high risk of bias. Blue represents some concerns of a risk of bias. The risk of bias was ‘unclear’ across all studies for the domains of baseline characteristics because of missing data in the studies, random housing because no details on this domain were reported, and random outcome assessment because no details of how cohorts of animals were selected to perform certain outcomes nor how the order of outcome assessment proceeded. Four studies did not report on allocation concealment, and two studies did not report on blinding investigators and outcome assessors and were deemed as having an ‘unclear’ risk of bias. 80% of studies exhibited a ‘high’ risk for incomplete outcome data. Similarly, three studies (60%) had a ‘high’ risk of selective outcome reporting since all expected outcomes discussed in the methods of the articles did not align with their results. Other potential sources of bias considered included the source of funding (industry funded), contamination of pooling drugs (additional treatment which might influence or bias the result), unit error analysis (all animals receiving the same intervention are caged together, but analysis was conducted as if every single animal was one experimental unit), design-specific risks of bias (reporting details of which animals performed the same or different outcomes), and the addition of new animals to replace dropouts from the original population. Two studies (40%) had a ‘high’ risk in at least one of these additional categories.
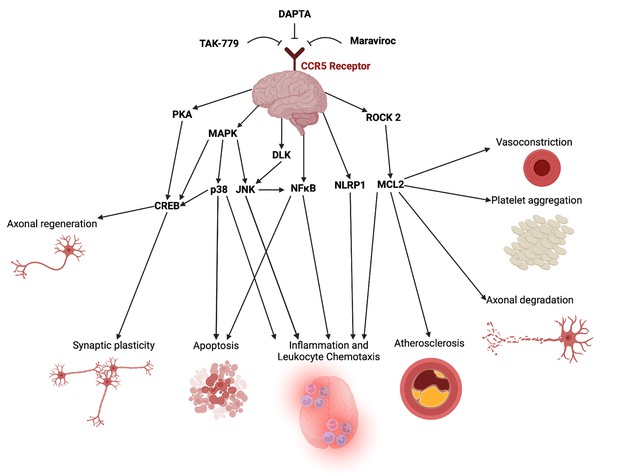
Potential mechanistic pathways and proposed domains of biological activity underlying CCR5 antagonist’s cerebroprotective and neural repair effects post-stroke described in the included studies.
A list of mechanistic evidence supporting these pathways was extracted from included studies in Appendix 1—table 1. This figure was created using BioRender.com.
Tables
Summary of study and animal model characteristics of included articles.
| Author | Year | Country | Species | Strain | Stroke type | Stroke model | Sex | Weight | Age |
|---|---|---|---|---|---|---|---|---|---|
| Li et al., 2016 | 2016 | China | Rat | Wistar | Ischaemic | Intraluminal suture | Male | 260–300 g | N/A |
| Takami et al., 2002 | 2002 | Japan | Mice | ddY | Ischaemic | Intraluminal suture | Male | N/A | 4 weeks |
| Chen et al., 2022 | 2022 | China | Mice | C57/BL6 | Ischaemic | Permanent middle cerebral artery occlusion | Male | 25–30 g | 8–10 weeks |
| Yan et al., 2021 | 2021 | China | Mice | CD1 | Haemorrhagic | Intracerebral haemorrhagic | Male | 30–40 g | N/A |
| Joy et al., 2019 | 2019 | USA | Mice | C57/BL6 | Ischaemic | Photothrombotic | Male | 25–30 g | 8–20 weeks |
Summary of intervention characteristics.
| Author | Drug | Dose (mg/kg) | Route | Timing | Doses(total #) | Outcomes measured(treatment n/control n) | Outcome window(post-stroke) |
|---|---|---|---|---|---|---|---|
| Li et al., 2016 | DAPTA (D-Ala-Peptide T-Amide) | 0.01 | SC | 15-min pre-stroke | 1 |
| 24 hr |
| Takami et al., 2002 | TAK-779 | 5 | ICV | 10-min pre-stroke | 1 |
| 48 hr |
| 50 | ICV | 10-min pre-stroke |
| ||||
| 0.25 | IV | 50-min post-stroke |
| ||||
| Chen et al., 2022 | Maraviroc | 20 | IP | 1.5-, 24-, and 48-hr post-stroke | 3 |
| 72 hr |
| Yan et al., 2021 | Maraviroc | 0.15 | IN | 1-hr post-stroke | 1 |
| 72 hr |
| 3 weeks | ||||||
| 25 days | ||||||
| 24-hr post-stroke |
| 72 hr | |||||
| Joy et al., 2019 | Maraviroc | 100 | IP | 24-hr post-stroke through daily injections for 9 weeks | 63 |
| 9 weeks |
| 8 weeks | ||||||
| 24-hr post-stroke then daily for 3 weeks | 21 |
| 9 weeks | ||||
| 3–4 weeks post-stroke then daily for 11 weeks | 56 |
| 11 weeks |
-
ICV = intracerebroventricular; IN = intranasal; IP = intraperitoneal; IV = intravenous; SC = subcutaneous.
Alignment of included preclinical studies with STAIR recommendations and CAMAROS trial parameters.
| Recommendation | Overall preclinical evidence | CAMAROS trial alignment | Notes |
|---|---|---|---|
| Candidate treatment qualification | |||
| Establish treatment dose–response | Yes | No |
|
| Treatment given after clinically relevant delayed times (1- to 4.5-hr post-stroke) | Yes | Yes |
|
| Both histologic and behavioural outcomes at acute (1–3 days) and chronic (7–30 days) time points | Yes | Yes |
|
| Treatment reaches presumed target and causes expected physiological effects that can be assessed with a clinically relevant biomarker | Yes | NA |
|
| Treatment able to pass the blood–brain barrier | Yes | NA |
|
| Preclinical assessment/validation | |||
| Aging/adult age | No | No |
|
| Male and female animals | No | No |
|
| Animals with comorbidities | No | No |
|
| Evidence from two or more laboratories | Yes | No |
|
| Gyrencephalic species | No | No |
|
| Tests during the awake phase of animals | No | No |
|
Alignment of included preclinical studies with additional STAIR/SRRR items and CAMAROS trial parameters.
| Recommendation | Preclinical evidence | CAMAROS alignment | Notes |
|---|---|---|---|
| Testing in both permanent and transient occlusion models | Yes | Yes |
|
| Monitoring of treatment effects on physiological parameters, including temperature, both during and after ischaemia | No | NA |
|
| Testing interaction with thrombolytics and other medications commonly administered in acute stroke | No | No |
|
| Animal model should produce infarction similar in relative size and location to that observed in humans | Yes | Yes |
|
| For studies claiming recovery effects, analysis of infarct volume should be performed to show equivalency of injury in the treated and stroke control groups | No | NA |
|
| For studies claiming recovery effects, tissue repair/neuroplasticity processes should be quantified and directly related to behavioural recovery | Yes | NA |
|
| For studies claiming recovery effects, initial impairment with spontaneous recovery that plateaus significantly below pre-stroke performance | No | NA |
|
| Behavioural effects should be assessed across a battery of domains, including both skilled and spontaneous upper limb and hindlimb use | No | No |
|
| Mechanism of action should be assessed through gain of function/loss of function studies and directly associated to behavioural effects | Yes | NA |
|
| Testing interactions of treatment with clinically inspired best practice, such as training or enrichment | No | No |
|
List of outcomes used to determine potential mechanisms of action, by study.
| Paper | Mechanistic evidence presented |
|---|---|
| Takami et al., 2002 – TAK-779 |
|
| Li et al., 2016 – DAPTA |
|
| Joy et al., 2019 – maraviroc |
|
| Yan et al., 2021 – maraviroc |
|
| Chen et al., 2022 – maraviroc |
|
Readiness of CCR5 antagonists for translation based on the PRIMED2 tool.
| PRIMED2 domain | TAK-779 | DAPTA | Maraviroc | CCR5 antagonism (all agents) |
|---|---|---|---|---|
| Sex of animals (0 – male OR female; 2 – both sexes) | 0 | 0 | 0 | 0 |
| Age of animals (0 – young only; 2 – older adults) | 0 | 0 | 0 | 0 |
| Species and strains (0 – one rodent species/strain; 1 – ≥2 rodent species/strains; 2 – rodents AND primates) | 0 | 0 | 1 | 1 |
| Reproducibility (0 – one species, one laboratory; 1 – ≥2 species, one lab OR ≥2 labs, one species; 2 – ≥2 species AND ≥2 labs) | 0 | 0 | 2 | 2 |
| Treatment time epoch (0 – no significant benefit; 1 – benefit in one epoch; 2 – benefit in ≥2 epochs) | 1 | 1 | 2 | 2 |
| Baseline comorbidities (0 – healthy animals only; 1 – one comorbid condition; 2 – ≥2 comorbid conditions) | 0 | 0 | 0 | 0 |
| Feasible time window (0 – treatment <45 min after ischemia onset; 2 – ≥45 min after ischemia onset) | 0 | 0 | 2 | 2 |
| Dose–response (0 – one dose; 2 – multiple doses with dose–response relationship) | 0 | 0 | 0 | 0 |
| Feasible route of delivery (0 – infeasible route [e.g., intraventricular injection]; 1 – semi-infeasible route [e.g., intraarterial injection]; 2 – feasible route [e.g., intravenous injection]) | 2 | 1 | 1 | 2 |
| Behavioural and/or long-term outcome assessment (0 – no benefit; 1 – behavioural OR other outcome ≥30 days post-stroke; 2 – behavioural AND outcomes ≥30 days post-stroke) | 0 | 0 | 2 | 2 |
| Typical infarct volume reduction magnitude 0 – small effect (Cohen’s d 0.2–0.39); 1 – medium effect (Cohen’s d 0.4–0.69); 2 – large effect (Cohen’s d≥0.7) | 2 | 2 | 2 | 2 |
| Readiness for translation score (0–7 – low; 8–15 – medium; 16–22 – high) | Low (5) | Low (4) | Medium (12) | Medium (13) |
GRIPP2 short form checklist.
| Section and topic | Item |
|---|---|
| 1: Aim | To conduct a preclinical systematic review assessing the effects of C-C chemokine receptor type 5 (CCR5) antagonists on motor and cognitive impairment following stroke. To collaborate with a panel of patients and caregivers with lived experience of stroke throughout the development and conduct of the preclinical systematic review. |
| 2: Methods | A panel of eight patients and caregivers with lived experience of stroke was recruited to join the research team through the Heart & Stroke Foundation and the Patient and Family Advocacy Program at The Ottawa Hospital. Recruitment advertisements were distributed to both organizations. The patients and caregivers were involved in developing the research question and the protocol (i.e., elements of the AMICO question presented in the Methods section); identifying data items for extraction; conduct of the review including screening and extraction; interpreting the review results; and contributed to edits to the final manuscript. Patients and caregivers attended monthly virtual meetings. These meetings provided background knowledge of preclinical stroke, systematic review conduct, and discussed research findings as the review progressed. Patients provided insights into their lived experiences with stroke and helped identify priority areas that they were interested in. Patients and caregivers were offered financial compensation and co-authorship in recognition of their contributions to the research project. |
| 3: Results | Patient engagement contributed to the study in several ways, including:
|
| 4: Discussion | Overall, patient engagement was successful in informing review development and conduct. Additionally, the researchers on the team learned from the patient panel. The patient panel brought a unique perspective to the planning and conduct of a preclinical systematic review. Additionally, members of the panel stated that the experience was useful for them as they gained new insights into preclinical research. |
| 5: Reflections | Engagement was embedded within the research project from the inception to dissemination, where patient partners were members of the larger team. Patient partners directed us to outcomes for the systematic review that critical clinically to patients in the chronic phase of stroke recovery. Their participation in the conduct of the review facilitated meaningful collaborations and discussion. |
Additional files
-
Supplementary file 1
Search strategy.
- https://cdn.elifesciences.org/articles/103245/elife-103245-supp1-v1.zip
-
Supplementary file 2
Data extraction forms.
- https://cdn.elifesciences.org/articles/103245/elife-103245-supp2-v1.zip
-
MDAR checklist
- https://cdn.elifesciences.org/articles/103245/elife-103245-mdarchecklist1-v1.docx