Trained immunity in the lung
Figures
Figure 1
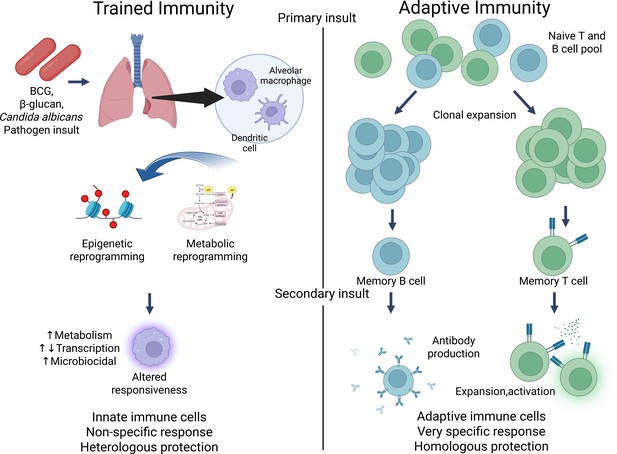
Contrasting trained immunity to adaptive immunity.
Figure generated using BioRender.com.
Tables
Table 1
Summary of trained immunity studies and lung diseases.
| Cell type(s) | Stimulus | Mechanism(s) | Outcomes | Notes | Study |
|---|---|---|---|---|---|
| Alveolar macrophages (AMs), monocyte-derived AMs (Mo-AMs) | Sequential infections: influenza virus followed by Streptococcus pneumoniae | Influenza leads to depletion of resident AMs through apoptosis, migration, or functional inactivation (‘alveolar macrophage disappearance reaction’). CCR2-dependent monocyte recruitment replenishes the AM niche with Mo-AMs. Mo-AMs undergo IL-6-mediated epigenetic training, enhancing early antibacterial responses. | Improved survival and reduced bacterial burden after secondary pneumococcal infection. Mo-AMs persist but gradually lose protective traits over time. | Trained immunity is transient (~2 months post-influenza). | Aegerter et al., 2020 |
| Resident alveolar macrophages (AMs) | PepO protein from Streptococcus pneumoniae | PepO protein stimulates complement C3 secretion and G-CSF production by AMs, without full activation. Establishes a trained phenotype by enhancing innate bactericidal function against unrelated pathogens. | Central-trained immunity established; increased resistance to bacterial pneumonia without inducing systemic inflammation. | Highlights possibility of trained immunity via non-lethal microbial components. | Xu et al., 2024 |
| Tissue-resident alveolar macrophages (TR-AMs) | Intranasal adenoviral vector administration | Local viral infection stimulates CD8+ T cells to produce IFN-γ, which primes TR-AMs to upregulate MIP-2 and KC chemokines. This enhances neutrophil recruitment to the airways during secondary bacterial infections. Training occurs without monocyte input (local imprinting). | Improved early bacterial clearance upon Streptococcus pneumoniae challenge. | Provides evidence that viral infections can directly induce trained immunity in TR-AMs. | Yao et al., 2018 |
| Mo-AMs replacing TR-AMs (especially in aged lungs) | Aging process, prior respiratory viral infections (influenza) | Aging leads to impaired TR-AM survival and impaired self-renewal capacity. Viral infections exacerbate depletion. CCR2-mediated recruitment of monocytes leads to replacement by Mo-AMs. Mo-AMs show increased glycolysis and a hyper-inflammatory phenotype compared to TR-AMs, contributing to tissue damage and chronic inflammation. | In aged mice, infections cause more severe lung injury due to predominance of glycolytic, inflammatory Mo-AMs rather than quiescent TR-AMs. | Emphasizes metabolic reprogramming (Warburg effect) and its detrimental effects in elderly lung immunity. | Li et al., 2022 |
| Resident airway macrophages | SARS-CoV-2 infection | Persistent chromatin remodeling around type I interferon (IFN-I) response genes, even after viral clearance. Increased accessibility of IRF and STAT transcription factor motifs. Suggests formation of ‘innate immune memory’ following viral pattern recognition. | Enhanced baseline antiviral state, potential impact on future respiratory infections. | Mechanisms still under investigation; likely involve both direct viral sensing and damage signals (DAMPs). | Lercher et al., 2024; Simonis et al., 2025 |
| Natural killer (NK) cells | Viral infections, BCG vaccination | NK cells acquire memory-like properties after infections. Enhanced IFN-γ, IL-1β, and IL-6 production upon secondary stimulation. Primed for faster and stronger responses. | Improved clearance of respiratory viruses; enhanced responses to secondary challenges. | NK cell-trained immunity impacts airway antiviral defense and broader innate immune memory. | Sun et al., 2009; Romee et al., 2012; Kleinnijenhuis et al., 2014 |
| Dendritic cells (DCs) | Cryptococcus neoformans infection, RSV infection | Exposure leads to epigenetic reprogramming of DCs. Increased IFN-γ and pro-inflammatory cytokine production upon secondary encounters. DC-mediated protection relies on cytokine production like IFN-γ, TNF-α, and IL-17a, as well as STAT1 pathway activation. | DCs play a crucial role in trained immunity and protection against reinfection. | Proper activation of DCs is crucial for protective immunity; impaired DC responses can lose protection. | Hole et al., 2019 |
| Dendritic cells (DCs) | Respiratory syncytial virus (RSV) infection | RSV-triggered TSLP induces epigenetic reprogramming in bone marrow-derived DCs, altering cytokine production and upregulating costimulatory molecules. This leads to an enhanced inflammatory phenotype and exacerbated allergic responses. | RSV-induced trained immunity via TSLP alters immune cell responses and can promote allergic diseases. | Innate immune memory may amplify allergic susceptibility and interfere with appropriate antiviral responses. | Hole et al., 2019; Malinczak et al., 2021 |
| Alveolar macrophages (AMs) and epithelial cells | β-glucan exposure, bleomycin-induced injury | β-glucan primes AMs and epithelial cells via soluble mediators. This leads to enhanced efferocytosis, increased SIRT1 expression, and tissue protection by reducing fibrosis and apoptosis. | β-glucan-induced trained immunity protects against injury and fibrosis, particularly in lung epithelial cells. | Enhanced tissue resilience, reduced apoptosis, and increased resistance to lung fibrosis. | Kang et al., 2024 |
Download links
A two-part list of links to download the article, or parts of the article, in various formats.
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Trained immunity in the lung
eLife 14:e104918.
https://doi.org/10.7554/eLife.104918





