Planar cell polarity coordination in a cnidarian embryo provides clues to animal body axis evolution
Figures
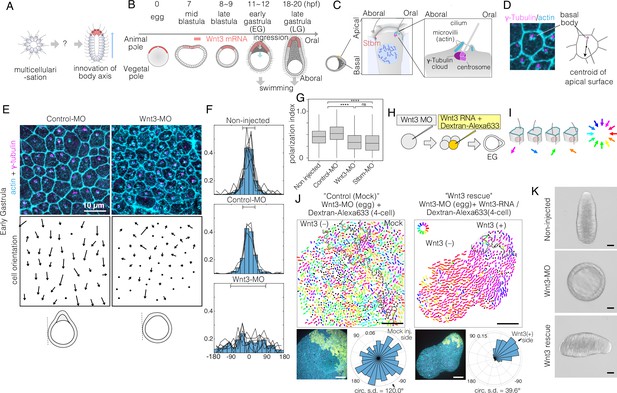
Local Wnt3 globally orients the body axis tissue polarity and morphogenesis.
(A) A key unanswered question of animal evolution is the origin of body axis patterning mechanisms, allowing initial multicellular assemblages to position specialised cell types (red) and coordinate cell polarity (represented in this diagram by oriented cilia and elongated shape). (B) Key stages of the early morphogenesis of Clytia embryos in relation to Wnt3 mRNA localisation (red). At the early gastrula (EG) stage, ectodermal cilia have formed, planar cell polarity (PCP) has developed along the future oral-aboral (OA)-axis, and the pointed morphology of the animal pole morphology can first be distinguished. Most axial elongation takes place during gastrulation. See also Figure 1—figure supplement 1. (C) Schematic representation of PCP in a single epidermal cell at the EG stage. Basal bodies are positioned on the oral side at the apical cortex (translational polarity). (D) Confocal image of the apical surface of a single epidermal cell stained for actin (cyan, phalloidin labelling) and γ-tubulin immunostaining (magenta), and a representation of the PCP vector, defined by the basal body position and its mirror image across the apical surface centroid as the initial and terminal points, respectively. (E) Local PCP coordination in control-MO- and Wnt3-MO-injected embryos at the EG stage. Bar: 10 µm. (Top) Confocal images as in D. (Bottom) PCP vector representation of the cells in the images. Dots represent cells with no clear polarisation or mitotic cells. Apical cell contours are wavy in Wnt3-MO embryos. (F) Distribution of cell orientation in individual scans (lines) and their average (histogram) for non-injected (373 cells, seven images), control-MO-injected (304 cells from three images) and Wnt3-MO-injected (225 cells from six images). The average orientation is defined as the origin (0°) for each scan. Horizontal bar: standard deviation. (G) The PCP polarisation index (degree of the polarisation of cells) by the length of the PCP vector, normalised by the cell size (****p<0.0001, Mann-Whitney U test). (H) Experimental procedures of the Wnt3-rescue experiment. Wnt3MO is injected into unfertilised eggs, then rescue mRNA solution with fluorescently labelled 10 kDa dextran is injected into a four-cell stage blastomere. (I) Circular colour-code representation of the PCP. (J) Wide-range PCP coordination observed in Wnt3-rescue experiments. Left: mock rescue control by water injection (N=9). Right: rescue by Wnt3 mRNA injection (N=27). Each set consists of three illustrations. Top: colour-wheel representation of the PCP. Cells derived from the Wnt3 or water-injected blastomere are at the top-right of each image, indicated with grey shading. Bar: 50 µm. Bottom-left: confocal thumbnail image. Cyan: actin; yellow: Dextran-Alexa Flour 647 (injected blastomere-derived cells). Bottom-right: radar plot of the cell orientation. The arrows in the radar show the circular mean of the PCP orientation within the single scan. circ s.d.: circular standard deviation. (K) Axial morphology of normal, Wnt3-MO-injected, and Wnt3-rescued embryos at late gastrula (LG) stage. Bar: 50 µm. Elongated body axis morphology was restored so that cells with injected Wnt3 mRNA were located at the induced oral (posterior). See also Figure 3C.
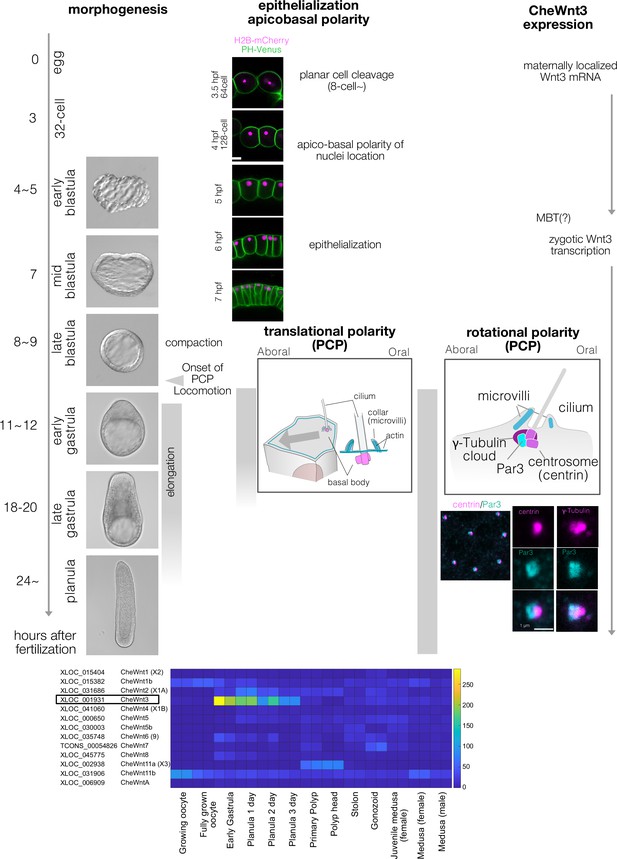
Clytia early embryogenesis stages and Wnt3 expression.
(A) Developmental stages of Clytia hemisphaerica early embryogenesis (18°C). The key events in morphogenesis, epithelialisation, and cell polarity are indicated. Morphologically, Clytia embryos form blastulae with monolayer blastomeres surrounding the central blastocoel. The blastocoel is formed from the 16- or 32-cell stages (3–3.5 hpf). The morphological apico-basal polarity of the blastomeres becomes prominent starting from the 128-cell stage, initially as apical localisation of the nuclei (4 hpf). The blastomeres epithelialise around the mid-blastula stage (7 hpf). The embryos become smaller (i.e. compaction) at the late blastula stage (8–9 hpf), when planar cell polarity (PCP) also starts to be coordinated, and the embryo begins to swim with ciliary beating. PCP is almost fully coordinated by the early gastrula stage (11–12 hpf), when the oral-aboral (OA) axis is morphologically prominent with a pointed-end shape at the oral end. Gastrulation occurs at the oral end by ingression of individual cells. The endodermal cells migrate from oral to aboral in the blastocoel. Body elongation along the OA-axis mainly occurs concomitantly with gastrulation and endoderm migration. Both PCP and endoderm formation are required for axial elongation. PCP is initially prominent as the eccentric location of the ciliary basal body at the oral side of each cell (translational PCP) in the early gastrula stage. The cilium is surrounded by actin-rich microvilli collars. Starting from the late gastrula stage (18–20 hpf), the basal body structure and surrounding collar also become polarised along the OA-axis (rotational polarity); the F-actin-positive collar is consistently reinforced at the aboral side, most likely mechanically supporting the directional power and recovery strokes along the OA-axis. It is also the most reliable PCP marker in later stages (Momose et al., 2012). The immunoreactivity of Par3 (anti-mouse Par3 antibody) was also detected at the aboral side of the ciliary body, suggesting a link between apico-basal cell polarity and PCP. (B) Expression profile of Clytia Wnt genes at different developmental stages from existing RNA-seq data (http://marimba.obs-vlfr.fr). Number of transcripts detected per million reads (TPM). The latest Wnt nomenclature based on Condamine et al. (DOI: 10.1016/j.ydbio.2019.09.001) and gene ID (XLOC) or transcript ID (TCONS) are indicated along with the previous names (Momose et al., 2008) in parentheses.
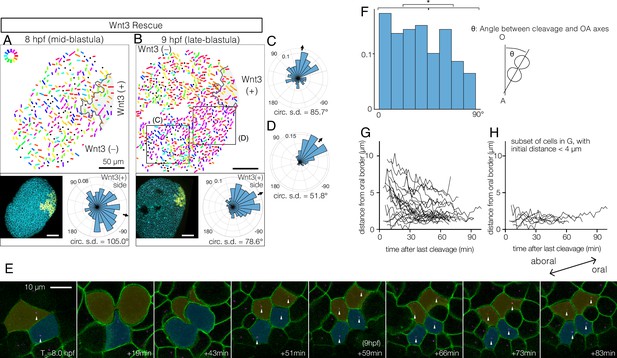
Wnt3-driven planar cell polarity (PCP) orientation precedes visible cell alignment.
(A, B) PCP orientation in Wnt3-rescue embryo at the mid-blastula stage (A: 8 hpf, N=10) and late-blastula stage (B: 9 hpf, B, N=10). Graphical PCP representations and radar histogram plots are as in Figure 1. (C, D) Radar plot of PCP in distal (C) and proximal (D) areas of the region shown in (B). (E) Basal body displacements after cell divisions. Bar: 10 µm. Cell membranes labelled by PH-Venus (green) and basal bodies with Poc1-mCherry (magenta). Arrows indicate basal bodies positioned on opposite sides of cleavage planes located after being engaged to the spindles. Aborally displaced basal bodies migrate towards the oral side. The average PCP defined the oral-aboral (OA)-axis. (F) Distribution of the angles between the cleavage orientation axis to the OA-axis defined by the PCP orientation (0–90°, n=131). Cleavages along the OA-axis (0–45°) are favoured (*p<0.05 chi-square test). (G) Directional migration of the basal body towards the oral cell boundary after cell cleavage. Each line indicates the distance of a single basal body to the oral edge of the cell after the last cleavage. Basal bodies migrate towards the oral cell boundary, suggesting PCP has been established by the time PCP becomes structurally manifest. (H) Subset of (G) where the initial distance to the oral edge is less than 4 µm after the cell division.
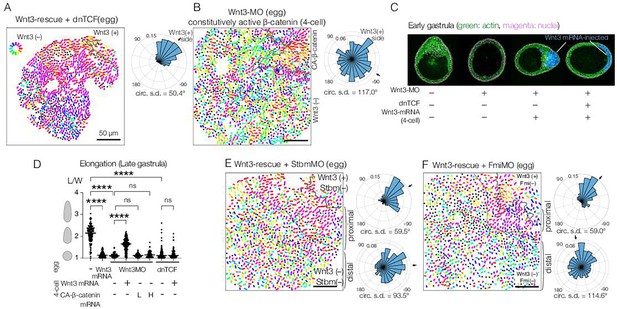
Planar cell polarity (PCP) orientation by Wnt3 is Wnt/β-catenin-independent, and its propagation across the body axis requires Stbm and Fmi.
(A) Wnt3-rescue with an additional injection of a dominant-negative form of TCF (dnTCF) into the egg, N=10. (B) Wnt3-rescue by the constitutively active form of β-catenin (CA-β-cat) instead of Wnt3 mRNA, N=7. (C) Onset of axial morphology in early gastrula (EG) embryos. Localised Wnt3 is necessary and sufficient for the onset of the elongation towards its source. Green: actin (phalloidin), magenta: nuclei (To-Pro3), and blue: Wnt3-mRNA/Dextran-injected cell lineage. (D) Roles of Wnt3 and β-catenin in the axial elongation in late gastrula (LG) embryos. The elongation was measured by the elongation index (L/W): the primary axis length (L) divided by its perpendicular length (W), ****p<0.0001, Mann-Whitney U test. Both local Wnt3 expression and β-catenin/TCF-dependent gastrulation were required for axial elongation. See Figure 3—figure supplement 1. (E, F) Wnt3-rescue experiments with additional injections of (E) Stbm-MO (N=23) and (F) Fmi-MO (N=6). Radar plots of the PCP orientation show the manually defined proximal area (proximal: between the dotted line and the Wnt3-positive region) and distal area (distal: opposite area to the Wnt3-positive region across the dotted line). PCP is correctly polarised without Stbm or Fmi close to the Wnt3-injected cell clone but not in the distant area.
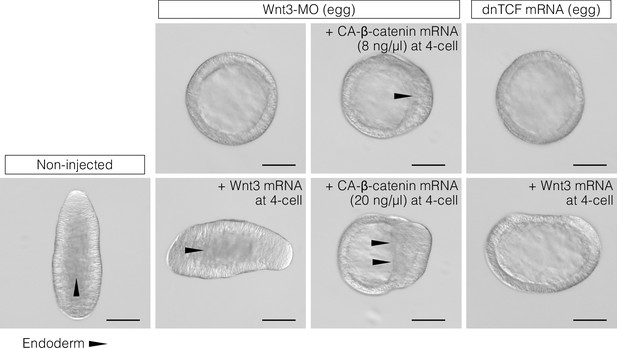
CheWnt3 is necessary and sufficient to orient the morphological oral-aboral (OA)-body axis, while the Wnt/β-catenin pathway acting under CheWnt3 is necessary but not sufficient.
DIC observation of non-injected (control) embryos and embryos injected with Wnt3-MO or a dominant-negative form of Clytia TCF (dnTCF) mRNA, with an additional injection of Clytia Wnt3 mRNA or a constitutively active form of Clytia β-catenin (CA-β-catenin). The images are representative of the results used for the measurements shown in Figure 3D. Bar: 100 µm. Triangles indicate the front line of endoderm migration from the oral (Wnt/β-catenin-activated) side towards the aboral side.
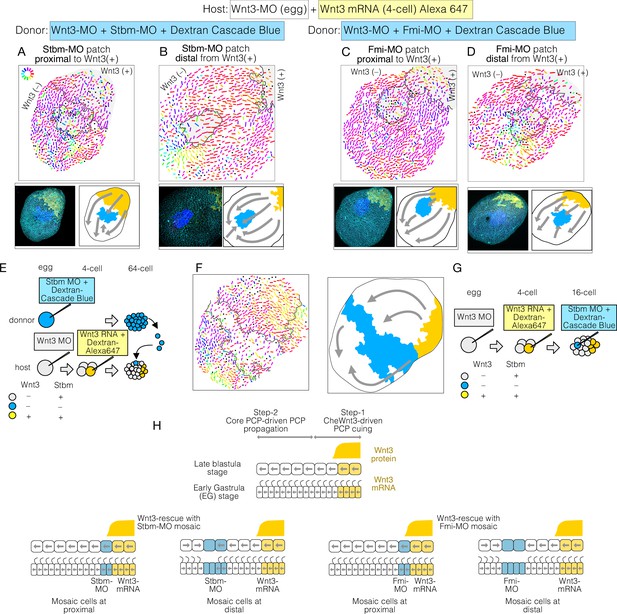
Planar cell polarity (PCP) behaviour in Stbm/Fmi-mosaic/ Wnt3-rescue experiments supports the two-step model for global PCP orientation.
(A–D) Wnt3-rescued early gastrula embryos, including Stbm(-) or Fmi(-) mosaic patches made by blastomere transplantation. (A, B) Blastomeres from Wnt3-MO/Stbm-MO double-injected embryos were transplanted as the donor into Wnt3-rescued host embryos at the 64-cell stage. They happened to be incorporated in close/proximal (A: 658 cells, N=4) or far/distal (B: 757 cells, N=4) positions with respect to the Wnt3 source, respectively. (C, D) The same experiment was conducted with Wnt3-MO/Fmi-MO-injected blastomeres as the donor, incorporated in proximal (C: 984 cells, N=3) and distal (D: 649 cells, N=3) positions, respectively. (E) Schematic drawing of the experimental procedure of the transplantation. (F) Wnt3-rescued early gastrula embryos with Stbm(-) mosaic patch generated by additional injection of Stbm-MO at the 16-cell stage (N=1). (G) Schematic drawing of the experimental procedures for (F). The flow of the PCP orientation is indicated by grey arrows in the bottom right drawings in (A–D) and right in (G). (H) Graphical summary of Stbm-MO and Fmi-MO mosaic experiments. The graphical representation and thumbnail confocal images indicate Wnt3-positive lineages and Stbm-MO- or Fmi-MO-injected lineages in yellow and blue. All vector representations are as in Figure 1.
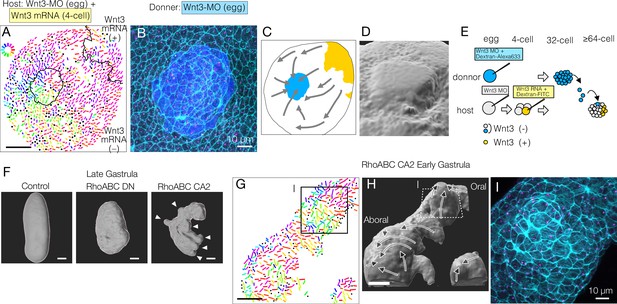
Mechanical strains can reorient planar cell polarity (PCP).
(A–E) PCP orientation induced by transplantation from a Wnt3-MO donor into the Wnt3-MO background of a Wnt3-rescue host. (A) Incomplete incorporation of host and donor cells into a smooth epidermis (N=3/8) causing long-range PCP orientation, as if the donor acts as an aboral cue, in addition to the Wnt3 oral cue. (A) PCP representation by colour-coded bars. See also the legends for Figure 1. (B) Confocal image of the apical cortex of the epidermis showing the rosette structure caused by incomplete incorporation of the donor cells. Both host and donor cells are elongated around the graft boundary. (C) Graphical summary of the PCP orientation: Wnt3-mRNA lineage in Wnt3-rescue host in yellow, Wnt3-MO graft in blue. (D) 3D reconstruction of the rosette structure from the confocal images by contouring cortical actin signals. (E) Schematic representation of the transplant experiment. (F) 3D representation of the late gastrula morphologies induced by mRNA injection of dominant-negative form (RhoABC-DN) and constitutively active forms (RhoABC-CA2) of the PCP effector RhoABC, which caused reduced elongation and extra protrusions, respectively. The overall oral-aboral (OA)-axis remained distinguishable based on morphology and gastrulation. (G–I) PCP is coordinated consistently with respect to the induced protrusions caused by RhoABC-CA2. (G) Colour-wheel representation of PCP in the RhoABC-CA2 injected early gastrula embryos; the presumed oral pole is to the top-right. (H) 3D reconstruction of the tissue morphology for the same specimen, with PCP flow represented by arrows. (I) Confocal image of the apical cortex of the epidermis at the induced protrusion, corresponding rectangles in G and H. Cortical actin fibres are circumferentially organised around the protrusion. Bars are 50 µm except for 10 µm in B and I. See Figure 1 for the circular colour-code representation of PCP orientation. Cyan: actin; magenta: γ-tubulin; blue: donor lineage marker Dextran for B and I.

Models for body axis symmetry-breaking during Clytia embryogenesis and planar cell polarity (PCP)-driven axis innovation scenarios in metazoan evolution.
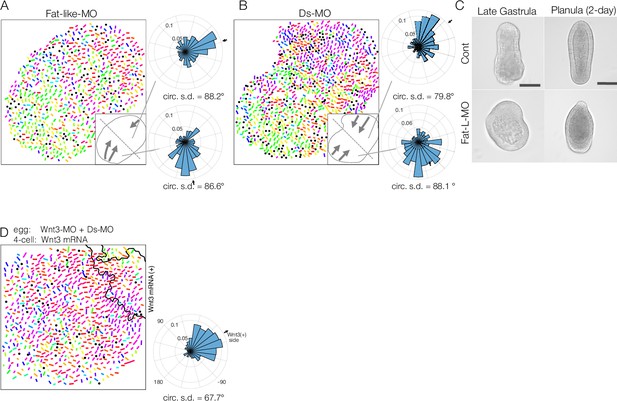
Fat-like and Dachsous play roles in long-range planar cell polarity (PCP) coordination for axial patterning in Clytia embryos.
(A, B) Long-range PCP coordination in early gastrula embryos injected with Fat-like-MO (CheFat1, A) or Ds-MO (CheDs, B). In both cases, while PCP is locally oriented in a specific direction, it is globally uncoordinated within the embryo. Two radar charts represent PCP orientation in the two halves of a single embryo. (C) Morphology of Fat-like-MO-injected embryos at the late gastrula and 2-day planula stages. Axial elongation is severely impaired in Fat-like-MO-injected embryos. Gastrulation from the oral end occurs, and oral-aboral (OA)-axis polarity is retained. (D) Wnt3-rescue by local mRNA injection can restore the PCP defect caused by Ds-MO injection. CheWnt3 and the Fat/Ds system may play partially overlapping roles in globally orienting PCP along the OA-axis, which remains morphologically distinct, unlike in embryos injected with Wnt3-MO or Stbm/Fmi-MO. (E) Local injection of Wnt3 partially restores PCP orientation defects caused by Dsh-MO injection. Fat/Ds in Clytia may contribute to global PCP orientation, partly overlapping with CheWnt3.
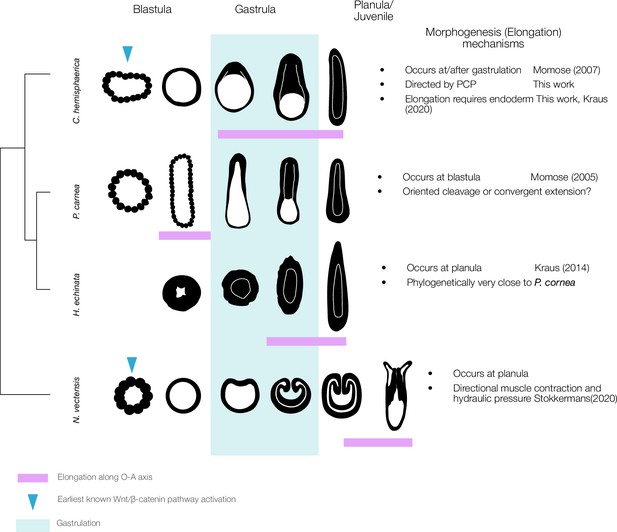
A wide heterochronic variation of axial elongation in phylum Cnidaria.
The axial elongation process occurs at different timings (indicated by the magenta box) during key developmental stages in four cnidarian model species: C. hemisphaerica, P. carnea, Hydractinia echinata, and Nematostella vectensis. The early gastrula stage is characterised by the onset of germ layer segregation and is typically the first stage to exhibit a distinct morphological OA-axis. Gastrulation patterns are also highly variable, including invagination, unipolar ingression, and delamination from the stereoblastula. Elongation occurs concomitantly with gastrulation in C. hemisphaerica, while in P. carnea it occurs before gastrulation. In contrast, in N. vectensis, elongation is mechanically driven by hydraulics resulting from directional muscle contraction coordinated along the OA-axis in planula stage (Stokkermans et al., 2022). Elongation is thus driven by tissue polarity that has already been established by this stage. The role of core PCP proteins in muscle PCP orientation remains to be studied in N. vectensis.
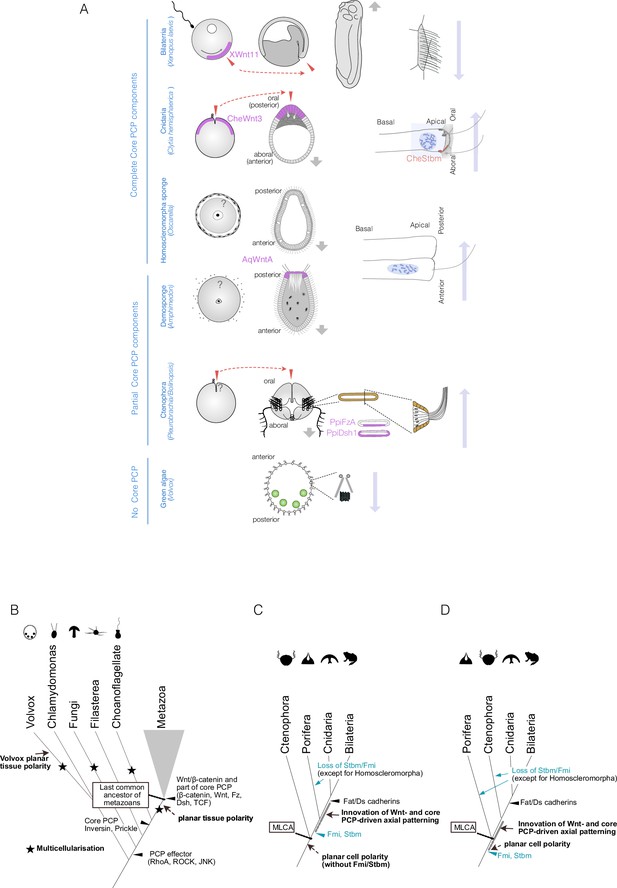
Planar cell polarity (PCP), or coordinated polarity of motile cilia, is tightly coupled to axial patterning across metazoans and has independently evolved in non-metazoan species.
(A) PCP associated with the body axis in metazoans and the multicellular green alga Volvox. Egg polarity and embryonic axial anatomy are indicated on the left. Cell polarity associated with the body axis is indicated on the right. The localisation of Wnt ligands or other localised PCP factors associated with egg polarity or body axis is indicated with a purple shade. In Clytia, Xenopus, and ctenophores, egg polarity determines the embryonic body axis, and these coordinations are shown in pairs of red arrows. Locomotion and fluid flow created by motile cilia are indicated with grey and long blue arrows, respectively. Volvox, a multicellular green alga that independently acquired multicellularity and lacks Wnt and core PCP proteins, displays clearly coordinated planar tissue polarity. PCP can therefore occur without Wnt and core PCP proteins. (B–D) Evolutionary scenarios for the emergence of the body axis and PCP based on Table 1. (B) Events in the common ancestor of metazoans and their outgroups. Multiple independent multicellularity events are indicated with stars (★). Key components of the Wnt/β-catenin and parts of the core PCP pathways (β-catenin, Wnt ligand, Frizzled, Dishevelled, TCF) predate the last common ancestor of metazoans (MLCA). Some core PCP proteins, such as Inversin (Diego)/Testin and Prickle, originated in the common ancestor of metazoans and choanoflagellates. All PCP effectors (e.g. Rho GTPases such as RhoA, Cdc42, ROCK) that regulate cytoskeletal arrangements are older, or as old as, the last common ancestor of fungi and metazoans (Opisthokonta) (Table 1, Supplementary file 1). (C) Metazoan evolution under the ctenophore-ancestor scenario. (D) Metazoan evolution under the sponge-ancestor scenario. In either case, the full set of core PCP proteins evolved in the sponge-cnidarian-bilaterian common ancestor. Stbm/Fmi was lost multiple times in sponges and would have been lost in ctenophores under the sponge-ancestor scenario. Wnt ligands and Dishevelled are also lost in the hexactinellid sponges Aphrocallistes vastus and Oopsacas minuta.
Videos
Live imaging of basal body migration at the blastula stage.
(193 µm x193 µm field of view, 570 times speed).
Tables
Presence and absence of Wnt/β-catenin and core planar cell polarity (PCP) protein genes in basal metazoan and non-metazoan species based on existing studies.
Homoscleromorpha sponges (Oscarella spp.) possess a complete set of core PCP proteins. PCP regulated by core PCP proteins may have originated in a sponge-cnidarian-bilaterian common ancestor, which is equivalent to the metazoan common ancestor in the sponge-ancestor scenario. The absence of PCP effectors in fungi and Capsaspora, and the lack of Inversin and Prickle orthologues in choanoflagellates, suggests that primitive PCP regulatory mechanisms predate the metazoan ancestor. Symbols: +: orthologue identified with fully conserved domains; –: orthologue absent; ±: gene with homologous sequence present but lacking complete domain structure.
| Core PCP | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wnt/β-catenin | Ft/Ds/Fj | |||||||||||||||
| β-Catenin | Axin | GSK3-β | TCF | Wnt | Frizzled/ Fzd | Dishevelled/Dvl | Flamingo/ Celsr | Strabismus/Vangl | Inversin | Prickle | Fat/Fat-like | Dachsous/Ds | Rho/ROCK | |||
| Amoeba | Dictyostelium discoideum | ±* | + | |||||||||||||
| Fungi | Allomyces macrogynus | ± § | + | + | ||||||||||||
| Filasterea | Capsaspora owczarzaki | + | + | |||||||||||||
| Choanoflagellate | Salpingoeca rosetta | ± § | + | ±† | – | – | + | + ¶ | + | |||||||
| Monosiga brevicollis | + | ± ‡ | – | – | – | – | + | + ¶ | ±** | + | ||||||
| Ctenophora | Mnemiopsis leidyi | + | – | + | + | + | + | + | – | – | – | + ¶ | + | |||
| Porifera | Homoscleromorpha | Oscarella carmela | + | + | + | + | + | + | + | + | + | + | + ¶ | ±** | + | |
| Oscarella lobularis | + | + | + | + | + | + | + | + | + ¶ | + | ||||||
| Calcerea | Sycon ciliatum | + | – | + | + | + | + | + | + | – | + | + ¶ | + | |||
| Demospongiae | Amphimedon queenslandica | + | + | + | + | + | + | + | – | – | + | + ¶ | + | + | ||
| Hexactinellida | Aphrocallistes vastus | + | + | + | + | – | + | – | – | – | + | + ¶ | + | |||
| Cnidaria | Anthozoa | Nematostella vectensis | + | + | + | + | + | + | + | + | + | + | + ¶ | + | + | + |
| Hydrozoa | Clytia hemisphaerica | + | + | + | + | + | + | + | + | + | + | + | +†† | + | + | |
| Hydra vulgaris | + | + | + | + | + | + | + | + | + | + | + | +†† | + | + | ||
| Placozoa | Tricoplax adherans | + | + | + | + | + | + | + | + | + | – | + | + | + | + | |
| Bilaterians | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
-
*
β-Catenin in Dictyostelium is a β-catenin-like protein called Aardvark, a component of the junctional complex involved in cell signalling (not mediated by GSK3).
-
†
TCF-like gene in choanoflagellates contains an HMG domain but is not a true TCF orthologue.
-
‡
Dsh-like gene in choanoflagellates is a PDZ domain-containing protein lacking DEP and DIX domains.
-
§
Choanoflagellate β-catenin is an armadillo repeat-containing protein.
-
¶
Prickle genes in ctenophores and sponges derive from a common Prickle-Testin ancestor. Choanoflagellates possess Testin orthologues but not Prickle.
-
**
Monosiga and Amphimedon have Lefftyrin cadherins that share EGF and LamG domains with Fat cadherins, but their domain arrangement is different – the EGF/LamG domains are adjacent to the transmembrane domain rather than at the N-terminus.
-
††
Clytia and Hydra have Fat1 (Fat-like) genes but not Fat4. Refer to Supplementary file 1 for the list of publications and gene accession numbers.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Sequence-based reagent | Wnt3MO | Momose et al., 2008 | Morpholino oligonucleotide | CCAAAACACACCAGTGTCGAGCCAT |
| Sequence-based reagent | Fmi-MO | This paper | Morpholino oligonucleotide | CCTCAAGCCATCTGAGCTTCATTTT |
| Sequence-based reagent | Stbm-MO | Momose et al., 2012 | Morpholino oligonucleotide | TCACTCCATCATCAAAATCATCCAT |
| Sequence-based reagent | Fat-like-MO | This paper | Morpholino oligonucleotide | CATCAAAATGTGAGACTTACCAGCC |
| Sequence-based reagent | Ds-MO | This paper | Morpholino oligonucleotide | TACGGTGATGGATAGTTCATCTTTC |
| Chemical compound, drug | Phalloidin Alexa Fluor 488 | Invitrogen | A12379 | 4 units/ml 0.13 µM in PBS |
| Chemical compound, drug | Phalloidin Alexa Fluor 647 | Invitrogen | A22287 | 4 units/ml 0.13 µM in PBS |
| Antibody | anti-Par3 (Rabbit polyclonal) | Merk Millipore | Cat# 07–330 RRID:AB_2101325 | IF (1:200) |
| Antibody | anti-γ Tubulin (Mouse monoclonal) | SIGMA | Cat# T5326 RRID:AB_532292 (GTU-88) | IF (1:500) |
| Antibody | anti-centrin (Mouse monoclonal) | Merck Millipore | Cat# 04–1624 RRID:AB_10563501 (20H5) | IF (1:200) |
| Recombinant DNA reagent | pCX3-Wnt3 | Momose et al., 2008 | XLOC_001931 | Wildtype (3 bp mismatch in MO target) |
| Recombinant DNA reagent | pCX3-dnTCF | This paper | XLOC_007658 | Dominant negative N-terminal 27 a.a. deletion |
| Recombinant DNA reagent | pCX3-CA-β-cat | This paper | XLOC_003560 | Constitutively active S97A, T101A, S105A |
| Recombinant DNA reagent | pCX3-RhoABC-DN | This paper | XLOC_044216 | Dominant negative T19N |
| Recombinant DNA reagent | pCX3-RhoABC-CA1 | This paper | XLOC_044216 | Constitutively active G14V |
| Recombinant DNA reagent | pCX3-RhoABC-CA2 | This paper | XLOC_044216 | Constitutively active Q63L |
| Recombinant DNA reagent | pMiniTol2-ACT2::PH-Venus | This paper | U09117.1 | PLC-delta-1 PH-domain-Venus fusion protein |
| Recombinant DNA reagent | pMiniTol2-ACT2::Poc1-CC | This paper | HM010924.1 | Clytia Poc1-mCherry fusion protein |
| Strain, strain background | Clytia hemisphaerica Z4C2 | EMBRC-Fr | Z4C2 | Male wildtype strain, discontinued |
| Strain, strain background | Clytia hemisphaerica Z4B | EMBRC-Fr | Z4B | Female wildtype strain, discontinued |
| Strain, strain background | Clytia hemisphaerica Z23 | EMBRC-Fr | Z23 | Male wildtype strain: (Z4C2 × Z4B) |
| Strain, strain background | Clytia hemisphaerica Z30 | EMBRC-Fr | Z30 | Female wildtype strain: (Z4C2 × Z4B) |
| Software, algorithm | ImageJ | ImageJ | RRID:SCR_003070 | |
| Software, algorithm | MATLAB | Mathworks | RRID:SCR_001622 | |
| Software, algorithm | Imaris | Oxford Instruments | RRID:SCR_007370 |
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/104508/elife-104508-mdarchecklist1-v1.docx
-
Supplementary file 1
Information on the presence of genes in key species, including accession numbers from sequence databases, or citations of studies reporting their absence.
- https://cdn.elifesciences.org/articles/104508/elife-104508-supp1-v1.xlsx





