Evolution of lateralized gustation in nematodes
eLife Assessment
Mackie and colleagues present a valuable comparison of lateralized gustation in two well-studied nematodes. Their results present convincing evidence that ASEL/R lateralization exists and is achieved by different means in P. pacificus compared to C. elegans. This work will be of interest to neurobiologists interested in how small nervous systems make sense of the environment, and how evolution can take multiple paths to asymmetry within a neuron class.
https://doi.org/10.7554/eLife.103796.3.sa0Valuable: Findings that have theoretical or practical implications for a subfield
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Convincing: Appropriate and validated methodology in line with current state-of-the-art
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
Animals with small nervous systems have a limited number of sensory neurons that must encode information from a changing environment. This problem is particularly exacerbated in nematodes that populate a wide variety of distinct ecological niches but only have a few sensory neurons available to encode multiple modalities. How does sensory diversity prevail within this constraint in neuron number? To identify the genetic basis for patterning different nervous systems, we demonstrate that sensory neurons in Pristionchus pacificus respond to various salt sensory cues in a manner that is partially distinct from that of the distantly related nematode Caenorhabditis elegans. Previously we showed that P. pacificus likely lacked bilateral asymmetry (Hong et al., 2019). Here, we show that by visualizing neuronal activity patterns, contrary to previous expectations based on its genome sequence, the salt responses of P. pacificus are encoded in a left/right asymmetric manner in the bilateral ASE neuron pair. Our study illustrates patterns of evolutionary stability and change in the gustatory system of nematodes.
Introduction
Nematodes form a vast array of ecological relationships, from specialized parasite–host dependencies to nematode–microbial interactions, each one demanding exquisitely fine-tuned sets of sensory palates that span multiple modalities (Bargmann, 2006; Hong and Sommer, 2006; Chaisson and Hallem, 2012; Wheeler et al., 2020; Rengarajan and Hallem, 2016; Koneru et al., 2016; Lo and Sommer, 2022; Lo et al., 2024). Yet, the number of sensory neurons across diverse nematode species seems to be constrained (White et al., 1986; Schafer, 2016; Hong et al., 2019). Several free-living and parasitic nematode species examined by serial section electron microscopy have nearly identical numbers of 12–13 pairs of head sensory neurons, known as the amphid neurons (Hong et al., 2019; Ward et al., 1975; Li et al., 2001; Bumbarger et al., 2009; Zhu et al., 2011). How does sensory diversity arise within this constraint in neuron number? When coupled with well-described neuronal anatomy, this conserved neuron count allows for detailed comparisons at the single-cell resolution and represents an opportunity to interrogate how sensory cues are processed by anatomically similar nervous systems to produce species-specific or developmental stage-dependent behavioral outputs. To identify the genetic basis for patterning different nervous systems and to understand the processes that underlie evolutionary changes in adapting to different environments, several comparative model systems have been developed to promote comparisons to the well-studied nematode Caenorhabditis elegans at the genetic and cellular levels, including the predatory entomophilic nematode, Pristionchus pacificus (Hong et al., 2019; Loer and Rivard, 2007; Baiocchi et al., 2017; Gang et al., 2020; Bryant et al., 2022).
As expected from their association with insects in the wild, the olfactory preferences of P. pacificus are distinct from those of C. elegans and the human parasite Strongyloides stercoralis (Hong and Sommer, 2006; Chaisson and Hallem, 2012; Hallem et al., 2011), but little is known about P. pacificus responses to water-soluble compounds. In C. elegans, the main salt receptor neuron class comprises of a bilateral pair of left and right ASE neurons (ASEL and ASER), which serve to induce an attractive locomotory response toward an increase in salt concentration (Bargmann and Horvitz, 1991). The gene che-1 (chemosensory defective) encodes a transcription factor that is exclusively expressed in the ASE neurons and is required for their proper differentiation, such that a che-1 mutant results in defective salt attraction (Dusenbery et al., 1975; Uchida et al., 2003; Chang et al., 2003; Etchberger et al., 2007).
One major role of C. elegans CHE-1 is to promote lateral asymmetry in the ASE neurons. The left and right ASE neurons asymmetrically express receptor-type guanylyl cyclases (rGCs, encoded by gcy genes) (Yu et al., 1997). This finding led to the realization that the ASE neurons are lateralized, such that the left and the right ASE neurons differentially respond to distinct salt ions (Pierce-Shimomura et al., 2001; Chang et al., 2004; Suzuki et al., 2008; Ortiz et al., 2009). This observation led in turn to the identification of a complex gene regulatory network that genetically programs the distinct sensory potentials of the left and right ASE neurons (Hobert, 2014), which includes a miRNA, lsy-6, at the top of this gene regulatory network (Hobert, 2014; Johnston and Hobert, 2003; Cochella and Hobert, 2012). However, the lsy-6 miRNA evolved selectively in the Caenorhabditis genus (Ahmed et al., 2013) and is absent in P. pacificus. Moreover, P. pacificus does not show an expansion of the ASEL-type and ASER-type rGCs, as C. elegans does (Hong et al., 2019). With these two genomic observations in mind, we had previously proposed that the ASE neurons are unlikely to be lateralized in P. pacificus (Hong et al., 2019).
However, we now revise this view in light of our work on mapping chemosensory responses in P. pacificus on the level of behavior and neuronal activity. We demonstrate that P. pacificus does in fact show lateralized chemosensory profiles, indicating that P. pacificus must have evolved independent means to establish ASE laterality. We also show that the tastant palate of P. pacificus is distinct from that of C. elegans, and that its dependence on ASE, as well as its terminal selector transcription factor che-1, have also diverged.
Results
P. pacificus and C. elegans differ in their behavioral responses to salts
Previous cross-species comparisons between P. pacificus and C. elegans indicated strong differences in olfaction preferences that reflect their divergent evolutionary histories and ecology (Hong and Sommer, 2006). To identify the neurons that mediate gustation, we first compared the chemosensory profiles of these two species toward water-soluble ions. In this survey, we found ammonium salts to be the strongest attractants to wildtype P. pacificus J4 to adult hermaphrodites (NH4Br, NH4Cl, and NH4I), with NH4I significantly more attractive to P. pacificus compared to C. elegans (Figure 1). Notably, in contrast to C. elegans (Ward, 1973), we find that P. pacificus is less attracted to NaCl and LiCl compared to the ammonium salts (Figure 1). Also, P. pacificus is repulsed by acetate salts (NaAc and NH4Ac), which induce attractive responses in C. elegans (Frøkjaer-Jensen et al., 2008). We conclude that P. pacificus and C. elegans display differences in their salt preferences.
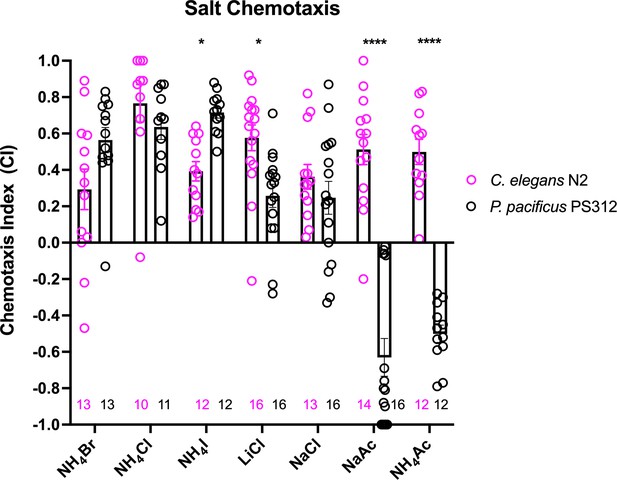
A comparison of chemotaxis responses to water-soluble ions between P. pacificus and C. elegans.
J4 to adult hermaphrodites from the two species responded to NH4I, LiCl, and acetates significantly differently. Using two-way ANOVA, significant difference found between wildtype P. pacificus and C. elegans for the same salt is indicated above each pair (*p < 0.05, ****p < 0.0001), while the differences within P. pacificus is as follows: all salts showed difference when compared to NaAc and to NH4Ac (****p < 0.0001), but not between NH4Ac and NaAc. Both LiCl and NaCl attraction are significantly lower than NH4Cl (*p < 0.05) and NH4I (***p < 0.001). Error bars denote standard error of the mean and the sample sizes are indicated on the bottom.
Ppa-che-1 shows similarities and differences to Cel-che-1 in both expression and function
The C. elegans che-1 mutant (chemotaxis-defective) was originally isolated for its inability to respond to a broad panel of salt tastants (Dusenbery et al., 1975; Ward, 1973), including those described above (Suzuki et al., 2008; Ortiz et al., 2009; Frøkjaer-Jensen et al., 2008). che-1 was found to encode for a Zn finger transcription factor that is exclusively expressed in the ASE neuron pair (Etchberger et al., 2007), which through laser ablations had been found to be the main salt receptor neurons (Bargmann et al., 1993). che-1 was found to control the entire differentiation of the ASE neurons, including the expression of putative receptors of the GCY receptor guanylyl cyclase family (Uchida et al., 2003; Chang et al., 2003; Etchberger et al., 2007; Etchberger et al., 2009).
To assess whether che-1 performs a similar function in salt perception for P. pacificus as for C. elegans, we analyzed the expression and function of the Ppa-che-1 ortholog. In a previous paper, we reported that the 5′ region of the sole Pristionchus che-1 ortholog directs reporter expression to the Ppa ASE and Ppa ASG neuron classes (Hong et al., 2019). Re-examination of our provisional cell identifications using a newly generated che-1p::GFP strain with stronger neurite expression revealed highly elaborated finger-like dendritic endings in the more anterior amphid neuron that could unambiguously be assigned to the AFD neurons (Figure 2A–C), prompting us to reassign expression of che-1 to ASE and AFD.
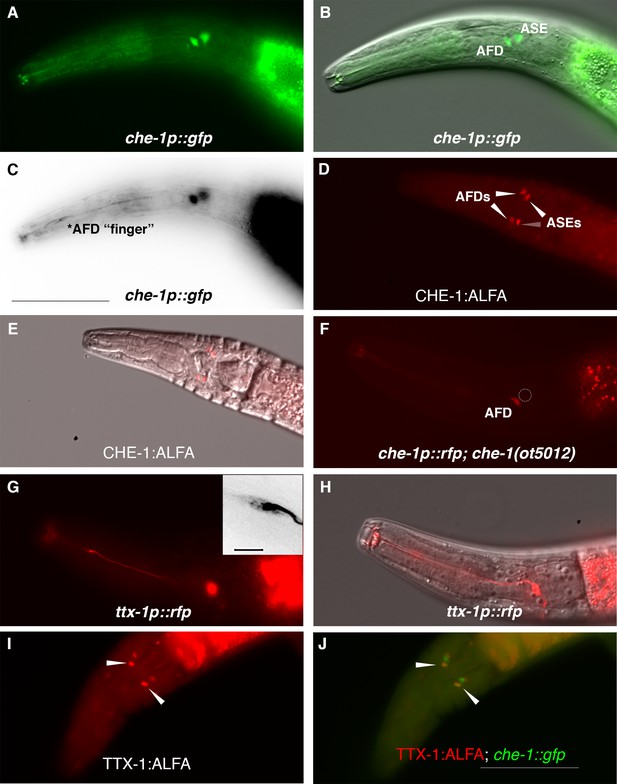
P. pacificus che-1 expression in ASE and AFD amphid neurons.
(A, B) The che-1::GFP marker in the che-1::RCaMP reporter strain is expressed in the ASE and AFD amphid neurons. (C) che-1::GFP expression is detectable in the morphologically distinct AFD neurons with ‘finger’-like dendritic endings. (D, E) Immunostaining of CHE-1::ALFA shows two pairs of amphid neuron cell bodies corresponding to the ASE and AFD neurons; dorsal–ventral view (n = 114). (F) The loss of che-1 results in loss che-1::RFP expression in the ASE (circle) while retaining reduced AFD expression. (G) ttx-1p::RFP expression in the AFD neurons with ‘finger’-like dendritic endings. Inset shows expanded inverted black–white image of the AFD dendritic ending. (H) AFD expression of the same ttx-1::RFP animal shown in (G) in a different plane with cell body in focus. (I, J) Immunostaining of TTX-1::ALFA (red) shows one pair of amphid neuron cell bodies co-localizing with the anterior pair of che-1::GFP-expressing AFD neurons (yellow); dorsal–ventral view (n = 13). Anterior is left and the scale bar in (C) represents 50 µm for all panels except for the G inset, which is 5 µm.
We confirmed that the che-1 reporter transgene indicates the full expression of the endogenous che-1 locus by tagging the endogenous che-1 locus with an ALFA-tag (Igreja et al., 2022). The che-1::ALFA animals showed staining in 2 pairs of head neurons whose position is consistent with being the ASE and AFD neurons (Figure 2D, E). By crossing the che-1 reporter transgene into a che-1 mutant background (see below), we also found that che-1 autoregulates its own expression especially in the ASE neurons (Figure 2F, GFP expression in 97% in WT (n = 36) vs 4% in che-1(−) (n = 48)), as it does in C. elegans (Etchberger et al., 2007). The reduction of che-1p::GFP in the AFDs was also observed (GFP expression in 100% in WT (n = 36) vs 33% in che-1(−) (n = 48)).
To provide further evidence that Ppa-CHE-1 is indeed expressed in the Ppa-AFD neurons, we analyzed the expression of the Ppa-ttx-1p::RFP reporter. In C. elegans, the OTX homeodomain transcription factor TTX-1 is a terminal selector expressed in the AFD neurons required for designating the AFD fate (Satterlee et al., 2001). We found ttx-1p::RFP to be strongly expressed in a pair of neurons with the hallmark finger-like dendritic ending of the AFD neurons (Figure 2G, H), as well as expression in other head and tail neurons (possibly RIP and RIB) and cells likely to be the pharyngeal marginal cells based on likely conservation with Cel-ttx-1 expression (Reilly et al., 2022). To confirm that endogenous TTX-1 protein expression is co-expressed with che-1p::GFP, we examined C-terminally tagged ttx-1:ALFA animals and found co-localization in the same anterior pair of amphid neurons, but no co-expression in the posterior pair of amphid neurons (Figure 2I, J, n = 15). Interestingly, the promoter expression in the posterior pair of amphid neurons in animals with both ttx-1p::RFP and gcy-22.3p::GFP reporters do co-localize in the ASER neurons (Figure 2—figure supplement 1), possibly due to differences between the expression patterns of the four possible ttx-1 splice forms. The transcriptional and protein co-expression of ttx-1 and che-1 in the AFD neurons unequivocally show the expression of che-1 in both AFD and ASE neurons in P. pacificus.
Next, we examined whether P. pacificus salt responsiveness shows similar che-1 dependence as in C. elegans. We generated two putative null alleles in the Ppa-che-1 homolog using CRISPR/Cas9 genome engineering, through introduction of small deletions in the first exon of the gene, thereby resulting in frameshift and premature stops (Figure 3A; Figure 3—figure supplement 2). Both Ppa-che-1 alleles exhibited defects in attraction toward NH4Cl and LiCl compared to wildtype (Figure 3B). However, unlike in C. elegans, Ppa-che-1 mutants showed no detectable difference in responses to NH4I, NaCl, and NaAc.
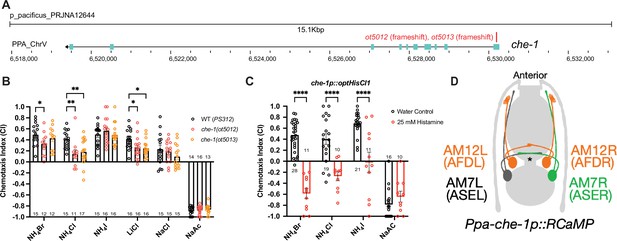
che-1 expressing amphid neurons are required for sensing water-soluble ions in P. pacificus.
(A) The che-1 locus with CRISPR/Cas9-induced mutations in Exon 1. (B) The che-1 mutants show defects in attraction toward NH4Br, NH4Cl, and LiCl. Sample sizes are indicated below for attractants and above for repellent. (C) The che-1p::HisCl1 animals lose attraction toward NH4Br, NH4Cl, and NH4I in a histamine-dependent manner. Sample sizes are indicated at the base of each bar. (D) A schematic map of the P. pacificus AM7 (ASE) and AM12 (AFD) amphid neurons that express the che-1p::RCaMP used in calcium imaging. The ASE axons are the only amphid axons in P. pacificus to cross each other over the dorsal lateral midline contralaterally. **p < 0.01, *p < 0.05, two-way ANOVA with Dunnett’s post hoc comparison showing alleles with significant difference to wildtype P. pacificus (PS312). ****p < 0.0001, two-way ANOVA showing significant difference between water control and histamine treatment.
We considered two different possibilities for the behavioral differences of Ppa-che-1 and Cel-che-1 mutants. P. pacificus may use sensory neurons other than ASE to sense these cues, or alternatively, Ppa-che-1 may not have the same fundamental impact on ASE function in P. pacificus as it does in C. elegans. To explore these different possibilities, we silenced che-1 expressing neurons by expressing codon-optimized HisCl1 channel under control of the che-1 promoter. Histamine-treated che-1p::HisCl1 animals showed complete loss of attraction to NH4Br, NH4Cl, and NH4I but did not significantly alter their repulsive response to NaAc (Figure 3C). As a control, we show that the presence of the che-1p::HisCl1 transgene was necessary for the knockdown of NH4Br attraction (Figure 3—figure supplement 1). These findings corroborate that NaAc is sensed by neurons other than ASE (or AFD, in which the che-1 promoter also drives HisCl1). Since NH4I sensation is affected by silencing of che-1(+) neurons but is unaffected in che-1 mutants, ASE function may be more greatly impacted by the silencing of ASE than by the loss of che-1.
The ASE neurons show left/right asymmetric responses to salt
To assess whether Ppa ASE neurons show the same lateralized response to salt ions as Cel ASE neurons, we generated transgenic P. pacificus lines that express RCaMP in ASE neurons and assessed calcium responses to attractive salts (Figure 4, Figure 4—figure supplements 1 and 2). Specifically, we looked for changes in calcium levels immediately after the addition and removal of specific salts. When 250 mM NH4Cl is administered, an ‘ON’ response is observed as calcium transiently increases in the left ASE neuron (ASEL). In contrast, an ‘OFF’ response was observed as calcium sharply dips in the right ASE neuron (ASER) before quickly returning to baseline when the salt was removed, which suggests hyperpolarization of this neuron (Figure 4A, B; Suzuki et al., 2008). However, when presented with a tenfold lower concentration of 25 mM NH4Cl, the ‘OFF’ response completely disappeared in ASER while the ‘ON’ response became more pronounced in ASEL. The ASER responses to 250 and 25 mM NaCl (Figure 4D) were very similar to the ‘OFF’ response (including hyperpolarization) observed for NH4Cl, but the ASEL responses differ between the two salts: instead of the ‘ON’ response expected of ASEL, we observed a relatively weak ‘OFF’ response without the characteristic hyperpolarization but accompanied by an attenuated ‘bump’, a profile that we classify as an ‘OFF-2’ response (Figure 4C). Finally, we examined the response to NH4I and found that it also elicited laterally asymmetric responses, but yet again distinct from the responses to both NH4Cl and NaCl, with ASEL showing a strong ‘ON’ response, and ASER showing an ‘ON–OFF’ biphasic response to 250 mM NH4I (Figure 4E, F; Kato et al., 2014; Wang et al., 2015). Interestingly, the ASER exhibited an ‘OFF’-only response to 25 mM NH4I, which was not observed for the same concentration of NH4Cl and NaCl, and thus may reflect the higher response to NH4I than to NH4Cl and NaCl in the behavior assays. Altogether, P. pacificus ASE neurons clearly show left/right asymmetric responses to salt attractants and these asymmetric responses show similarities and differences to the C. elegans ASE taste neurons (see Discussion).
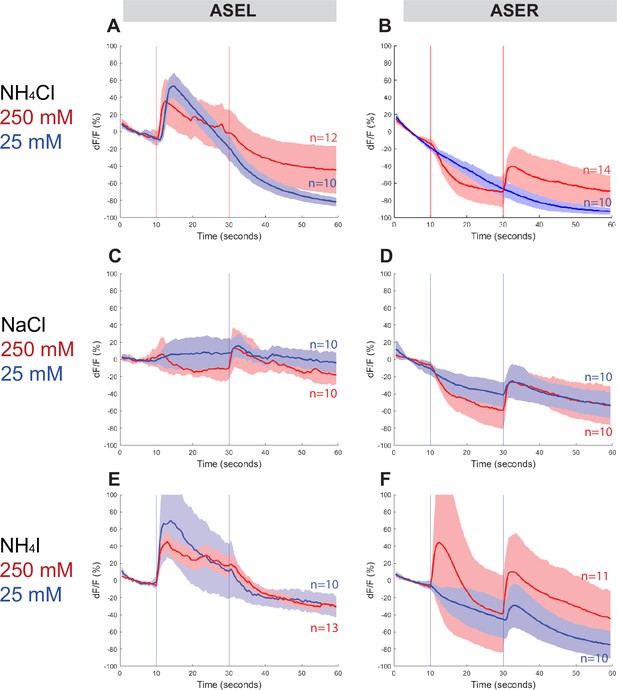
ASEL and ASER responses to different concentrations of NH4Cl, NaCl, and NH4I.
Average percent change in RCaMP fluorescence (dF/F) over time (seconds) of tracked ASE (left and right) sensory neurons in P. pacificus. Salts were presented at 10 s (‘ON’, left vertical line) for a duration of 20 s, and then removed (‘OFF’, right vertical line) for the remaining 30 s; the total recording time was 60 s. (A, B) ASEL and ASER neuron responses to high (250 mM, red) compared to low (25 mM, blue) concentrations of NH4Cl. (C, D) ASEL and ASER neuron responses to NaCl. (E, F) ASEL and ASER neuron responses to NH4I. Shaded ribbons represent 95% confidence intervals. Shaded ribbons in (E, F) have been cropped to maintain consistent y-axes across the plots, allowing for easier comparison. Sample sizes are indicated (n).
The AFD neurons also respond to salts in P. pacificus
The RCaMP line that we used to assess calcium responses in ASE is also expressed in AFD, allowing us to simultaneously examine the calcium responses in the AFD (AM12) neurons (Figure 5, Figure 2—figure supplement 1, Figure 5—figure supplements 1 and 2, Figure 7—figure supplement 1). Surprisingly, we detected a distinctly ‘ON–OFF’ biphasic response to all three salt types at both concentrations. Specifically, although we observed weaker or comparable responses in AFD neurons when compared to either ASE neuron’s response toward 250 mM NH4Cl, NaCl, and NH4I (Figure 5A, B, E, F, I, J), the AFD responses were more robust than ASER toward 25 mM NH4Cl, NaCl, and NH4I (Figure 5D, H, L). Specifically, AFD neurons responded strongly to 25 mM NaCl when neither one of the ASE neurons showed a positive response (Figure 5G, H). Averaging the calcium transients separately by AFD left versus right did not result in significant differences in the shape of the neuronal calcium responses, with the exception of the AFDR responses to higher versus lower concentrations of NH4I (Figure 5—figure supplement 2, Figure 7—figure supplement 1). We have not further pursued whether these AFD responses are a reflection of a direct perception of salt or a secondary consequence of communication of salt-perceptive neurons (like ASE) to AFD.
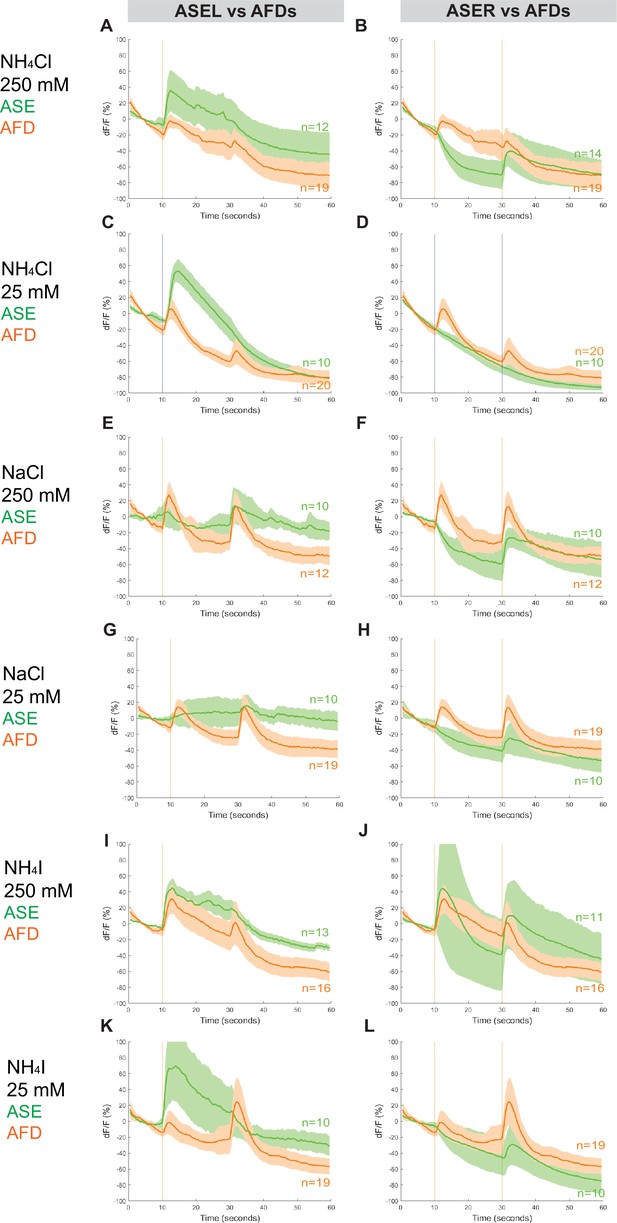
Combined AFD responses in comparison to ASE left or right neuron responses to NH4Cl, NaCl, and NH4I.
Average percent change in RCaMP fluorescence (dF/F) over time (seconds) as described in Figure 4. Averaged combined AFD (left and right neurons, orange) compared to left or right ASE (green) responses to (A, B) 250 mM NH4Cl, (C, D) 25 mM NH4Cl, (E, F) 250 mM NaCl, (G, H) 25 mM NaCl, (I, J) 250 mM NH4I, and (K, L) 25 mM NH4I. Shaded ribbons represent 95% confidence intervals. Shaded ribbons in (J, K) have been cropped to maintain consistent y-axes across the plots, allowing for easier comparison. Sample sizes are indicated (n).
A target of che-1, the guanylyl cyclase gcy-22.3, is required for ASER salt response
We further explored the asymmetric salt perception by the ASE neurons, which in C. elegans is largely mediated through distinct receptor-type guanylyl cyclases (rGC proteins, encoded by gcy genes), which confer salt specificity via their extracellular domains (Ortiz et al., 2009). Our previous genome survey of Ppa homologs of gcy genes has revealed patterns that made us question whether Ppa gcy genes are convincing candidates for lateralized chemotactic responses. Specifically, we noted that ASER-expressed C. elegans gcy genes and ASEL-expressed C. elegans gcy genes have only expanded in the Caenorhabditis genus (Hong et al., 2019). One outlier to this pattern is the Cel-gcy-22 gene, which is expressed in ASER, but has not expanded in C. elegans (Hong et al., 2019; Ortiz et al., 2006). However, this gene has duplicated several times in P. pacificus, resulting in 5 putative Ppa-gcy-22 paralogs (Hong et al., 2019). We fused the promoter of one of these paralogs, Ppa-gcy-22.3, to gfp and found that transgenic animals express GFP exclusively in ASER (Figure 6A), identical to the C. elegans gcy-22 ortholog (Ortiz et al., 2006). We confirmed its expression in ASER by analyzing animals that carry both the Ppa-gcy-22p::gfp reporter and the Ppa-che-1p::rfp reporter, showing a unilateral overlap of these reporters in ASER (Figure 6B).

The laterally asymmetric expression of gcy-22.3 is dependent on the zinc finger transcription factor CHE-1.
(A) The gcy-22.3::GFP marker is expressed exclusively in the right ASE neuron (ASER) (n > 200). (B) The gcy-22.3::GFP marker co-localizes with che-1::RFP expression in the ASER. (C) gcy-22.3::GFP expression is absent in the che-1(ot5012) mutant (n = 55). Anterior is left and dorsal is top. Scale bar in (A) represents 50 µm for all panels.
To assess whether Ppa-gcy-22.3 is a potential effector of Ppa-che-1 function, we crossed the gcy-22.3 reporter into che-1(ot5012) mutants. We found expression of gcy-22.3 was eliminated (Figure 6C), leading us to conclude that gcy-22.3 is a potential effector of che-1 function, identical to its homolog in C. elegans.
To determine whether and which of the observed salt responses is mediated by the ASER-expressing gcy-22.3, we generated a putative gcy-22.3 null mutant through CRISPR/Cas9 genome editing (2 bp complex deletion that introduces a frameshift) (Figure 7A) and examined its response to the higher salt concentration. The ‘OFF’ response to 250 mM NH4Cl was notably abolished in the ASER neuron in the loss-of-function gcy-22.3 mutant, while the ‘ON’ response in the ASEL remained intact (Figure 7B, C). However, the responses to 250 mM NaCl were not significantly reduced in either the ASEL or ASER neuron in the gcy-22.3 mutant (Figure 7D, E). Furthermore, the gcy-22.3 mutation also reduced the ‘ON’ portion of the AFD biphasic response following presentation of 250 mM NH4Cl and NaCl (Figure 7F, G). We also examined the behavioral responses toward individual salt ions in gcy-22.3 mutants, including a second loss-of-function allele csu182. Overall, we found no significant differences between wildtype and mutants in responses toward individual ions, and only the attraction to NH4Cl was slightly enhanced in both alleles (Figure 8), which was unexpected given the lack of calcium response observed in gcy-22.3(csu181) toward NH4Cl. Our findings show that while proper ASE function is critical for salt attraction, defects in individual gcy genes do not lead to a major impact on the worms’ ability to track toward attractive salts.
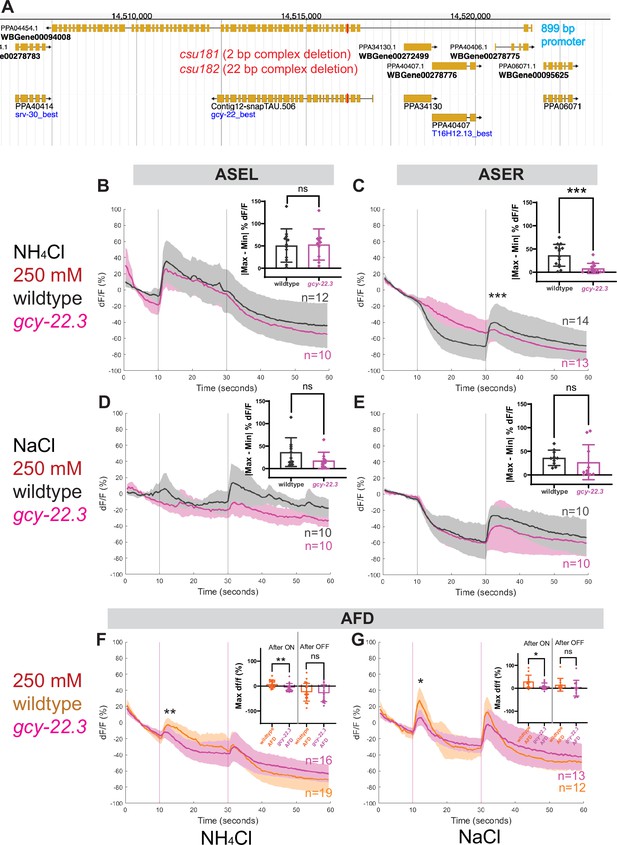
ASE and AFD responses in wildtype compared to gcy-22.3 mutants.
Average percent change in RCaMP fluorescence (dF/F) over time (seconds) as described in Figure 4. (A) http://pristionchus.org/ Genome Browser view of the gcy-22.3 locus with the CRISPR/Cas9-induced mutations indicated with a red bar (Exon 5 of PPA04454 or Exon 4 of Contig12-snapTAU.506). (B, C) ASEL and ASER neuron responses to 250 mM NH4Cl wildtype (gray) and gcy-22.3 mutants (magenta). (D, E) ASEL and ASER neuron responses to 250 mM NaCl in wildtype (gray) and gcy-22.3 mutants (magenta). AFD (combined left and right neurons) responses (F) to 250 mM NH4Cl and (G) to 250 mM NaCl in wildtype (orange) and gcy-22.3 mutants (magenta). Shaded ribbons represent 95% confidence intervals. Sample sizes are indicated (n). For ASEL/R comparisons, bar plots represent the difference between the minimum % dF/F value 10 s pre-stimulus and maximum % dF/F value 10 s post-stimulus for either the (B) ON or (C, E) OFF stimulus. For AFD comparisons, bar plots represent maximum % dF/F values 10 s after the ON and OFF stimulus. *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant difference. ‘ns’ indicate no significant difference. Comparisons between different genotypes were analyzed using unpaired t-test or Mann–Whitney test.
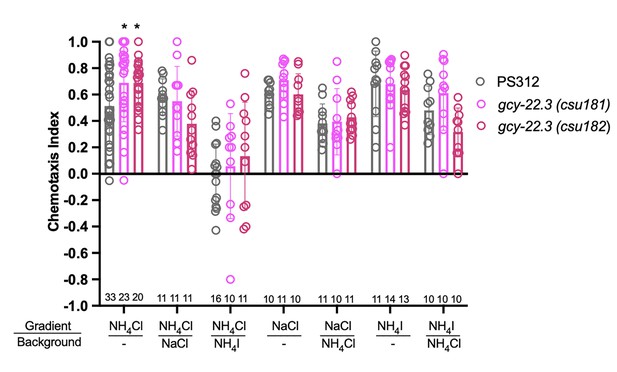
Chemotaxis responses to individual ions in gcy-22.3 mutants.
Young adult hermaphrodite responses to salt gradients with or without various salts in background. *p < 0.05. Significant differences were found between wildtype PS312 and gcy-22.3 mutants to NH4Cl by two-way ANOVA and Dunnett’s test. Each assay involves a minimum of 10 animals. Sample sizes for each condition are indicated on the bottom. Error bars denote standard error of the mean.
Discussion
Our study has revealed several insights into the substrates of evolutionary changes between two distantly related nematode species, P. pacificus and C. elegans. We used intracellular calcium levels as our readout for neuronal activity with a genetically encoded calcium sensor in two pairs of che-1-expressing amphid sensory neurons – the first calcium imaging study in P. pacificus. We show that three neuron types (ASE left, ASE right, and AFD neurons) each have distinct calcium responses to specific ion concentrations, revealing diversity at the single neuron level. We have identified the first laterally asymmetric marker in P. pacificus, gcy-22.3p::GFP, with its expression limited to ASER homolog (AM7). Our unexpected discovery that neuronal asymmetry is present in the ASE homologs between two distantly related nematode species, despite the lack of a lsy-6 homolog, suggests that functional lateralization in P. pacificus may be mediated by a different genetic pathway compared to C. elegans.
P. pacificus and C. elegans have diverged taste palates
We have shown that while C. elegans is attracted to acetate salts, P. pacificus avoids these acetates. Previous studies have shown that ammonium acetate (NH4Ac) is sensed both as a water-soluble compound as well as a volatile odorant and is mediated by different signaling pathways (Frøkjaer-Jensen et al., 2008). Like C. elegans, it is therefore likely that ammonium and acetate ions involve a different set of neurons in P. pacificus (i.e. non-che-1 expressing neurons), based on the finding that histamine-treated che-1p::HisCl1 animals did not significantly attenuate their repulsive response to NaAc. We find that sodium chloride is a common attractant for both P. pacificus and C. elegans, although the magnitude of response is lower than previously published results (Suzuki et al., 2008). This result is likely due to differences in generating the salt gradients. Nevertheless, we find that P. pacificus neurons have a distinct response to this salt when compared to those observed in C. elegans neurons (Table 1). The P. pacificus ASEL neuron responds to a decrease in NaCl concentration (likely sodium), as evidenced by the ‘OFF-2’ response profile, whereas the C. elegans ASEL neuron responds to an increase in sodium concentration. However, in both nematodes, the ASER neuron responds to a decrease in chloride concentration. Additionally, the P. pacificus ASER neuron exhibits a unique ON–OFF response to ammonium iodide, whereas in C. elegans, no ON–OFF type of response is seen in the ASE neurons – the C. elegans ASER neuron responds only to a decrease in iodide concentration. Based on our calcium imaging and chemotaxis results on the gcy-22.3 mutants, attraction to ammonium ions (NH4+) is likely mediated in part by the ASER neuron in P. pacificus. Pristionchus species are entomophilic and most frequently found to be associated with beetles in a necromenic manner and thus insect cadavers could be sources of ammonium in the soil (Koneru et al., 2016; Herrmann et al., 2007; Herrmann et al., 2006; Fielding et al., 2013). Additionally, ammonium salts could represent a biological signature of other nematodes that the predatory morph of P. pacificus could interpret as prey. In P. pacificus, nutritional state has a measurable role in the mouth-form polyphenism decision between predatory and non-predatory morphs (Piskobulu et al., 2025). Collectively, our findings highlight the divergence of the P. pacificus salt sensory neurons compared to those observed in C. elegans.
Comparison of ASEL/R responses between P. pacificus and C. elegans.
| P. pacificus | C. elegans | |||
|---|---|---|---|---|
| ASEL | ASER | ASEL | ASER | |
| NH4Cl (1–80 mM) | ON | x | x (Suzuki et al., 2008; Ortiz et al., 2009) | OFF (Suzuki et al., 2008; Ortiz et al., 2009) |
| NH4Cl (81–250 mM) | ON | OFF | ? | ? |
| NaCl (1–80 mM) | x | OFF | ON (Suzuki et al., 2008; Herrmann et al., 2007; Herrmann et al., 2006; Fielding et al., 2013) | OFF (Suzuki et al., 2008; Herrmann et al., 2007; Herrmann et al., 2006; Fielding et al., 2013) |
| NaCl (81–250 mM) | OFF-2 | OFF | ON (Herrmann et al., 2006) | OFF (Herrmann et al., 2006) |
| NH4I (1–80 mM) | ON | OFF | x (Ortiz et al., 2009) | OFF (Ortiz et al., 2009) |
| NH4I (81–250 mM) | ON | ON-OFF | ? | ? |
-
‘x’ minimal to no response.
-
‘?’ represents unknown.
P. pacificus ASE neurons have narrower sensitivity range
The sensitivity range of the P. pacificus ASER responses is significantly less compared to the ASEL responses, as well as to C. elegans ASER responses. Whereas the C. elegans ASER has a 40-fold sensitivity range in the ‘OFF’ response to the removal of various concentrations of NaCl (1–40 mM) (Suzuki et al., 2008; Rabinowitch et al., 2014; Shindou et al., 2019; Watteyne et al., 2020), the P. pacificus ASER showed the ‘OFF’ response only to 250 mM NH4Cl but not to a tenfold reduction in concentration of NH4Cl (25 mM). For the ASEL in contrast, the response to 25 mM was just as strong as to 250 mM NH4Cl (tenfold) and comparable to the eightfold concentration range observed for C. elegans ASEL toward NaCl. Alternatively, the magnitude of these sensitivity differences may be also partially due to differences among calcium indicators (i.e. GCaMP and Cameleon), but multiple P. pacificus che-1p::GCaMP strains did not exhibit sufficient basal fluorescence to allow for image tracking and direct comparison. The narrower sensitivity range in P. pacificus chemosensation was also observed for attraction to volatile odors (Hong and Sommer, 2006), which span only 10-fold, versus up to 10,000-fold in attractive odors for C. elegans (Bargmann and Horvitz, 1991).
P. pacificus taste neurons exhibit a unique biphasic response
Although the left-ON and right-OFF responses are conserved in the ASE neurons in both species, the biphasic response of ASER to 250 mM NH4I has not been observed in either of the ASE neurons toward salts. In C. elegans, hyperosmotic stimulus such as 1 M glycerol, or high concentrations or long duration of CuSO4 exposure both result in biphasic responses by the ASH neurons that sense noxious chemicals (Wang et al., 2015; Chronis et al., 2007). Olfactory neurons that mediate avoidance behavior such as the AWB neurons can also exhibit a biphasic response to the presence and removal of a high concentration isoamyl alcohol that normally elicits avoidance behavior (Yoshida et al., 2012). The neuron-specific response can also be dependent on the concentration of the chemical compound, since the ASER to 25 mM NH4I was a weak ‘OFF’ rather than a biphasic one. In contrast to the salt-dependent response types by P. pacificus ASE neurons, the P. pacificus AFD neurons also show exclusively biphasic responses with various amplitudes. Further characterization will help determine if biphasic responses can also be found in other sensory neuron types in P. pacificus, specifically those neurons mediating avoidance behavior.
AFD are potentially polymodal neurons
Broadly speaking, C. elegans chemosensory neurons have been classically characterized as specialized neurons for dedicated modalities such as water-soluble chemicals (ASE), volatile odorants (AWA, AWB, and AWC), noxious chemicals (ASH), pheromones (ADL, ADF, and ASK), and light (ASJ) and temperature (AFD) (Bargmann, 2006; Bargmann et al., 1993; Mori and Ohshima, 1995; Sengupta et al., 1996; Macosko et al., 2009; Liu et al., 2010). Advances in multi-neuron calcium recordings have since shown that a given odor within a certain concentration range is detected by different ensembles of the 12 amphid neuron classes, including the AFD neurons (Leinwand and Chalasani, 2013; Lin et al., 2023; Yemini et al., 2021). Unexpectedly, we found that the P. pacificus AFD neurons exhibit a distinctive biphasic response to all three salts tested (NH4Cl, NaCl, and NH4I), which differ from the C. elegans AFD calcium responses (Yemini et al., 2021; Zaslaver et al., 2015). Moreover, the loss of the receptor gcy-22.3 reduced the AFD ‘ON’ response to NH4Cl and NaCl, indicating ASER contributes to the AFD response. The strong positive AFD response to 25 mM NaCl that is absent in both ASE neurons further supports the likelihood that AFD receives inputs from other amphid neurons. The integration of thermosensation and chemosensation is important for memory-regulated behavior. In C. elegans, maximum chemotaxis indices toward NH4Cl occurs when there is concordance between cultivation temperature and assay temperature (Kuhara et al., 2008). The C. elegans AFD neurons are also important for gustatory aversive learning in NaCl avoidance (Watteyne et al., 2020). Given the influence of environmental temperature on the P. pacificus mouth-form plasticity and the wide range of micro-climates that wild strains of P. pacificus have been isolated from Leaver et al., 2016; Sieriebriennikov et al., 2017; Leaver et al., 2022, temperature and taste preferences could be regulated at multiple genetic levels during crucial developmental decisions.
Changes in the gene regulatory architecture of sensory neuron specification
We found that the key regulator of Cel ASE identity, che-1, is also expressed in Ppa ASE and may play a similar role as a terminal selector in this neuron type, based on its effect on ASE-mediated behavior and regulation of the Ppa-gcy-22.3 gene. However, the stronger behavioral effect of silencing of che-1 expressing neurons compared to a che-1 mutant background could either indicate that che-1 does not have as broad a role in controlling ASE differentiation in P. pacificus versus C. elegans. It is possible that a developmental loss of ASE differentiation may result in compensatory changes in the chemosensory system during early development, as was observed in the C. elegans mating pheromone response by males (White et al., 2007). It is also worthwhile to note that Cel-gcy-22 stands out for being located on a separate chromosome (Chr. V) compared to the other ASER-type gcy genes (gcy-1, gcy-4, gcy-5 on Chr. II) as well as the Cel-gcy-22 mutant having defects in chemoattraction toward a wide range of salt ions (Ortiz et al., 2009; Ortiz et al., 2006). Given that the five P. pacificus gcy-22-like paralogs are located on three separate chromosomes (Chr. I, IV, and X), it is likely that they emerged from independent and repeated gene duplication events after the separation of Caenorhabditis and Pristionchus lineages. Although the promoter fusion reporters of other gcy genes have been uninformative due to lack of expression (Ppa-gcy-22.1, Ppa-gcy-7.1, Ppa-gcy-7.2, and Ppa-gcy-5), it is likely that ASEL-specific gcy genes as well as additional ASER-specific gcy-22-like genes exist. Finding other genes with left–right specific expression could help to identify genetic determinants affecting lateral asymmetry.
Unexpectedly, we found that unlike in C. elegans, the Ppa-che-1 gene is also expressed in the AFD neurons. Since we cannot record neural activity in AFD in a che-1 mutant (the che-1p::RCaMP driver fails to be expressed sufficiently in che-1 mutants due to autoregulation), and do not yet have molecular markers for Ppa AFD neurons, we cannot assess whether che-1 affects AFD neuron differentiation.
Perhaps the most striking difference in the gene regulatory architecture of ASE neuron specification is the apparent lack of the key regulator of ASE asymmetry in P. pacificus, the miRNA lsy-6. In C. elegans, the expression of lsy-6 exclusively in ASEL is prepatterned via an early embryonic Notch signal (Cochella and Hobert, 2012) and serves to downregulate the homeodomain transcription factor cog-1 in the ASEL neuron (Johnston et al., 2005). Through a network of downstream regulatory events, asymmetry of rGCs eventually becomes established (Hobert, 2014). cog-1 and several asymmetrically expressed downstream effectors of cog-1, such as the die-1, lim-6, and fozi-1 transcription factors are conserved in P. pacificus, but whether the function of these factors in controlling P. pacificus ASE laterality is conserved remains to be determined. In this context it is intriguing to note that another prominent gene regulatory pathway that is controlled by miRNAs in C. elegans, the heterochronic pathway (let-7s and lin-4), appears to have diverged in P. pacificus as well, despite the conservation of the overall physiological readouts of this pathway (temporal patterning of cell lineage divisions) (Sharma et al., 2024). It is tempting to speculate that miRNA-meditated regulatory process is particularly labile.
In conclusion, our work illustrates how comparative behavioral and genetic analyses in nematodes is a powerful strategy to uncover substrates of evolutionary change in simple nervous systems.
Materials and methods
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Recombinant DNA reagent | pMM5 | This paper | Ppa-che-1pei::optRCaMP | |
| Recombinant DNA reagent | pHC30 | This paper | Ppa-che-1pei::optHisCl1 | |
| Recombinant DNA reagent | pVL2 | This paper | Ppa-gcy-22.3p::GFP | |
| Strain (P. pacificus) | Ppa-che-1p::HisCl1 | This paper | Ppa-che-1pei::optHisCl1; Ppa-egl-20p::turboRFP (promoter with first exon and intron) | csuEx83/RLH336 |
| Strain (P. pacificus) | Ppa-che-1::2xALFA | This paper | Ppa-che-1::ALFA (C-terminal tagged to last exon) | RLH325 |
| Strain (P. pacificus) | Ppa-che-1p::RCaMP; che-1p::GFP | This paper | Ppa-che-1pei::optRCaMP; Ppa-che-1pei::optGFP; Ppa-egl-20p::turboRFP | csuEx93/RLH335 |
| Strain (P. pacificus) | Ppa-ttx-1p::RFP | This paper | Ppa-ttx-1pei::RFP (promoter with first exon and intron) | csuEx96/RLH352 |
| Strain (P. pacificus) | Ppa-ttx-1::2xALFA | This paper | Ppa-ttx-1::ALFA (C-terminal tagged to Exon 18) | RLH280 |
Nematode strains
Request a detailed protocolP. pacificus and other nematode strains were maintained at ~20°C on NGM plates seeded with E. coli OP50 for food as described previously (Cinkornpumin et al., 2014); these are derived from standard C. elegans culture methods (Brenner, 1974). P. pacificus and other nematode strains used are listed in Supplementary file 1A.
Chemotaxis assays
Request a detailed protocolThe assay for assessing response to salt gradients was adapted from C. elegans and P. pacificus chemotaxis assays (Hong and Sommer, 2006; Bargmann and Horvitz, 1991; Ortiz et al., 2006). Overnight salt gradients were established on 10 cm chemotaxis plates containing 20 ml agar (5 mM KPO4, 1 M CaCl2, 3% Bacto-agar, and 1 mM MgSO4) by adding 10 µl of 2.5 M salt solutions for 16 hr. Alternatively, agar containing 25 mM (NH4Cl and NH4I) or 50 mM (NaCl) were used to test for responses to individual salt ions. Following the establishment of the overnight point gradient, another 4 µl of the same salt solution or water control was added to reinforce the gradient 4 hr before the assay. Just prior to the assay, 1 µl of 1 M sodium azide was added to both the attractive salt (A) and the control (C) spots. P. pacificus J4 to adult hermaphrodites from near-saturated cultures were washed 3× with distilled water and collected by centrifuging at 2000 rpm for 2 min. Approximately 200 worms were loaded onto the edge of each assay plate between the gradient sources, and at least 10 combined worms have to reach the scoring arenas to be considered. At least 10 assays constituted each experimental trial, and multiple trials were conducted and averaged for each condition. The chemotaxis index for each end-point assay plate is defined as (A − C)/(A+C). To conduct conditional knockdowns of neurons, 5 M histamine dihydrochloride (Sigma-Aldrich H7250) stock solution in sterilized deionized water (Arrowhead CA) was filter-sterilized and top plated onto the agar plates to a final histamine concentration of 25 mM histamine approximately 10 min before loading the worms to commence the assay. Most assays lasted 3–4 hr at room temperature to allow P. pacificus sufficient time to reach the scoring arenas, with ~40% of the animals participating. We excluded an outlier due to likely scoring error (value = –0.64) in the chemotaxis assay for gcy-22.3(csu182) on NH4Cl (Figure 8). When using the csuEx83[Ppa-che-1p::optHisCl1] strain, only animals expressing Ppa-egl-20p::RFP tail marker from the extrachromosomal array were scored. Because of P. pacificus’ strong aversion to acetate, we could not easily assess the individual contributions of salt ions in a saturated background of ammonium acetate as conventionally practiced in C. elegans studies (Ortiz et al., 2009).
Ppa-che-1p::HisCl1 strain
Request a detailed protocolTo make the Ppa-che-1p::optHisCl1, the P. pacificus codon-optimized histamine-gated chloride channel sequence used in C. elegans (HisCl1) (Pokala et al., 2014; Pokala and Flavell, 2022) was designed using (https://hallemlab.shinyapps.io/Wild_Worm_Codon_Adapter/; Bryant and Hallem, 2021), was custom synthesized (Twist Bioscience), and subsequently inserted behind the che-1 promoter (3.1 kb containing the first exon and intron) (Hong et al., 2019) to create the pHC30 plasmid construct. This Ppa-che-1p::optHisCl1 plasmid (2 ng/µl) along with PS312 genomic DNA (80 ng/µl) and Ppa-egl-20p::RFP co-injection marker (2 ng/µl) were individually digested with HindIII and assembled as the injection mix to create csuEx83.
Ppa-che-1p::RCaMP reporter strain
Request a detailed protocolTo make the Ppa-che-1pei::optRCaMP, we generated a transgenic worm strain expressing the codon-optimized genetically encoded calcium indicator (GECI), jRCaMP1a, in the neurons of interest (Kerr et al., 2000; Nakai et al., 2001). jRCaMP1a is an improved red GECI based on mRuby with comparable sensitivity to GCaMP6 (Dana et al., 2016). The codon-optimized RCaMP sequence was custom synthesized (Twist Bioscience), and then subcloned using the plasmid pJET1.2/blunt (Thermo Fisher Scientific) to create the pMM2 plasmid construct. Using Gibson Assembly (E2611, New England Biolab), the codon-optimized RCaMP sequence was introduced downstream of the Ppa-che-1 promoter sequence with the first exon and intron sequences to create the pMM5 plasmid construct. Separately, we also generated a Ppa-che-1pei::optGFP transcriptional reporter with codon-optimized GFP (pMM3) to enable the localization of the che-1-expressing neurons during video acquisition (Han et al., 2020). The Ppa-che-1pei::optRCaMP (2 ng/µl) and Ppa-che-1pei::optGFP constructs (1 ng/µl), along with PS312 genomic DNA (80 ng/µl) and Ppa-egl-20p::RFP (1.5 ng/µl) were individually digested with HindIII and assembled as the injection mix to create csuEx93. Despite multiple attempts, we were unable to generate an equivalent che-1pei::GCaMP transgenic line with sufficient basal level of GCaMP expression for a comparison to the RCaMP calcium dynamics.
Promoter fusion reporter strains
Request a detailed protocolThe Ppa-gcy-22.3p::GFP construct contains 899 bp of the upstream promoter of the PPA04554 transcript is fused to the codon-optimized GFP (pVL2). A shorter gcy-22.3 transcript with different first two exons is also predicted, which is more similar in length to other rGC genes in P. pacificus (Contig12-snapTAU.506). The Ppa-ttx-1pei::RFP construct contains 1935 bp of the upstream promoter along with the first exon and intron sequences of Ppa-ttx-1 (PPA26714) fused to the codon-optimized RFP, excluding the first two codons (pDC14). pVL2 (2 ng/µl) or pDC14 (2 ng/µl), along with PS312 genomic DNA (80 ng/µl) were individually digested with HindIII to create the injection mixes to generate the independent reporter strains, csuEx90[Ppa-gcy-22.3p::GFP], as well as Ppa-ttx-1pei::RFP(csuEx94) and Ppa-ttx-1pei::RFP(csuEx96). csuEx96 showed less gland cell expression (which can occlude neuronal expression) and stronger AFD expression than csuEx94.
CRISPR mutagenesis generated mutants
CRISPR/Cas9 mutagenesis was used to generate mutations (Han et al., 2020; Nakayama et al., 2020). crRNA and primer sequences, and induced mutations, are included in Supplementary file 1C and .
che-1 alleles (PPA01143)
Request a detailed protocolTarget crRNA, tracrRNA, and Cas9 nuclease were purchased from IDT Technologies (San Diego, CA). crRNA and tracrRNA were hydrated to 100 µM with IDT Duplex Buffer, and equal volumes of each (0.61 µl) were combined and incubated at 95°C for 5 min, then 25°C for 5 min. Cas9 protein (0.5 µl of 10 µg/µl) was added, then the mix was incubated at 37°C for 10 min. Ppa-egl-20p::RFP was used as a co-injection marker. To reach a final total volume of 40 µl, the Cas9–crRNA–tracrRNA complex was combined with pZH009 (Ppa-egl-20p::RFP) DNA to reach 50 ng/µl final concentration using nuclease-free water. F1 progeny were screened for the presence of Ppa-egl-20p::RFP expression in the tail and candidate F1’s were sequenced to identify heterozygotes (Nakayama et al., 2020). ot5012 has a 4-bp insertion while ot5013 has an 8-bp complex insertion/deletion, and both mutations cause frameshift mutations and premature stop codons. Each allele was outcrossed two times to wildtype before characterization.
gcy-22.3 alleles (PPA04454)
Request a detailed protocolTarget crRNA, tracrRNA, and Cas9 nuclease were purchased from IDT Technologies (San Diego, CA). crRNA (RHL1400) and tracrRNA were hydrated to 100 µM with IDT Duplex Buffer, and equal volumes of each (0.61 µl) were combined and incubated at 95°C for 5 min, then 25°C for 5 min. Cas9 protein (0.5 µl of 10 µg/µl) was added, then the mix was incubated at 37°C for 10 min. Ppa-egl-20p::RFP was used as a co-injection marker. To reach a final total volume of 40 µl, the Cas9–crRNA–tracrRNA complex was combined with pZH009 (Ppa-egl-20p::RFP) DNA to reach 50 ng/µl final concentration using nuclease-free water. F1 progeny were screened for the presence of Ppa-egl-20p::RFP expression in the tail and candidate F1’s were sequenced to identify heterozygotes (Nakayama et al., 2020). csu181 has a 2-bp complex deletion, while csu182 has a 22-bp complex deletion, both mutations cause frameshifts and premature stop codons. Each allele was outcrossed two times to wildtype before characterization.
ALFA C-terminal tagging and immunostaining
Request a detailed protocolFor induction of site-specific insertions via CRISPR/Cas9-mediated mutagenesis, target crRNA, tracrRNA, and Cas9 nuclease were treated as described above. Single-stranded DNA repair template containing the ALFA nanobody tag (RHL1551 for CHE-1 and RHL1519 for TTX-1) with 35 bp of homology arms on the 5′ and 3′ sides were purchased from IDT. To minimize sequence identity, the two copies of the ALFA sequence contain silent mutations. The crRNA (RHL1396 for che-1 and RHL1514 for ttx-1) and tracrRNA were hydrated to 100 µM with IDT Duplex Buffer, and equal volumes of each (0.61 µl) were combined and incubated at 95°C for 5 min, then 25°C for 5 min. The ALFA tag was C-terminally inserted to the longest ttx-1 splice form in the last 18th exon (ppa_stranded_DN30925_c0_g1_i5) (Trinity 2016 transcripts can be found on http://pristionchus.org/). F1 animals expressing the co-injection marker egl-20::optRFP were lysed and checked by PCR for insertions.
Non-starved healthy cultures of ALFA-tagged CHE-1 (csu226[Ppa-che-1::2xALFA]) or ALFA-tagged TTX-1 (RLH280[Ppa-ttx-1::2xALFA]) (from six 6 cm plate cultures) were washed with M9 and filtered (Sartorius 84 g/m2, Grade 392) and processed as previously described (Igreja et al., 2022). In brief, mixed stage worms were fixed overnight at 4°C on a nutator with 500 µl fixation buffer (4% paraformaldehyde in PBS). The worms were then incubated overnight at 37°C with 500 µl 4% β-mercaptoethanol dissolved in 1% Triton X-100 in 0.1 M Tris pH 7.4, and digested in 200 μl of collagenase buffer with 200 units of collagenase type IV (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) at 37°C for ~3.5 hr. The partially digested worms were subsequently washed three times with 500 µl PBST. After collagenase treatment the centrifugation between the washes was done at low speed, 1000 RCF. After washes, the worms were stained in 50 µl 1% BSA in PBST with the primary antibody (1:100 FluoTag-X2 anti-ALFA-AZdye568, N1502, NanoTag Biotechnologies, Göttigen, Germany), overnight at 4°C in a nutator. Following the washes, worms were resuspended in 50 µl VectaShield mounting medium (H-1000, Vector Laboratories, USA) containing DAPI (Molecular Probes, Thermo Fisher Scientific) and gently mounted on freshly prepared 3% Noble agar pads. Images were acquired on a Leica DM6000 microscope.
Calcium imaging
Request a detailed protocolTo conduct calcium imaging, worms were trapped and imaged within a microfluidic PDMS chip while delivering stimuli directly to the nose of an immobilized worm, as previously described (Chronis et al., 2007; Chalasani et al., 2007). The microfluidic chip was connected to a programmable valve controller (ValveBank) that enables the user to toggle between ‘stimulant ON’ and ‘stimulant OFF’ states. Specifically, the ValveBank allows controlled switching of flow from the two outer buffer channels in the chip such that either the control solution (stimulant OFF) or the stimulant solution (stimulant ON) flows over the worm nose (Figure 4—figure supplement 3). Buffer solution (for the outer two channels) consisted of M9 buffer with 0.1% Tween-20 and 1 µg/ml fluorescein (Stiernagle, 2006; Liu et al., 2018). The worm loading solution consisted of M9 buffer with 0.1% Tween-20 and 1.5 mM tetramisole hydrochloride to immobilize the animal. Water-soluble stimulant solutions consisted of the following water-soluble compounds dissolved in nanopure/milli-Q water: ammonium chloride (NH4Cl), ammonium iodide (NH4I), and sodium chloride (NaCl) at concentrations of 250 and 25 mM. 750 mM NaCl elicited very inconsistent responses. The water-soluble stimulant solution was made by diluting the water-soluble compound in nanopure/milli-Q water with 0.1% Tween-20. The control solution consisted of 0.1% Tween-20 nanopure/milli-Q water. Worms were exposed to the control solution, stimulant solution, and then control solution using a 60-s program: 10 s stimulant OFF, 20 s stimulant ON, and 30 s stimulant OFF. As a negative control, animals were exposed only to the control solution, without switching channels, for the duration of the recording. For each animal, the orientation of the nose and vulva were recorded and used as a guide to determine the ventral and dorsal sides of the worm, and subsequently, the left and right sides of the worm. Accounting for the plane of focus of the neuron pairs as viewed through the microscope, it was then determined whether the imaged neuron was the worm’s left or right neuron of each pair. Images were captured using a Zeiss Axio Observer Z1 inverted fluorescence microscope and a pco.panda 4.2 sCMOS camera. Changes in fluorescence intensity were measured in the neurons of interest while the worm was exposed to green light. Images were processed using MetaMorph software version 7.10.5.476. Images were captured at 500 ms exposure time at 2 fps because the baseline fluorescence in Ppa-che-1p::optRCaMP worms was too dim to capture viable data using the standard 100 ms exposure time. Baseline F₀ was measured as the average background-subtracted fluorescence from the first 9 s of each recording and change in fluorescence intensity was calculated as dF/F = (F − F₀)/F₀, as described (Liu et al., 2018). Data were analyzed and plotted using custom scripts generated in MATLAB versions R2021a–R2024a. This code is available at https://github.com/honglabcsun/Calcium-Imaging, copy archived at Mackie and Hong Lab CSUN, 2025.
For bar plot comparisons between wildtype and gcy-22.3 mutants, we calculated minimum pre-stimulus and maximum post-stimulus % dF/F values using custom scripts generated in MATLAB. Min–Max data were exported as text files, manually converted and organized into an XLSX file (Microsoft Excel Office16) and imported into Prism GraphPad software (version 10) for data visualization and statistical analysis.
Nomenclature
Request a detailed protocolThroughout the results section, P. pacificus genes will be referred to without the Ppa- prefix; if necessary for comparison to another species such as C. elegans (Cel-) the Ppa- prefix will then be used.
Materials availability
Request a detailed protocolThe plasmids and viable nematode strains will be made available upon request.
Data availability
All data generated or analyzed during this study are included in the manuscript and supporting files. Raw data and code is available at https://github.com/honglabcsun/Calcium-Imaging, copy archived at Mackie and Hong Lab CSUN, 2025.
References
-
Conserved miRNAs are candidate post-transcriptional regulators of developmental arrest in free-living and parasitic nematodesGenome Biology and Evolution 5:1246–1260.https://doi.org/10.1093/gbe/evt086
-
The neural basis of heat seeking in a human-infective parasitic wormCurrent Biology 32:2206–2221.https://doi.org/10.1016/j.cub.2022.04.010
-
Three-dimensional reconstruction of the amphid sensilla in the microbial feeding nematode, acrobeles complexus (nematoda: rhabditida)The Journal of Comparative Neurology 512:271–281.https://doi.org/10.1002/cne.21882
-
Chemosensory behaviors of parasitesTrends in Parasitology 28:427–436.https://doi.org/10.1016/j.pt.2012.07.004
-
The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuronGenes & Development 21:1653–1674.https://doi.org/10.1101/gad.1560107
-
Diet influences rates of carbon and nitrogen mineralization from decomposing grasshopper frass and cadaversBiology and Fertility of Soils 49:537–544.https://doi.org/10.1007/s00374-012-0702-5
-
A sensory code for host seeking in parasitic nematodesCurrent Biology 21:377–383.https://doi.org/10.1016/j.cub.2011.01.048
-
The bacterial community of entomophilic nematodes and host beetlesMolecular Ecology 25:2312–2324.https://doi.org/10.1111/mec.13614
-
Adaptation to environmental temperature in divergent clades of the nematode Pristionchus pacificusEvolution; International Journal of Organic Evolution 76:1660–1673.https://doi.org/10.1111/evo.14520
-
Neuropeptide signaling remodels chemosensory circuit composition in Caenorhabditis elegansNature Neuroscience 16:1461–1467.https://doi.org/10.1038/nn.3511
-
Predator-secreted sulfolipids induce defensive responses in C. elegansNature Communications 9:1128.https://doi.org/10.1038/s41467-018-03333-6
-
Vitamin B12 and predatory behavior in nematodesVitamins and Hormones 119:471–489.https://doi.org/10.1016/bs.vh.2022.01.006
-
Evolution of neuronal patterning in free-living rhabditid nematodes I: Sex-specific serotonin-containing neuronsThe Journal of Comparative Neurology 502:736–767.https://doi.org/10.1002/cne.21288
-
SoftwareCalcium-imaging, version swh:1:rev:adeaf83b08822e58d9081c5390b8f09940280a6aSoftware Heritage.
-
A high signal-to-noise Ca(2+) probe composed of a single green fluorescent proteinNature Biotechnology 19:137–141.https://doi.org/10.1038/84397
-
Screening for CRISPR/Cas9-induced mutations using a co-injection marker in the nematode Pristionchus pacificusDevelopment Genes and Evolution 230:257–264.https://doi.org/10.1007/s00427-020-00651-y
-
High nutritional conditions influence feeding plasticity in pristionchus pacificus and render worms non-predatoryJournal of Experimental Zoology. Part B, Molecular and Developmental Evolution 344:94–111.https://doi.org/10.1002/jez.b.23284
-
Recording and quantifying C. elegans behaviorMethods in Molecular Biology 2468:357–373.https://doi.org/10.1007/978-1-0716-2181-3_20
-
Olfactory circuits and behaviors of nematodesCurrent Opinion in Neurobiology 41:136–148.https://doi.org/10.1016/j.conb.2016.09.002
-
The role of DAF-21/Hsp90 in mouth-form plasticity in pristionchus pacificusMolecular Biology and Evolution 34:1644–1653.https://doi.org/10.1093/molbev/msx106
-
Off-response in ASH neurons evoked by CuSO4 requires the TRP channel OSM-9 in Caenorhabditis elegansBiochemical and Biophysical Research Communications 461:463–468.https://doi.org/10.1016/j.bbrc.2015.04.017
-
Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.?2UUThe Journal of Comparative Neurology 160:313–337.https://doi.org/10.1002/cne.901600305
-
The structure of the nervous system of the nematode Caenorhabditis elegansPhilosophical Transactions of the Royal Society of London. B, Biological Sciences 314:1–340.https://doi.org/10.1098/rstb.1986.0056
-
The sensory circuitry for sexual attraction in C. elegans malesCurrent Biology 17:1847–1857.https://doi.org/10.1016/j.cub.2007.09.011
-
Odour concentration-dependent olfactory preference change in C. elegansNature Communications 3:739.https://doi.org/10.1038/ncomms1750
-
Sensory neuroanatomy of Parastrongyloides trichosuri, a nematode parasite of mammals: Amphidial neurons of the first-stage larvaThe Journal of Comparative Neurology 519:2493–2507.https://doi.org/10.1002/cne.22637
Article and author information
Author details
Funding
National Institutes of Health (SC1GM140970)
- Ray L Hong
National Institutes of Health (R56MH096881)
- Steven J Cook
Howard Hughes Medical Institute
- Oliver Hobert
The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Acknowledgements
This research is funded by NIH SC1GM140970 to RLH, NIH R56MH096881 to SHC. OH is funded by the HHMI. MM and RLH contributed in conception, design, and acquisition of work. VL, HRC, DLC, IMD, NRK, KTQ, and SJC contributed to data acquisition. RLH, OH, and SHC contributed to the analysis and writing of the work. The authors declare that they have no competing interests. We would also like to thank I Martinez and C Igreja for technical assistance, and M Barsegyan for assistance with data acquisition. All data needed to evaluate the conclusions in the paper are present in the paper and the supplementary materials.
Version history
- Preprint posted:
- Sent for peer review:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Version of Record published:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.103796. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2025, Mackie et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 900
- views
-
- 55
- downloads
-
- 4
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 4
- citations for umbrella DOI https://doi.org/10.7554/eLife.103796






