Assessing healthy vaccinee effect in COVID-19 vaccine effectiveness studies: a national cohort study in Qatar
Figures
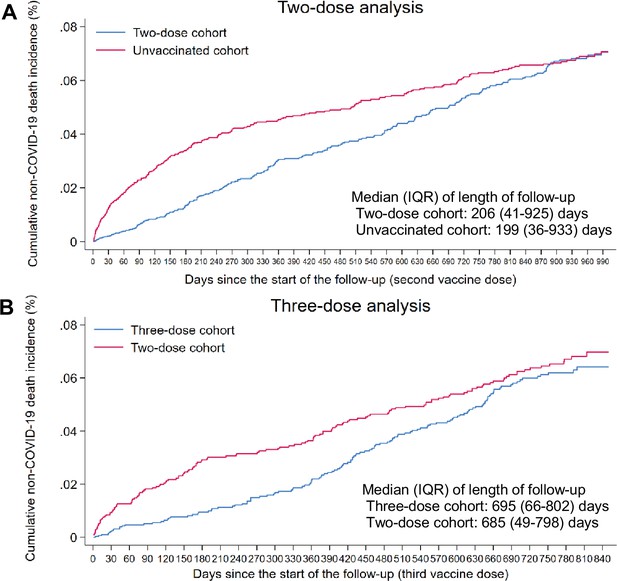
Cumulative incidence of non-COVID-19 death in the matched (A) two-dose cohort compared to the unvaccinated cohort and (B) three-dose cohort compared to the two-dose cohort.
-
Figure 1—source data 1
Data used to generate Figure 1A and B.
- https://cdn.elifesciences.org/articles/103690/elife-103690-fig1-data1-v1.xlsx
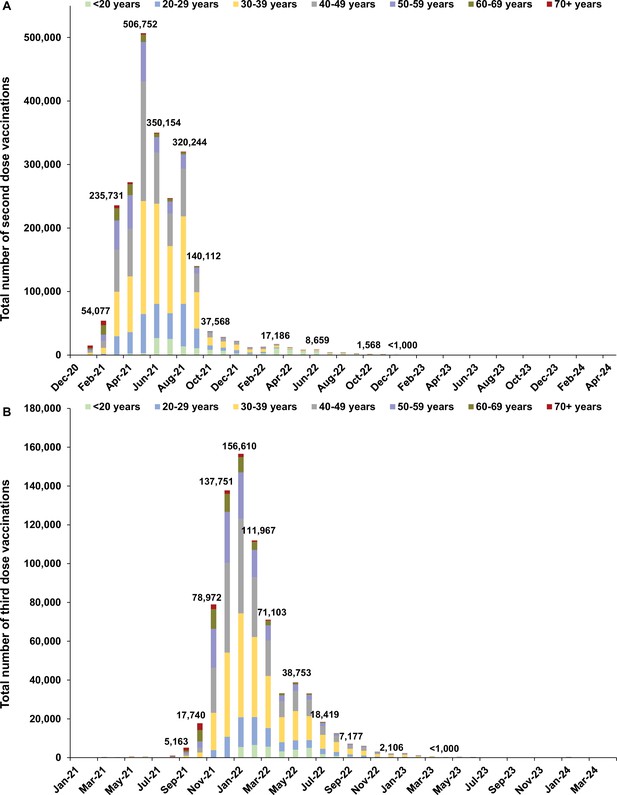
Distribution of vaccinations.
Number of (A) second dose and (B) third dose vaccinations by calendar month.
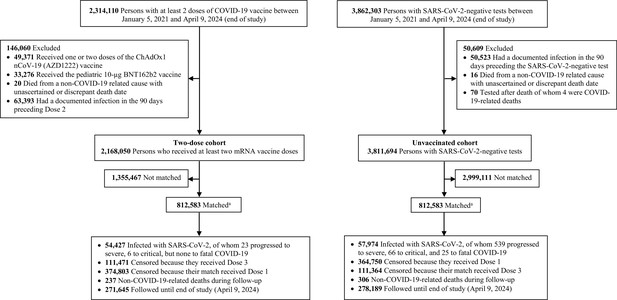
Flowchart describing the study population selection process for investigating an indication effect or a healthy vaccinee effect among recipients of primary series vaccination compared to those with no vaccination in Qatar.
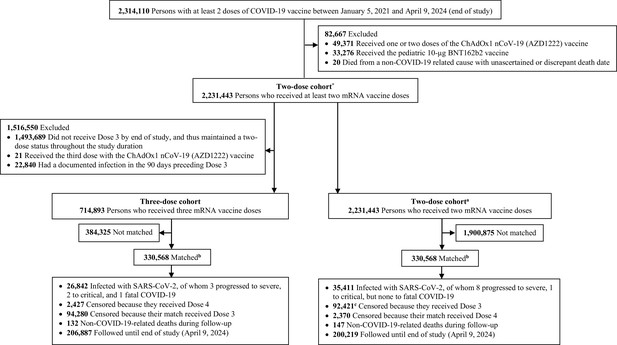
Flowchart describing the study population selection process for investigating an indication effect or a healthy vaccinee effect among recipients of booster (third dose) vaccination compared to recipients of primary series vaccination in Qatar.
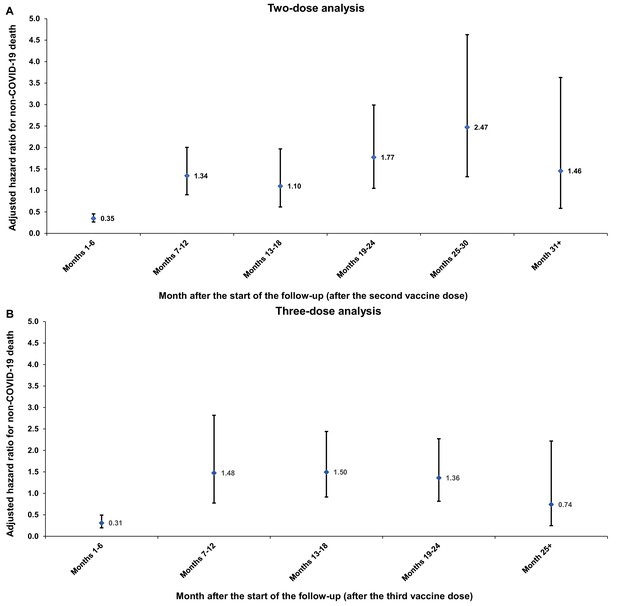
Adjusted hazard ratios for incidence of non-COVID-19 death in the (A) two-dose analysis and (B) three-dose analysis, by 6-month interval of follow-up.
Error bars indicate the corresponding 95% confidence intervals.
-
Figure 2—source data 1
Data used to generate Figure 2A and B.
- https://cdn.elifesciences.org/articles/103690/elife-103690-fig2-data1-v1.xlsx
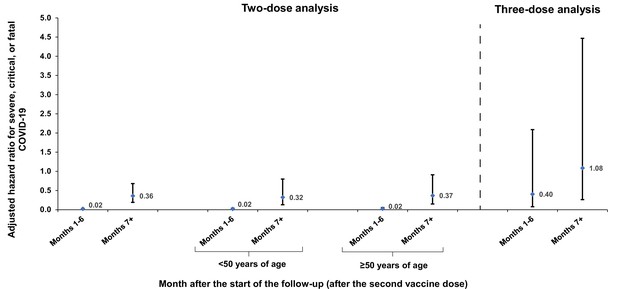
Adjusted hazard ratios for incidence of severe, critical, or fatal COVID-19 in the two-dose and three-dose analyses, by 6-month interval of follow-up.
Error bars indicate the corresponding 95% confidence intervals.
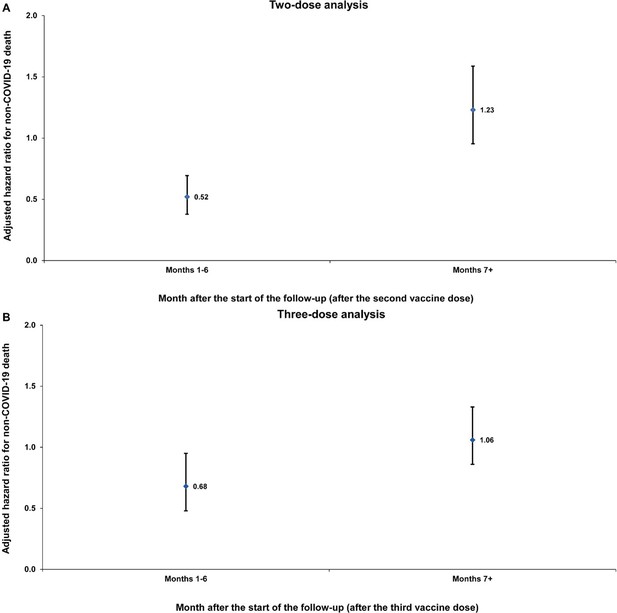
Sensitivity analysis.
Adjusted hazard ratios for incidence of non-COVID-19 death in the entire population without matching on a SARS-CoV-2-negative test among controls. Results are shown for the (A) two-dose analysis and the (B) three-dose analysis, in the first 6 months of follow-up and the period thereafter.
Tables
Baseline characteristics of the full and matched cohorts for investigating an indication effect or a healthy vaccinee effect among recipients of primary series or booster (third dose) vaccination in Qatar.
| Two-dose analysis | Three-dose analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Full eligible cohorts | Matched cohorts* | Full eligible cohorts | Matched cohorts† | ||||||||
| Two-dose | Unvaccinated | SMD‡ | Two-dose | Unvaccinated | SMD‡ | Three-dose | Two-dose | SMD‡ | Three-dose | Two-dose | SMD‡ | |
| N=2,168,050 | N=3,811,694 | N=812,583 | N=812,583 | N=714,893 | N=2,231,443 | N=330,568 | N=330,568 | |||||
| Median age (IQR)—years | 38 (31–45) | 32 (24–41) | 0.50§ | 34 (28–41) | 33 (27–40) | 0.07§ | 40 (33–49) | 38 (31–45) | 0.21§ | 38 (32–45) | 39 (34–47) | 0.01§ |
| Age group—no. (%) | ||||||||||||
| 0–19 years | 106,156 (4.9) | 622,215 (16.3) | 0.58 | 69,673 (8.6) | 69,673 (8.6) | 0.00 | 33,216 (4.6) | 107,885 (4.8) | 0.23 | 9,221 (2.8) | 9,221 (2.8) | 0.00 |
| 20–29 years | 326,484 (15.1) | 909,809 (23.9) | 191,420 (23.6) | 191,420 (23.6) | 72,966 (10.2) | 334,458 (15.0) | 40,015 (12.1) | 40,015 (12.1) | ||||
| 30–39 years | 809,250 (37.3) | 1,228,030 (32.2) | 326,985 (40.2) | 326,985 (40.2) | 239,713 (33.5) | 834,373 (37.4) | 139,067 (42.1) | 139,067 (42.1) | ||||
| 40–49 years | 576,564 (26.6) | 660,453 (17.3) | 158,847 (19.5) | 158,847 (19.5) | 204,224 (28.6) | 595,300 (26.7) | 98,080 (29.7) | 98,080 (29.7) | ||||
| 50–59 years | 244,963 (11.3) | 268,839 (7.1) | 51,661 (6.4) | 51,661 (6.4) | 107,990 (15.1) | 252,382 (11.3) | 36,284 (11.0) | 36,284 (11.0) | ||||
| 60–69 years | 80,555 (3.7) | 92,395 (2.4) | 12,014 (1.5) | 12,014 (1.5) | 43,815 (6.1) | 82,558 (3.7) | 7,355 (2.2) | 7,355 (2.2) | ||||
| 70+ years | 24,078 (1.1) | 29,953 (0.8) | 1,983 (0.2) | 1,983 (0.2) | 12,969 (1.8) | 24,487 (1.1) | 546 (0.2) | 546 (0.2) | ||||
| Sex | ||||||||||||
| Male | 1,599,920 (73.8) | 2,682,394 (70.4) | 0.08 | 593,856 (73.1) | 593,856 (73.1) | 0.00 | 467,443 (65.4) | 1,645,973 (73.8) | 0.18 | 245,116 (74.1) | 245,116 (74.1) | 0.00 |
| Female | 568,130 (26.2) | 1,129,300 (29.6) | 218,727 (26.9) | 218,727 (26.9) | 247,450 (34.6) | 585,470 (26.2) | 85,452 (25.9) | 85,452 (25.9) | ||||
| Nationality¶ | ||||||||||||
| Bangladeshi | 306,251 (14.1) | 269,021 (7.1) | 0.30 | 68,102 (8.4) | 68,102 (8.4) | 0.00 | 66,000 (9.2) | 312,475 (14.0) | 0.39 | 37,670 (11.4) | 37,670 (11.4) | 0.00 |
| Egyptian | 106,392 (4.9) | 184,152 (4.8) | 40,791 (5.0) | 40,791 (5.0) | 59,691 (8.3) | 109,910 (4.9) | 18,103 (5.5) | 18,103 (5.5) | ||||
| Filipino | 201,002 (9.3) | 277,459 (7.3) | 76,146 (9.4) | 76,146 (9.4) | 99,405 (13.9) | 209,620 (9.4) | 40,680 (12.3) | 40,680 (12.3) | ||||
| Indian | 531,366 (24.5) | 1,074,425 (28.2) | 268,830 (33.1) | 268,830 (33.1) | 222,135 (31.1) | 549,694 (24.6) | 121,774 (36.8) | 121,774 (36.8) | ||||
| Nepalese | 233,558 (10.8) | 347,108 (9.1) | 68,279 (8.4) | 68,279 (8.4) | 28,584 (4.0) | 239,262 (10.7) | 20,694 (6.3) | 20,694 (6.3) | ||||
| Pakistani | 103,600 (4.8) | 223,498 (5.9) | 46,416 (5.7) | 46,416 (5.7) | 34,161 (4.8) | 106,177 (4.8) | 14,548 (4.4) | 14,548 (4.4) | ||||
| Qatari | 195,030 (9.0) | 319,209 (8.4) | 64,135 (7.9) | 64,135 (7.9) | 40,519 (5.7) | 199,550 (8.9) | 23,062 (7.0) | 23,062 (7.0) | ||||
| Sri Lankan | 75,586 (3.5) | 127,750 (3.4) | 21,827 (2.7) | 21,827 (2.7) | 20,759 (2.9) | 77,913 (3.5) | 10,988 (3.3) | 10,988 (3.3) | ||||
| Sudanese | 45,213 (2.1) | 78,528 (2.1) | 17,594 (2.2) | 17,594 (2.2) | 12,920 (1.8) | 46,586 (2.1) | 4,140 (1.3) | 4,140 (1.3) | ||||
| Other nationalities** | 370,052 (17.1) | 910,544 (23.9) | 140,463 (17.3) | 140,463 (17.3) | 130,719 (18.3) | 380,256 (17.0) | 38,909 (11.8) | 38,909 (11.8) | ||||
| Coexisting conditions | ||||||||||||
| 0 | 1,809,569 (83.5) | 3,352,859 (88.0) | 0.14 | 746,840 (91.9) | 746,840 (91.9) | 0.00 | 540,392 (75.6) | 1,860,263 (83.4) | 0.20 | 311,376 (94.2) | 311,376 (94.2) | 0.00 |
| 1 | 183,168 (8.4) | 261,898 (6.9) | 45,414 (5.6) | 45,414 (5.6) | 78,872 (11.0) | 189,770 (8.5) | 12,288 (3.7) | 12,288 (3.7) | ||||
| 2 | 86,673 (4.0) | 102,968 (2.7) | 13,988 (1.7) | 13,988 (1.7) | 44,676 (6.2) | 89,926 (4.0) | 5,049 (1.5) | 5,049 (1.5) | ||||
| 3 | 39,989 (1.8) | 42,960 (1.1) | 3,842 (0.5) | 3,842 (0.5) | 22,684 (3.2) | 41,422 (1.9) | 1,149 (0.3) | 1,149 (0.3) | ||||
| 4 | 22,810 (1.1) | 23,715 (0.6) | 1,602 (0.2) | 1,602 (0.2) | 13,504 (1.9) | 23,539 (1.1) | 558 (0.2) | 558 (0.2) | ||||
| 5 | 13,035 (0.6) | 13,575 (0.4) | 657 (0.1) | 657 (0.1) | 7,590 (1.1) | 13,415 (0.6) | 122 (<0.01) | 122 (<0.01) | ||||
| ≥6 | 12,806 (0.6) | 13,719 (0.4) | 240 (<0.01) | 240 (<0.01) | 7,175 (1.0) | 13,108 (0.6) | 26 (<0.01) | 26 (<0.01) | ||||
| Prior infection status†† | ||||||||||||
| No prior infection | 1,957,313 (90.3) | – | – | 764,366 (94.1) | 764,366 (94.1) | 0.00 | 591,083 (82.7) | – | – | 287,773 (87.1) | 287,773 (87.1) | 0.00 |
| Prior pre-omicron infection | 208,058 (9.6) | – | 46,631 (5.7) | 46,631 (5.7) | 96,567 (13.5) | – | 33,864 (10.2) | 33,864 (10.2) | ||||
| Prior omicron infection | 2,463 (0.1) | – | 1,548 (0.2) | 1,548 (0.2) | 24,690 (3.5) | – | 8,624 (2.6) | 8,624 (2.6) | ||||
| Prior pre-omicron and omicron infections | 216 (<0.01) | – | 38 (<0.01) | 38 (<0.01) | 2,553 (0.4) | – | 307 (0.1) | 307 (0.1) | ||||
-
IQR, interquartile range; SMD, standardized mean difference.
-
*
Cohorts were matched exactly one-to-one by sex, 10-year age group, nationality, type of coexisting conditions, and prior infection status. Persons who received their second vaccine dose in a specific calendar week in the two-dose cohort were additionally matched to persons who had a record for a SARS-CoV-2-negative test in that same calendar week in the unvaccinated cohort, to ensure that matched pairs had presence in Qatar over the same time period.
-
†
Cohorts were matched exactly one-to-one by sex, 10-year age group, nationality, type of coexisting conditions, prior infection status, and calendar week of the second vaccine dose. Persons who received their third vaccine dose in a specific calendar week in the three-dose cohort were additionally matched to persons who had a record for a SARS-CoV-2-negative test in that same calendar week in the two-dose cohort, to ensure that matched pairs had presence in Qatar over the same time period.
-
‡
SMD is the difference in the mean of a covariate between groups divided by the pooled standard deviation. An SMD≤0.1 indicates adequate matching.
-
§
SMD is for the mean difference between groups divided by the pooled standard deviation.
-
¶
Nationalities were chosen to represent the most populous groups in Qatar.
-
**
These comprise up to 183 other nationalities in the unmatched and 148 other nationalities in the matched two-dose analyses, and up to 169 other nationalities in the unmatched and 111 other nationalities in the matched three-dose analyses.
-
††
Ascertained at the start of follow-up. Accordingly, distribution is not available for the unmatched unvaccinated cohort in the two-dose analysis and unmatched two-dose cohort in the three-dose analysis, as the start of follow-up for each person in these reference/control cohorts is determined by that of their match after the matching process is completed.
Hazard ratios for incidence of non-COVID-19 death, SARS-CoV-2 infection, and severe, critical, or fatal COVID-19 in the (A) two-dose analysis and (B) three-dose analysis.
| (A) Two-dose analysis | Two-dose cohort* | Unvaccinated cohort* |
|---|---|---|
| Sample size | 812,583 | 812,583 |
| Number of non-COVID-19 death | 237 | 306 |
| Number of incident infections | 54,427 | 57,974 |
| Number of severe, critical, or fatal COVID-19 disease | 29 | 630 |
| Total follow-up time (person-weeks) | 46,028,318 | 46,275,391 |
| Non-COVID-19 death | ||
| Incidence rate of non-COVID-19 death (per 10,000 person-weeks; 95% CI) | 0.05 (0.05–0.06) | 0.07 (0.06–0.07) |
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 0.77 (0.65–0.91) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)† | 0.76 (0.64–0.90) | |
| SARS-CoV-2 infection | ||
| Unadjusted hazard ratio for SARS-CoV-2 infection (95% CI) | 0.93 (0.92–0.94) | |
| Adjusted hazard ratio for SARS-CoV-2 infection (95% CI)‡ | 0.89 (0.88–0.90) | |
| Effectiveness against SARS-CoV-2 infection (95% CI)‡ | 10.7 (9.6–11.7) | |
| Severe, critical, or fatal COVID-19 disease | ||
| Unadjusted hazard ratio for severe, critical, or fatal COVID-19 disease (95% CI) | 0.05 (0.03–0.07) | |
| Adjusted hazard ratio for severe, critical, or fatal COVID-19 disease (95% CI)‡ | 0.04 (0.03–0.06) | |
| Effectiveness against severe, critical, or fatal COVID-19 disease (95% CI)‡ | 95.9 (94.0–97.1) | |
| (B) Three-dose analysis | Three-dose cohort§ | Two-dose cohort§ |
| Sample size | 330,568 | 330,568 |
| Number of non-COVID-19 death | 132 | 147 |
| Number of incident infections | 26,842 | 35,411 |
| Number of severe, critical, or fatal COVID-19 disease | 6 | 9 |
| Total follow-up time (person-weeks) | 24,015,307 | 23,088,912 |
| Non-COVID-19 death | ||
| Incidence rate of non-COVID-19 death (per 10,000 person-weeks; 95% CI) | 0.05 (0.05–0.07) | 0.06 (0.05–0.07) |
| Unadjusted hazard ratio for non-COVID-19 death (95% CI)¶ | 0.87 (0.68–1.10) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)¶ | 0.85 (0.67–1.07) | |
| SARS-CoV-2 infection | ||
| Unadjusted hazard ratio for SARS-CoV-2 infection (95% CI) | 0.74 (0.72–0.75) | |
| Adjusted hazard ratio for SARS-CoV-2 infection (95% CI)** | 0.74 (0.72–0.75) | |
| Effectiveness against SARS-CoV-2 infection (95% CI)** | 26.3 (25.2–27.5) | |
| Severe, critical, or fatal COVID-19 disease | ||
| Unadjusted hazard ratio for severe, critical, or fatal COVID-19 disease (95% CI) | 0.64 (0.23–1.81) | |
| Adjusted hazard ratio for severe, critical, or fatal COVID-19 disease (95% CI)** | 0.66 (0.23–1.86) | |
| Effectiveness against severe, critical, or fatal COVID-19 disease (95% CI)** | 34.1 (−46.4–76.7) | |
-
CI, confidence interval; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
-
*
Cohorts were matched exactly one-to-one by sex, 10-year age group, nationality, type of coexisting conditions, and prior infection status. Persons who received their second vaccine dose in a specific calendar week in the two-dose cohort were additionally matched to persons who had a record for a SARS-CoV-2-negative test in that same calendar week in the unvaccinated cohort, to ensure that matched pairs had presence in Qatar over the same time period.
-
†
Adjusted for sex, 10-year age group, nationality, number of coexisting conditions, prior infection status, and calendar week of the second vaccine dose for the two-dose cohort or SARS-CoV-2-negative test for the unvaccinated cohort.
-
‡
Adjusted for sex, 10-year age group, nationality, number of coexisting conditions, prior infection status, calendar week of the second vaccine dose for the two-dose cohort or SARS-CoV-2-negative test for the unvaccinated cohort, and testing rate.
-
§
Cohorts were matched exactly one-to-one by sex, 10-year age group, nationality, type of coexisting conditions, prior infection status, and calendar week of the second vaccine dose. Persons who received their third vaccine dose in a specific calendar week in the three-dose cohort were additionally matched to persons who had a record for a SARS-CoV-2-negative test in that same calendar week in the two-dose cohort, to ensure that matched pairs had presence in Qatar over the same time period.
-
¶
Adjusted for sex, 10-year age group, nationality, number of coexisting conditions, prior infection status, and calendar week of the second vaccine dose.
-
**
Adjusted for sex, 10-year age group, nationality, number of coexisting conditions, prior infection status, calendar week of the second vaccine dose, and testing rate.
Subgroup analyses.
Hazard ratios for incidence of non-COVID-19 death stratified by age group, clinical vulnerability status, and prior infection status in the (A) two-dose analysis and (B) three-dose analysis.
| (A) Two-dose analysis | Two-dose cohort* | Unvaccinated cohort* |
|---|---|---|
| Age | ||
| <50 years of age | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 0.91 (0.73–1.12) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)† | 0.89 (0.72–1.11) | |
| ≥50 years of age | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 0.59 (0.44–0.78) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)† | 0.56 (0.42–0.75) | |
| Clinical vulnerability status | ||
| Less clinically vulnerable to severe COVID-19 | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 0.99 (0.80–1.23) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)† | 0.98 (0.79–1.22) | |
| More clinically vulnerable to severe COVID-19 | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 0.53 (0.41–0.70) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)† | 0.51 (0.39–0.68) | |
| Prior infection status | ||
| No prior infection | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 0.76 (0.64–0.90) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)† | 0.74 (0.63–0.89) | |
| Prior pre-omicron infection | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 1.05 (0.48–2.30) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)† | 1.00 (0.45–2.20) | |
| Prior omicron infection | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | -- ‡ | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)† | -- ‡ | |
| Prior pre-omicron & omicron infections | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | -- ‡ | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)† | -- ‡ | |
| (B) Three-dose analysis | Three-dose cohort § | Two-dose cohort § |
| Age | ||
| <50 years of age | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 0.90 (0.67–1.21) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)¶ | 0.90 (0.67–1.20) | |
| ≥50 years of age | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 0.80 (0.54–1.18) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)¶ | 0.76 (0.51–1.13) | |
| Clinical vulnerability status | ||
| Less clinically vulnerable to severe COVID-19 | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 0.91 (0.68–1.23) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)¶ | 0.91 (0.67–1.22) | |
| More clinically vulnerable to severe COVID-19 | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 0.78 (0.54–1.15) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)¶ | 0.76 (0.52–1.12) | |
| Prior infection status | ||
| No prior infection | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 0.80 (0.63–1.03) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)¶ | 0.79 (0.61–1.01) | |
| Prior pre-omicron infection | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 1.63 (0.71–3.73) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)¶ | 1.63 (0.71–3.72) | |
| Prior omicron infection | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | 1.32 (0.30–5.90) | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)¶ | 1.32 (0.30–5.91) | |
| Prior pre-omicron & omicron infections | ||
| Unadjusted hazard ratio for non-COVID-19 death (95% CI) | -- ‡ | |
| Adjusted hazard ratio for non-COVID-19 death (95% CI)¶ | -- ‡ | |
-
CI, confidence interval; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
-
*
Cohorts were matched exactly one-to-one by sex, 10-year age group, nationality, type of coexisting conditions, and prior infection status. Persons who received their second vaccine dose in a specific calendar week in the two-dose cohort were additionally matched to persons who had a record for a SARS-CoV-2-negative test in that same calendar week in the unvaccinated cohort, to ensure that matched pairs had presence in Qatar over the same time period.
-
†
Adjusted for sex, 10-year age group, nationality, number of coexisting conditions, prior infection status (where applicable), and calendar week of the second vaccine dose for the two-dose cohort or SARS-CoV-2-negative test for the unvaccinated cohort.
-
‡
Could not be estimated because of no or small number of events.
-
§
Cohorts were matched exactly one-to-one by sex, 10-year age group, nationality, type of coexisting conditions, prior infection status, and calendar week of the second vaccine dose. Persons who received their third vaccine dose in a specific calendar week in the three-dose cohort were additionally matched to persons who had a record for a SARS-CoV-2-negative test in that same calendar week in the two-dose cohort, to ensure that matched pairs had presence in Qatar over the same time period.
-
¶
Adjusted for sex, 10-year age group, nationality, number of coexisting conditions, prior infection status (where applicable), and calendar week of the second vaccine dose.
Sensitivity analyses.
Hazard ratios for incidence of non-COVID-19 death among Qataris with and without matching on a SARS-CoV-2-negative test among controls in the (A) two-dose analysis and (B) three-dose analysis.
| (A) Two-dose analysis | Two-dose cohort | Unvaccinated cohort |
|---|---|---|
| Sensitivity analysis I-Restricting analysis to Qataris* | ||
| Unadjusted hazard ratiofor non-COVID-19 death (95% CI) | 0.29 (0.19–0.43) | |
| Adjusted hazard ratiofor non-COVID-19 death (95% CI)† | 0.29 (0.19–0.43) | |
| Sensitivity analysis II-Restricting analysis to Qataris and not matching by a SARS-CoV-2-negative test among controls‡ | ||
| Unadjusted hazard ratiofor non-COVID-19 death (95% CI) | 0.40 (0.31–0.51) | |
| Adjusted hazard ratiofor non-COVID-19 death (95% CI)§ | 0.38 (0.30–0.50) | |
| (B) Three-dose analysis | Three-dose cohort | Two-dose cohort |
| Sensitivity analysis I-Restricting analysis to Qataris¶ | ||
| Unadjusted hazard ratiofor non-COVID-19 death (95% CI) | 0.77 (0.44–1.33) | |
| Adjusted hazard ratiofor non-COVID-19 death (95% CI)** | 0.76 (0.43–1.32) | |
| Sensitivity analysis II-Restricting analysis to Qataris and not matching by a SARS-CoV-2-negative test among controls†† | ||
| Unadjusted hazard ratiofor non-COVID-19 death (95% CI) | 0.77 (0.52–1.12) | |
| Adjusted hazard ratiofor non-COVID-19 death (95% CI)** | 0.77 (0.53–1.13) | |
-
CI, confidence interval; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
-
*
Cohorts were matched exactly one-to-one by sex, 10-year age group, type of coexisting conditions, and prior infection status. Persons who received their second vaccine dose in a specific calendar week in the two-dose cohort were additionally matched to persons who had a record for a SARS-CoV-2-negative test in that same calendar week in the unvaccinated cohort, to ensure that matched pairs had presence in Qatar over the same time period.
-
†
Adjusted for sex, 10-year age group, number of coexisting conditions, prior infection status, and calendar week of the second vaccine dose for the two-dose cohort or SARS-CoV-2-negative test for the unvaccinated cohort.
-
‡
Cohorts were matched exactly one-to-one by sex, 10-year age group, type of coexisting conditions, and prior infection status.
-
§
Adjusted for sex, 10-year age group, number of coexisting conditions, and prior infection status.
-
¶
Cohorts were matched exactly one-to-one by sex, 10-year age group, type of coexisting conditions, prior infection status, and calendar week of the second vaccine dose. Persons who received their third vaccine dose in a specific calendar week in the three-dose cohort were additionally matched to persons who had a record for a SARS-CoV-2-negative test in that same calendar week in the two-dose cohort, to ensure that matched pairs had presence in Qatar over the same time period.
-
**
Adjusted for sex, 10-year age group, number of coexisting conditions, prior infection status, and calendar week of the second vaccine dose.
-
††
Cohorts were matched exactly one-to-one by sex, 10-year age group, type of coexisting conditions, prior infection status, and calendar week of the second vaccine dose.





