NR2F2 is required in the embryonic testis for fetal Leydig cell development
Figures
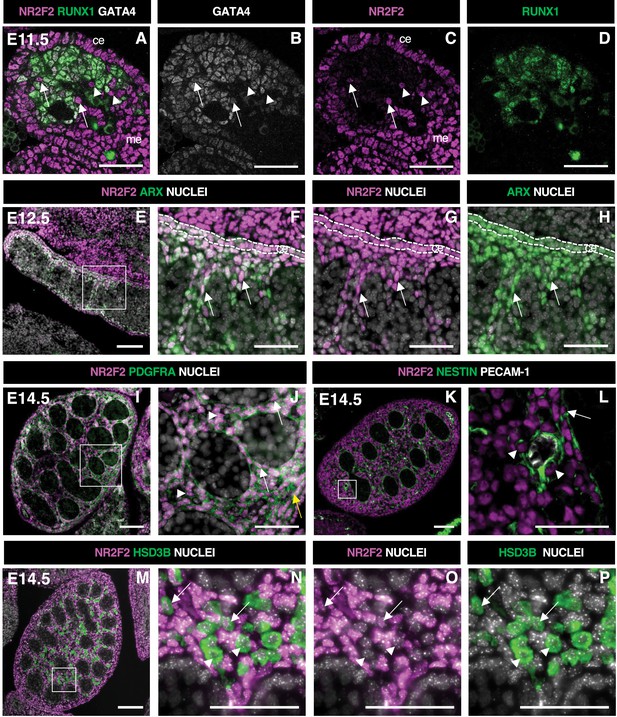
NR2F2 is expressed in steroidogenic progenitors of the fetal testis.
(A–D) Immunodetection of NR2F2, RUNX1, and GATA4 on embryonic day 11.5 (E11.5) (18 tail somites) XY gonad. NR2F2 is detected in coelomic epithelium (ce), mesonephros (me), and RUNX1 negative cells that are either GATA4 positive (arrows in A–C) or GATA4 negative (arrowheads in A–C). (E–H) Immunodetection of NR2F2 and ARX on E12.5 XY gonad. NR2F2 is co-expressed with ARX in the coelomic epithelium (ce, dotted lines in F–H) and in interstitial cells between the testis cords (arrows in F–H). (I,J) Immunodetection of NR2F2 and PDGFRA on E14.5 XY gonad. NR2F2 is detected in PDGFRA positive cells, including interstitial progenitors (arrowheads in J), peritubular myoid cell surrounding testis cords (arrows in J), and cells of the future tunica albuginea (yellow arrow in J). (K,L) Immunodetection of NR2F2, NESTIN, and PECAM-1 on E14.5 XY gonad. NR2F2 is detected in NESTIN positive interstitial progenitors, including perivascular cells (arrowheads in L) and peritubular myoid cells (arrow in L). (M–P) Immunodetection of NR2F2 and HSD3B on E14.5 XY gonad. NR2F2 is absent from the majority of HSD3B positive fetal Leydig cells (arrowheads in N–P) and is only detected at low levels in a few HSD3B positive elongated cells (arrows in N–P). Data are representative of triplicate biological replicates. Scale bar = 50 µm in A–D, F–H, J, L, and N–P. Scale bar = 100 µm in E, I, K, M.
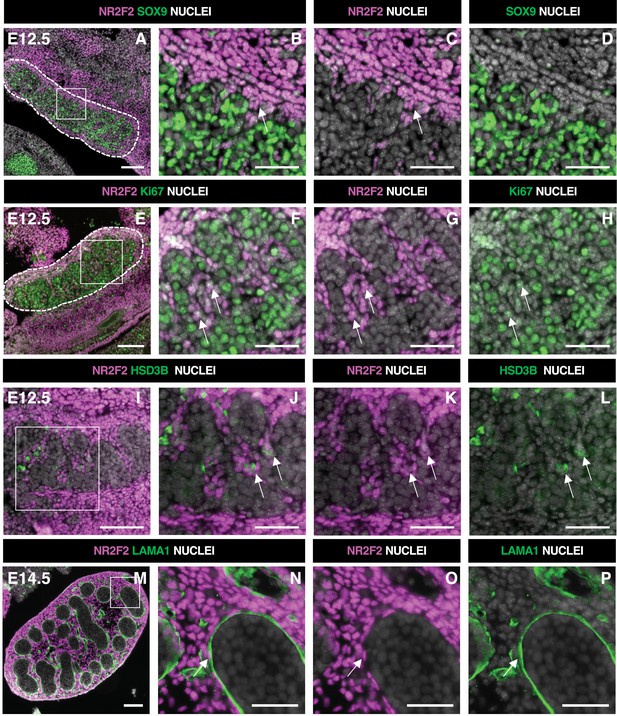
NR2F2 is expressed in steroidogenic progenitors of the fetal testis.
(A–D) Immunodetection of NR2F2 and SOX9 on embryonic day 12.5 (E12.5) XY gonad (outlined by dotted lines in A). NR2F2 is detected in interstitial cells outside the testis cords (arrows in B and C). (E–H) Immunodetection of NR2F2 and Ki67 on E12.5 XY gonad (outlined by dotted lines in E). NR2F2 positive cells are proliferating (arrows in F–H). (I–L) Immunodetection of NR2F2 and HSD3B on E12.5 XY gonad. NR2F2 is detected at low levels in HSD3B positive elongated cells (arrows in J–L). (M–P) Immunodetection of NR2F2 and LAMA1 on E14.5 XY gonad. NR2F2 is expressed in peritubular myoid cells lining the basement membrane outside the testis cords (arrow in N–P). Data are representative of triplicate biological replicates. Scale bar = 50 µm in B–D, F–H, J–L, and N–P. Scale bar = 100 µm in A, E, I, M.
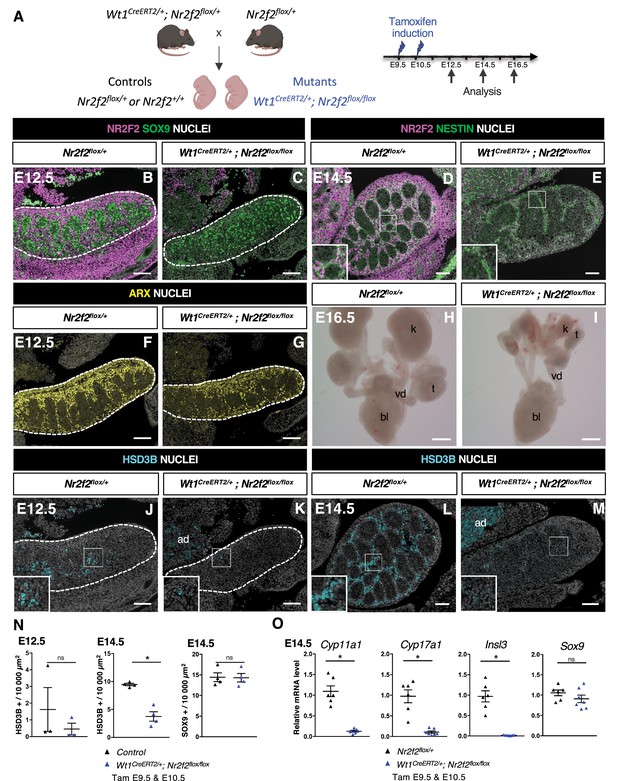
NR2F2 deletion by Wt1CreERT2 impairs Sertoli cell differentiation and fetal Leydig cell (FLC) development.
(A) Generation of Wt1CreERT2; Nr2f2flox/flox mutants. Tamoxifen was administered at embryonic day 9.5 (E9.5) and E10.5, and embryos were recovered at E12.5, E14.5, and E16.5. (B,C) Immunodetection of NR2F2 and SOX9 on E12.5 Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes (outlined by dotted lines) after tamoxifen treatment at E9.5 and E10.5. NR2F2 is efficiently deleted in the gonad and mesonephros. (D,E) Immunodetection of NR2F2 and NESTIN on E14.5 Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes. NR2F2 is efficiently deleted in NESTIN1 positive cells. (F,G) Immunodetection of ARX on E12.5 Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes (outlined by dotted lines). Interstitial cells are generated in Wt1CreERT2; Nr2f2flox/flox mutants. (H,I) Macroscopic view of the urogenital tract of XY E16.5 Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes dissected after tamoxifen treatment at E9.5 and E10.5. Testes (t) and kidneys (k) are hypoplastic in Wt1CreERT2; Nr2f2flox/flox mutants. bl: bladder. vd: vas deferens. (J,K) Immunodetection of HSD3B on E12.5 Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes (outlined by dotted lines). ad: adrenal. (L,M) Immunodetection of HSD3B on E14.5 Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes. ad: adrenal. (N) Quantification of the number of HSD3B positive cells per surface unit and of the number of SOX9 positive cells per surface unit in control (wild-type or Nr2f2flox/+) and Wt1CreERT2; Nr2f2flox/flox testes. Each triangle represents the mean number of HSD3B or SOX9 positive cells per surface unit of one individual measured on at least two sections per gonad. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (O) Quantification of Cyp11a1, Cyp17a1, Insl3, and Sox9 transcripts in Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes treated with tamoxifen at E9.5 and E10.5 and dissected at E14.5 after normalization to Sdha and Tbp by RT-qPCR. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. Immunodetection data are representative of triplicate biological replicates. Scale bar = 100 µm in B–G, J–M. Scale bar = 500 µm in H, I.
-
Figure 2—source data 1
Source data for cell counts and RT-qPCR data in Figure 2.
- https://cdn.elifesciences.org/articles/103783/elife-103783-fig2-data1-v1.xlsx
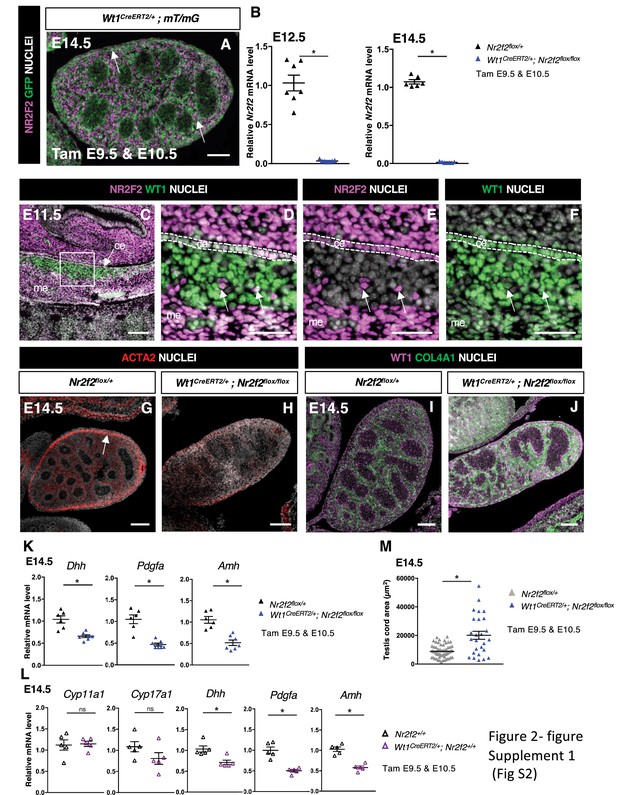
NR2F2 deletion by Wt1CreERT2 impairs Sertoli cell differentiation and fetal Leydig cell (FLC) development.
(A) Immunodetection of NR2F2 and GFP on embryonic day 14.5 (E14.5) XY Wt1CreERT2;mT/mG gonad after tamoxifen was administered at E9.5 and E10.5. Upon Cre-mediated recombination, GFP is expressed in all somatic cells of the gonad, including NR2F2 positive cells (arrows). (B) Quantification of Nr2f2 transcripts after normalization to Sdha and Tbp by RT-qPCR in Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes dissected at E12.5 and E14.5 after tamoxifen treatment at E9.5 and E10.5. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (C–F) Immunodetection of NR2F2 and WT1 on E11.5 XY gonad. NR2F2 is co-expressed with WT1 in the coelomic epithelium (ce), in the mesonephros (me), and in interstitial steroidogenic progenitors (arrows). (G,H) Immunodetection of ACTA2 on E14.5 Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes. The expression in the future tunica albuginea (arrow in G) is impaired in Wt1CreERT2; Nr2f2flox/flox mutants. (I,J) Immunodetection of WT1 and COL4A1 on E14.5 Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes. Testis cords are enlarged and abnormally shaped in Wt1CreERT2; Nr2f2flox/flox mutants. (K) Quantification of Dhh, Pdgfa, and Amh transcripts in Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes treated with tamoxifen at E9.5 and E10.5 and dissected at E14.5 after normalization to Sdha and Tbp by RT-qPCR. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (L) Quantification of Cyp11a1, Cyp17a1, Dhh, Pdgfa, and Amh transcripts in Nr2f2+/+ and Wt1CreERT2/+ testes treated with tamoxifen at E9.5 and E10.5 and dissected at E14.5 after normalization to Sdha and Tbp by RT-qPCR. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (M) Quantification of the testis cord area in control testes (n=60 testis cords from 2 Nr2f21 and 1 Nr2f2flox/+ embryos, average testis cord number per embryo = 21) and Wt1CreERT2; Nr2f2flox/flox testes (n=26 testis cords from 3 embryos, average testis cord number per embryo = 9) dissected at E14 after tamoxifen treatment at E9.5 and E10.5. Testis cords were identified on sections by immunodetection with anti-SOX9 antibodies. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05. Immunodetection data are representative of triplicate biological replicates. Scale bar = 50 µm in D–F. Scale bar = 100 µm in A, C, G–J.
-
Figure 2—figure supplement 1—source data 1
Source data for testis cord measurements and RT-qPCR data in Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/103783/elife-103783-fig2-figsupp1-data1-v1.xlsx
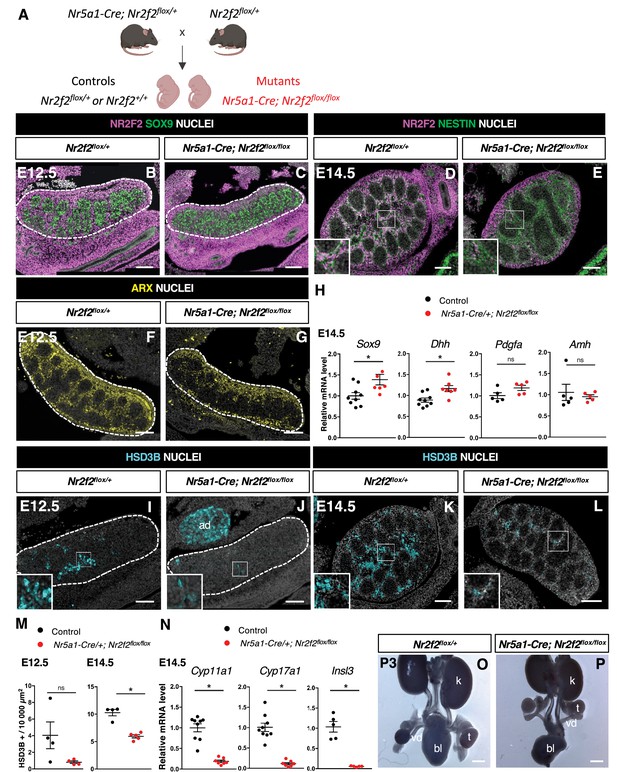
NR2F2 deletion by Nr5a1-Cre impairs fetal Leydig cell (FLC) development.
(A) Generation of Nr5a1-Cre; Nr2f2flox/flox mutants. (B,C) Immunodetection of NR2F2 and SOX9 on embryonic day 12.5 (E12.5) Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes (outlined by dotted lines). NR2F2 is deleted in interstitial cells but is still present in the outermost layer of the testis. (D,E) Immunodetection of NR2F2 and NESTIN on E14.5 Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. NR2F2 is still detected in NESTIN1 positive cells. (F,G) Immunodetection of ARX on E12.5 Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes (outlined by dotted lines). Interstitial cells are generated in Nr5a1-Cre; Nr2f2flox/flox mutants. (H) Quantification of Sox9, Dhh, Pdgfa, and Amh transcripts after normalization to Sdha and Tbp in control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox by RT-qPCR at E14.5. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (I,J) Immunodetection of HSD3B on E12.5 Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes (outlined by dotted lines). ad: adrenal. (K,L) Immunodetection of HSD3B on E14.5 Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. (M) Quantification of the number of HSD3B positive cells per surface unit in control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox testes at E12.5 and E14.5. Each circle represents the mean number of HSD3B positive cells per surface unit of one individual measured on at least two sections per gonad. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (N) Quantification of Cyp11a1, Cyp17a1, and Insl3 after normalization to Sdha and Tbp in control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox by RT-qPCR at E14.5. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (O,P) Macroscopic view of the urogenital tract of XY P3 Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox mutants. The testes are in abdominal position in Nr5a1-Cre; Nr2f2flox/flox mutants. k: kidney, t: testis, bl: bladder. vd: vas deferens. Immunodetection data are representative of triplicate biological replicates. Scale bar = 100 µm in B–G, I–L. Scale bar = 500 µm in O, P.
-
Figure 3—source data 1
Source data for cell counts and RT-qPCR data in Figure 3.
- https://cdn.elifesciences.org/articles/103783/elife-103783-fig3-data1-v1.xlsx
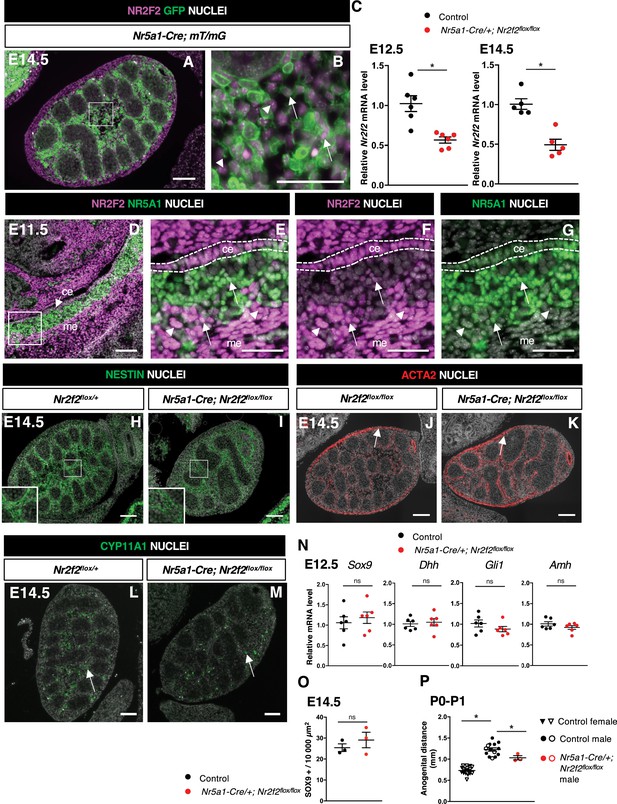
NR2F2 deletion by Nr5a1-Cre impairs fetal Leydig cell (FLC) development.
(A,B) Immunodetection of NR2F2 and GFP on embryonic day 14.5 (E14.5) XY Nr5a1-Cre;mT/mG gonad. Upon Cre-mediated recombination, GFP is expressed in somatic cells of the gonad including NR2F2 positive cells (arrows); however, the outermost layer and some interstitial NR2F2 positive cells (arrowheads) do not express GFP. (C) Quantification of Nr2f2 transcripts after normalization to Sdha and Tbp by RT-qPCR in control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox testes dissected at E12.5 and E14.5. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05. (D–G) Immunodetection of NR2F2 and NR5A1 on E11.5 XY gonad. NR2F2 is co-expressed with NR5A1 in the coelomic epithelium (ce) and in interstitial steroidogenic progenitors (arrows). NR2F2 positive cells that do not express NR5A1 are likely steroidogenic progenitors of mesonephric (me) origin (arrowheads). (H,I) Immunodetection of NESTIN on E14.5 Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. Images corresponding to the samples shown in Figure 3D and E. (J,K) Immunodetection of ACTA2 on E14.5 Nr2f2flox/flox and Nr5a1-Cre; Nr2f2flox/flox testes. White arrows indicate cells that will form the tunica albuginea. (L,M) Immunodetection of CYP11A1 (arrows) on E14.5 Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. (N) Quantification of Sox9, Dhh, Gli1, and Amh transcripts after normalization to Sdha and Tbp by RT-qPCR in control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox testes at E12.5. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. ns indicates p-value>0.05. (O) Quantification of the number of SOX9 positive cells per surface unit in control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox testes at E14.5. Each circle represents the mean number of SOX9 positive cells per surface unit of one individual measured on at least two sections per gonad. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. ns indicates p-value>0.05. (P) Anogenital distance (distance between the anus and the external genitalia measured with an electronic caliper) in control females (wild-type or Nr2f2flox/+, inverted triangles), control males (wild-type or Nr2f2flox/+, black circles), and Nr5a1-Cre; Nr2f2flox/flox males (red circles) measured at P0 (solid symbols) and P1 (open symbols). Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05. Immunodetection data are representative of triplicate biological replicates. Scale bar = 50 µm in B, E–G. Scale bar = 100 µm in A, D, H–M.
-
Figure 3—figure supplement 1—source data 1
Source data for cell counts, anogenital distance measurments and RT-qPCR data in Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/103783/elife-103783-fig3-figsupp1-data1-v1.xlsx
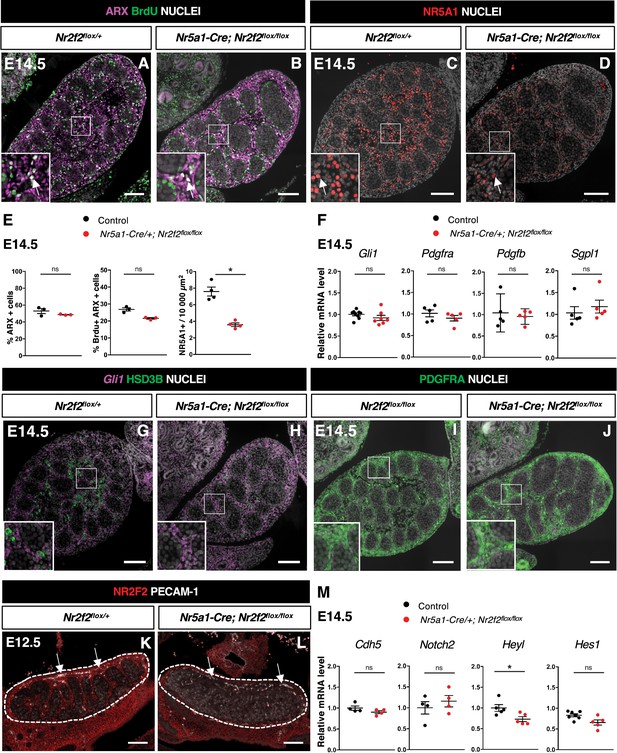
NR2F2 function is required for the initiation of fetal Leydig cell (FLC) differentiation.
(A,B) Immunodetection of ARX and BrdU (arrows) on embryonic day 14.5 (E14.5) XY Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. (C,D) Immunodetection of NR5A1 on E14.5 XY Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. Arrows indicate strong expression of NR5A1 positive FLC. (E) Quantification of the percentage of ARX positive cells (number of ARX positive nuclei relative to the total number of nuclei labeled by DAPI), of the percentage of ARX positive cells labeled by BrdU (number of nuclei positive for ARX and BrdU relative to the number of ARX positive nuclei) and of the number of NR5A1 positive cells per surface unit in E14.5 XY control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox testes. Each circle represents the mean percentage of ARX+ or ARX+/BrdU+ or NR5A1+ cells per surface unit of one individual measured on at least two sections per gonad. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (F) Quantification of Gli1, Pdgfra, Pdgfb, and Sgpl1 transcripts after normalization to Sdha and Tbp in control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox by RT-qPCR at E14.5. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (G,H) In situ hybridization detection of Gli1 transcripts and immunodetection of HSD3B protein on E14.5 XY Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. (I,J) Immunodetection of PDGFRA on E14.5 XY Nr2f2flox/flox and Nr5a1-Cre; Nr2f2flox/flox testes. (K,L) Immunodetection of PECAM-1 and NR2F2 on E12.5 XY Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. PECAM-1 is expressed in germ cells and in endothelial cells (white arrows). (M) Quantification of Cdh5, Notch2, Heyl, and Hes1 transcripts after normalization to Sdha and Tbp in control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox by RT-qPCR at E14.5. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. Immunodetection data are representative of triplicate biological replicates. Scale bar = 100 µm.
-
Figure 4—source data 1
Source data for cell counts and RT-qPCR data in Figure 4.
- https://cdn.elifesciences.org/articles/103783/elife-103783-fig4-data1-v1.xlsx
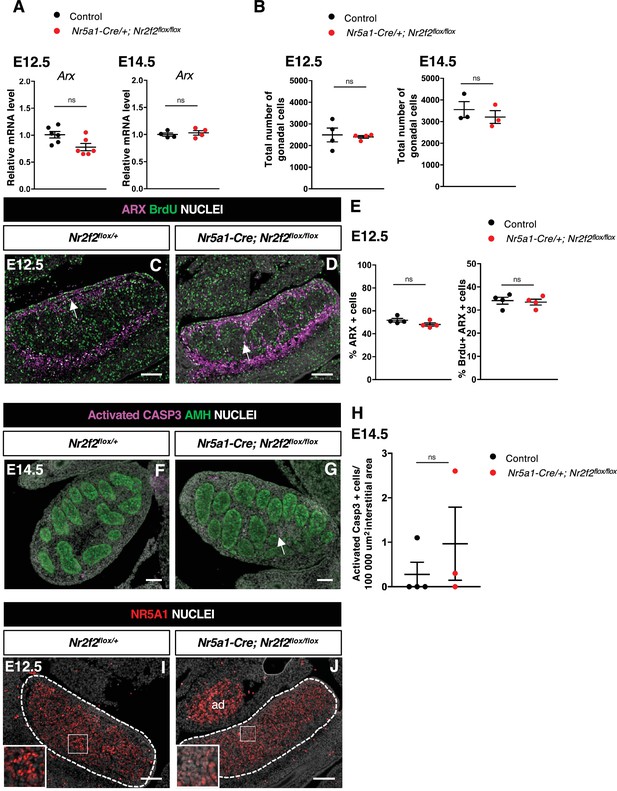
NR2F2 function is required for the initiation of fetal Leydig cell (FLC) differentiation.
(A) Quantification of Arx transcripts after normalization to Sdha and Tbp by RT-qPCR at embryonic day 14.5 (E14.5) and E12.5. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (B) Quantification of the total number of gonadal cells labeled by DAPI per gonadal section on E14.5 and E12.5 XY control (wild-type or Nr2f2flox/+), and Nr5a1-Cre; Nr2f2flox/flox testes. Each circle represents the mean percentage of gonadal cells of one individual measured on at least two sections per gonad. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (C,D) Immunodetection of ARX and BrdU (arrows) on E12.5 XY Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. (E) Quantification of the percentage of ARX positive cells (number of ARX positive nuclei relative to the total number of nuclei labeled by DAPI) and of the percentage of ARX positive cells labeled by BrdU (number of nuclei positive for ARX and BrdU relative to the number of ARX positive nuclei) on E12.5 XY control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox testes. Each circle represents the mean percentage of ARX+ or ARX+/BrdU+ cells per surface unit of one individual measured on at least two sections per gonad. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (F,G) Immunodetection of activated caspase 3 (arrow in F) and AMH on E14.5 XY Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. (H) Quantification of the number of caspase 3 positive cells per 100,000 µm2 of interstitial area (area outside the testis cords) on E14.5 XY control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox testes. Each circle represents the mean number of activated caspase 3 positive cells per surface unit of one individual measured on at least two sections per gonad. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. ns indicates p-value>0.05. (I,J) Immunodetection of NR5A1 on E12.5 XY Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. ad: adrenal. Immunodetection data are representative of triplicate biological replicates. Scale bar = 100 µm.
-
Figure 4—figure supplement 1—source data 1
Source data for cell counts and RT-qPCR data in Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/103783/elife-103783-fig4-figsupp1-data1-v1.xlsx
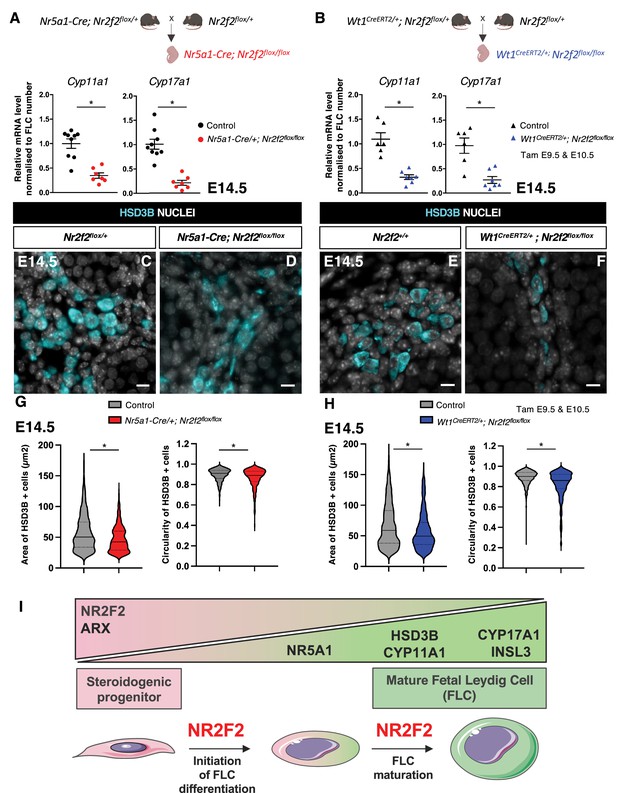
NR2F2 function is required for fetal Leydig cell (FLC) maturation.
(A) RT-qPCR quantification of Cyp11a1 and Cyp17a1 transcripts in control (wild-type or Nr2f2flox/+) and Nr5a1-Cre; Nr2f2flox/flox testes after normalization to Sdha and Tbp and to the number of FLC as quantified by HSD3B immunofluorescence at embryonic day 14.5 (E14.5). Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (B) RT-qPCR quantification of Cyp11a1 and Cyp17a1 transcripts in Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox gonads after normalization to Sdha and Tbp and to the number of FLC as quantified by HSD3B immunofluorescence at E14.5. Data are shown as means ± SEM. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (C,D) Immunodetection of HSD3B on E14.5 XY Nr2f2flox/+ and Nr5a1-Cre; Nr2f2flox/flox testes. (E,F) Immunodetection of HSD3B on E14.5 XY Nr2f2flox/+ and Wt1CreERT2; Nr2f2flox/flox testes after tamoxifen was administered at E9.5 and E10.5. (G) Quantification of the area and circularity of HSD3B positive cells in two E14.5 control (wild-type or Nr2f2flox/+, 737 cells, gray violin plot) and three Nr5a1-Cre; Nr2f2flox/flox (486 cells, red violin plot) testes. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (H) Quantification of the area and circularity of HSD3B positive cells in three E14.5 control (wild-type or Nr2f2flox/+, 1485 cells, gray violin plot) and three Wt1CreERT2; Nr2f2flox/flox (474 cells, blue violin plot) testes. Statistical significance was assessed by Mann-Whitney U two-tailed test. * indicates p-value≤0.05; ns indicates p-value>0.05. (I) Summary figure: NR2F2 is expressed in spindle-shaped interstitial steroidogenic progenitors together with ARX and is progressively downregulated upon FLC differentiation. NR2F2 is required for the initiation of FLC differentiation (marked by the upregulation of NR5A1) and for FLC maturation (characterized by the increase in cytoplasmic volume and the high expression of steroidogenic enzymes and Insl3). Immunodetection data are representative of triplicate biological replicates. Scale bar = 10 µm.
-
Figure 5—source data 1
Source data for cell measurements and RT-qPCR data in Figure 5.
- https://cdn.elifesciences.org/articles/103783/elife-103783-fig5-data1-v1.xlsx
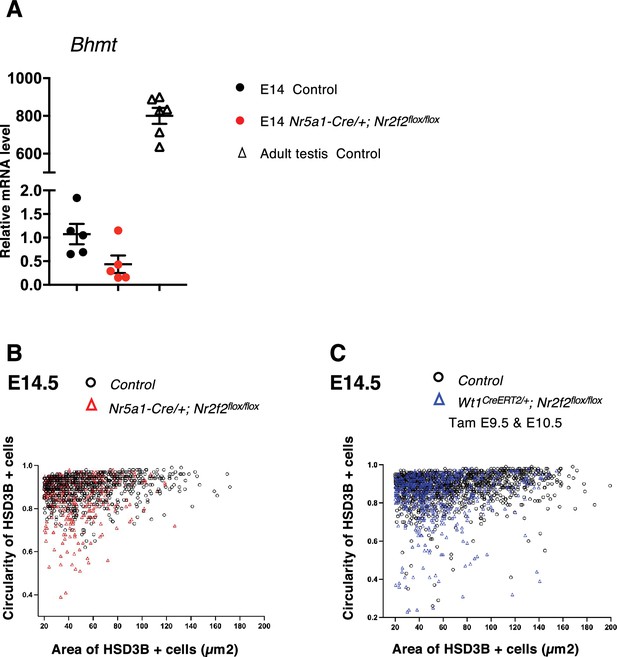
NR2F2 function is required for fetal Leydig cell (FLC) maturation.
(A) Quantification of Bhmt transcripts after normalization to Sdha and Tbp by RT-qPCR at embryonic day 14.5 (E14.5) in XY control (wild-type or Nr2f2flox/+), and Nr5a1-Cre; Nr2f2flox/flox testes and in wild-type adult testes. (B) Quantification of the circularity and area of HSD3B positive cells in two E14.5 control (wild-type or Nr2f2flox/+, 737 cells, black open circles) and three Nr5a1-Cre; Nr2f2flox/flox (486 cells, red open triangles) testes. (C) Quantification of the circularity and area of HSD3B positive cells in three E14.5 control (wild-type or Nr2f2flox/+, 1485 cells, black open circles) and three Wt1CreERT2; Nr2f2flox/flox (474 cells, blue open triangles) testes.
-
Figure 5—figure supplement 1—source data 1
Source data for RT-qPCR data in Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/103783/elife-103783-fig5-figsupp1-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Mus musculus) | Nr2f2 | MGI | MGI:1352452 | |
| Genetic reagent (Mus musculus) | Nr2f2tm1Vc | Dr. M. Vasseur-Cognet | MGI:3578106 | |
| Genetic reagent (Mus musculus) | Wt1tm2(cre/ERT2)Wtp | Dr. William T Pu | MGI:7528785 | |
| Genetic reagent (Mus musculus) | Tg(Nr5a1-cre)2Klp | Dr. Keith L Parker | MGI:5493455 | |
| Chemical compound | Tamoxifen | Sigma-Aldrich | T5648 | 200 mg/kg body weight |
| Chemical compound | 5-Bromo-2'-deoxy-uridine | Sigma-Aldrich | B5002 | 50 mg/kg body weight |
| Chemical compound | Paraformaldehyde | EMS | 15710-S | 4% in PBS |
| Antibody | Anti-ACTA2 (Mouse monoclonal) | Gift from Dr. Chaponnier | IF (1:500) | |
| Antibody | Anti- Activated Caspase 3 (Rabbit polyclonal) | R&D Systems | AF835 (RRID:AB_2243952) | IF (1:200) |
| Antibody | Anti- AMH (Mouse monoclonal) | Bio-Rad | MCA2246 (RRID:AB_2226471) | IF (1:50) |
| Antibody | Anti- ARX (Rabbit polyclonal) | Gift from Pr. Morohashi and Dr. Inoue | IF (1:200) | |
| Antibody | Anti-COL4A1 (Rabbit polyclonal) | Abcam | ab19808 (RRID:AB_445160) | IF (1:400) |
| Antibody | Anti-CYP11A1 (Rabbit polyclonal) | Gift from Dr. Wilhelm | IF (1:200) | |
| Antibody | Anti-GATA4 (Goat polyclonal) | Santa Cruz Biotechnology | Sc-1237 (RRID:AB_2108747) | IF (1:200) |
| Antibody | Anti-GFP (Chicken polyclonal) | Abcam | Ab13970 (RRID:AB_300798) | IF (1:200) |
| Antibody | Anti-HSD3B (Goat polyclonal) | Santa Cruz Biotechnology | Sc-30820 (RRID:AB_2279878) | IF (1:200) |
| Antibody | Anti-HSD3B (Rabbit polyclonal) | Invitrogen | PA5-76669 (RRID:AB_2720396) | IF (1:500) |
| Antibody | Anti-Ki67 (Rabbit monoclonal) | Spring Bioscience | M3062 (RRID:AB_11219741) | IF (1:200) |
| Antibody | Anti-LAMA1 (Rabbit polyclonal) | Sigma-Aldrich | L9393 (RRID:AB_477163) | IF (1:200) |
| Antibody | Anti-NESTIN (Rabbit) | BioLegend | 839801 (RRID:AB_2565443) | IF (1:1000) |
| Antibody | Anti-NR2F2 (Mouse monoclonal) | R&D Systems | PP-H7147-00 (RRID:AB_2155627) | IF (1:200) |
| Antibody | Anti-NR5A1 (Rabbit polyclonal) | Cosmo Bio | KO611(RRID:AB_2861370) | IF (1:200) |
| Antibody | Anti-PDGFRA (Rabbit polyclonal) | Santa Cruz Biotechnology | SC-338 (RRID:AB_631064) | IF (1:200) |
| Antibody | Anti-PECAM-1 (Goat polyclonal) | Santa Cruz Biotechnology | Sc-1506 (RRID:AB_2161037) | IF (1:200) |
| Antibody | Anti-RUNX1 (Rabbit monoclonal) | Abcam | ab92336 (RRID:AB_2049267) | IF (1:500) |
| Antibody | Anti-SOX9 (Rabbit polyclonal) | Sigma-Aldrich | HPA001758 (RRID:AB_1080067) | IF (1:250) |
| Antibody | Anti-WT1 (Goat polyclonal) | R&D Systems | AF5729 (RRID:AB_2216239) | IF (1:200) |
| Commercial assay or kit | BrdU detection kit | Roche | 11 296 736 001 | |
| Commercial assay or kit | RNAscope Multiplex Fluorescent Reagent Kit v2 | Bio-Techne | 323110 | |
| Sequence-based reagent | Gli1 probe | Bio-Techne | 311001 | |
| Commercial assay or kit | RNeasy Micro Kit | QIAGEN | 74004 | |
| Commercial assay or kit | SYBR Green I Master | Roche | 04887352001 | |
| Software, algorithm | RefFinder | https://www.ciidirsinaloa.com.mx/RefFinder-master/?type=reference | ||
| Software, algorithm | OMERO | https://www.openmicroscopy.org/omero/ | ||
| Software, algorithm | GraphPad Prism | Graphpad Software, Inc, La Jolla, CA, USA | GraphPad Prism 10.2.1 |





