Phosphorylation and activation of ubiquitin-specific protease-14 by Akt regulates the ubiquitin-proteasome system
Figures
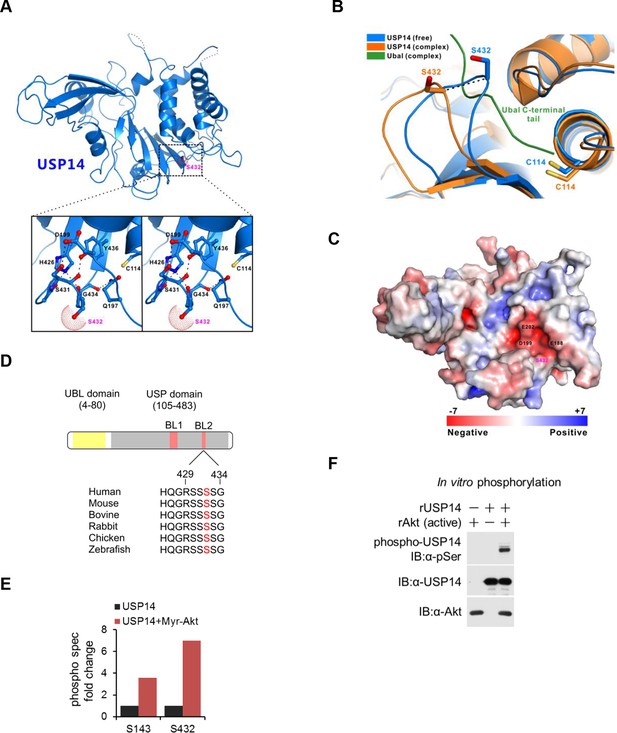
Structural basis of ubiquitin-specific protease-14 (USP14) activation by phosphorylation of Ser432.
(A) Detailed view of blocking loop 2 (BL2), which occludes the active site of USP14 (PDB access code 2AYN). The BL2 loop, which contains Ser432, is shown in stick model, in the apo form. (B) Combined ribbon representation and stick model showing a comparison of the conformations of the BL2 loop contained in the apo form (blue, PDB access code 2AYN) and in the USP14- Ub-aldehyde (Ubal) adduct (orange, PDB access code 2AYO). In this drawing, the Ser432 and Cys114 residues are shown in stick model, and the bound Ubal (a ubiquitin derivative in which the C-terminal carboxylate is replaced by an aldehyde) in the complex is drawn in green. (C) A surface charge potential representation (contoured at ± 7 kT/eV; blue/red) of USP14 (PDB accession 2AYN) showing that the S432 residue is very close to a highly negatively charged patch mainly formed by the acidic E188, D199, and E202 residues. When S432 is phosphorylated, the negatively charged phosphate group may induce a repulsive force, thereby relieving inhibition of the catalytic activity of USP14. (D) USP14 domain organization and sequence alignment of the Akt phosphorylation site within USP14 orthologs from different species. Two BLs (BL1 and BL2) covering the USP14 active site are shown. The Akt phosphorylation site in USP14 from different species as predicted by Scansite. (E) S432 is the major phosphorylation site in USP14. HEK293T cells were treated as in Figure 1—figure supplement 1B, followed by electrospray ionization mass spectrometry (ESI-MS) analysis. Spectral counts were determined by ESI-MS. (F) Akt phosphorylates USP14 in vitro. Bacterially expressed and purified USP14 was incubated with active Akt in the presence of ATP. Reaction products were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and phosphorylated species were detected by a phospho-Ser antibody.
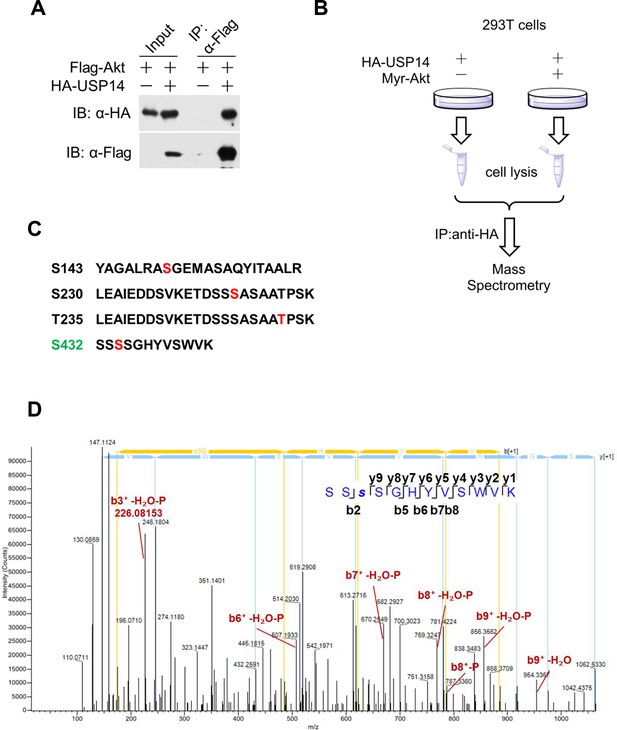
Akt phosphorylates ubiquitin-specific protease-14 (USP14).
(A) Akt interacts with USP14. HEK293T cells were transfected with indicated plasmids for 24 hr. The cell lysates were collected for co-immunoprecipitation and western blotting analysis. (B) Schematic representation of mass spectrometry assay to determine USP14 phosphorylation sites by Akt. (C) Four phosphorylation sites of USP14 were determined by mass spectrometry. (D) The representative MS/MS spectrum of phosphorylated tryptic peptide ‘SSSphosSGHYVSWVK’ of human USP14 protein. The peptide sequence ‘SSSphosSGHYVSWVK’ containing phosphorylated S432 was identified by shotgun analysis using mass spectrometry when USP14 was coexpressed with Myr-Akt in HEK293T cells. Fragmentation ion of the amide bond of the peptide result in formation of “b” ion and “y” ion series corresponding to the N- and C-terminal fragments respectively. Representative ions with phosphorylation and H2O loss were manually labeled in red on the spectrum.
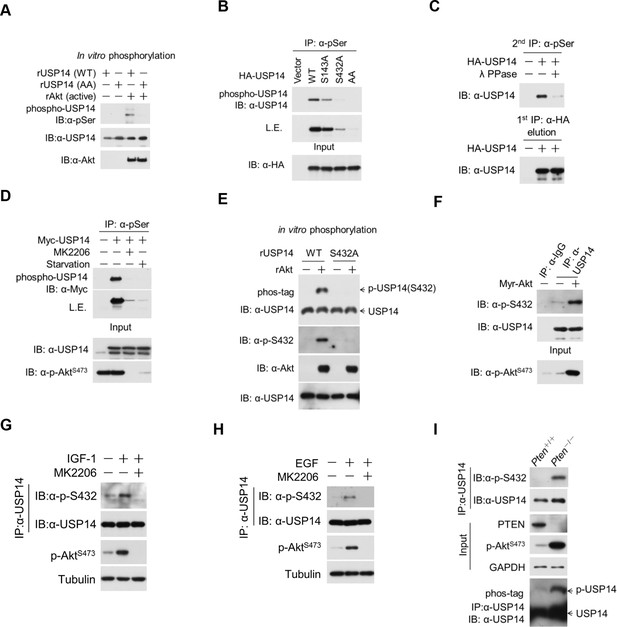
Ubiquitin-specific protease-14 (USP14) is phosphorylated at Ser432 by activated Akt.
(A) In vitro phosphorylation of USP14 at S432 by Akt. Bacterially expressed and purified wild type USP14 or AA mutant incubated with active Akt in the presence of ATP. Reaction products were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and phosphorylation was detected by the phospho-Ser antibody. (B) Akt phosphorylates USP14 at S432 in vivo. Western blot analysis of whole cell lysate and immunoprecipitates derived from HEK293T cells transfected with wild type USP14, USP14 S143A, USP14 S432A, and USP14 S143A/S432A (AA) constructs using the phospho-Ser antibody. L.E., long exposure. (C) Immunoprecipitation (IP) and IB analysis of HEK293T cells transfected with HA-USP14 and Myr-Akt and preincubated with or without λ-phosphatase as indicated. (D) Inhibition of Akt decreased exogenous USP14 phosphorylation. HEK293T cells were transfected with Myc-USP14 for 20 hr then treated with 1 μM MK2206 or deprived of serum for another 4 hr before harvest. (E) In vitro kinase assay to detect Akt phosphorylation of USP14 by phospho-Ser432-specific antibody and phos-tag-containing gels. Bacterially expressed and purified wild type USP14 or S432A mutant was incubated with active Akt in the presence of ATP. The reaction products were resolved by SDS-PAGE, and USP14 phosphorylation was detected using an antibody that specifically recognizes Ser432 phosphorylation of USP14 or determined by differential migration on phos-tag gels. (F) In vivo detection of endogenous USP14 Ser432 phosphorylation by anti-p-Ser432-specific antibody. Western blot analysis of immunoprecipitates derived from H4 cells transfected with or without Myr-Akt plasmids using the anti-p-Ser432-specific antibody. (G, H) Phosphorylation of endogenous USP14 S432 upon stimulation with insulin-like growth factor (IGF-1) or epidermal growth factor (EGF). HEK293T cells were serum-starved and pretreated with Akt inhibitor MK2206 (1 μM) for 30 min before stimulation with IGF-1 (100 ng/mL) for 30 min (G) or EGF (100 ng/mL) for 1 hr (H). The cell lysates were immunoprecipitated with USP14 antibody and western-blotted with anti-p-S432 antibody. (I) Phosphorylation of endogenous USP14 S432 in Pten knockout cells with high activity of Akt. Lysates from mouse embryonic fibroblasts (MEFs) with indicated genotypes were immunoprecipitated with USP14 antibody and then Western blotted with p-S432 antibody. The differential migration of phospho-USP14 on phos-tag-containing gels was determined as shown in the bottom panel.
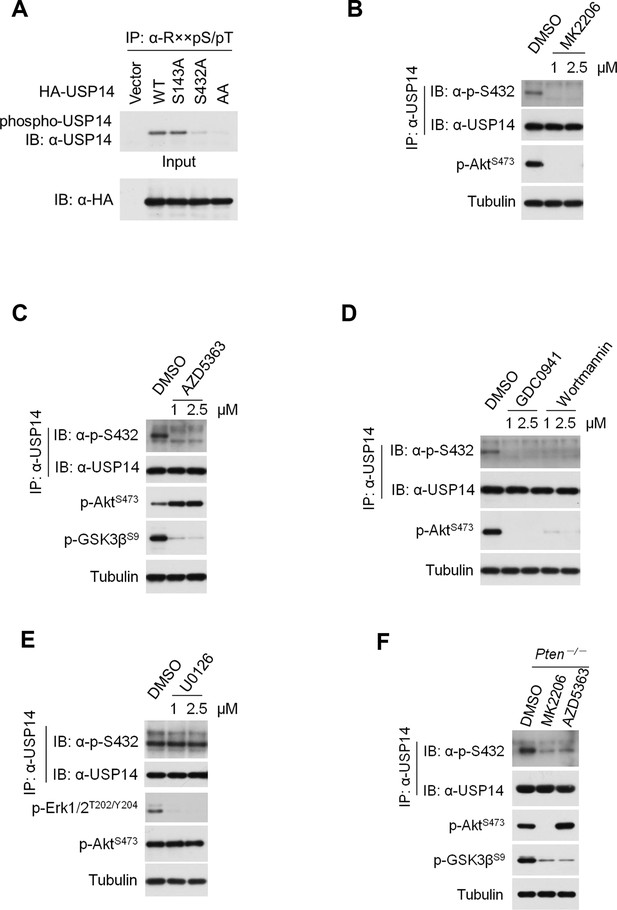
Ubiquitin-specific protease-14 (USP14) is phosphorylated at Ser432 by Akt.
(A) Akt phosphorylates USP14 at S432 in vivo. Western blotting analysis of whole cell lysate and immunoprecipitates derived from HEK293T cells transfected with wild type USP14, USP14 S143A, USP14 S432A, and USP14 S143A/S432A (AA) constructs using an Akt phosphorylation-consensus motif (R××S/T) antibody. (B, C) Inhibition of Akt decreases USP14 S432 phosphorylation levels. H4 cells were treated with different concentration of Akt inhibitors MK2206 (B) or AZD5363 (C) as indicated for 4 hr. The cell lysates were collected for immunoprecipitation and western blotting analysis. (D) Inhibition of phosphoinositide 3-kinases (PI3K) decreases USP14 S432 phosphorylation levels. H4 cells were treated with different concentration of PI3K inhibitors GDC0941 or Wortmannin as indicated for 4 hr. The cell lysates were collected for immunoprecipitation and western blotting analysis. (E) ERK1/2 inhibition has no effect on USP14 S432 phosphorylation. H4 cells were treated with different concentration of ERK1/2 inhibitor U0126 as indicated for 4 hr. The cell lysates were collected for immunoprecipitation and western blotting analysis. (F) Pten–/–mouse embryonic fibroblast (MEF) cells were treated with 1 μM Akt inhibitors for 4 hr, then cell lysates were collected for immunoprecipitation and western blotting analysis.
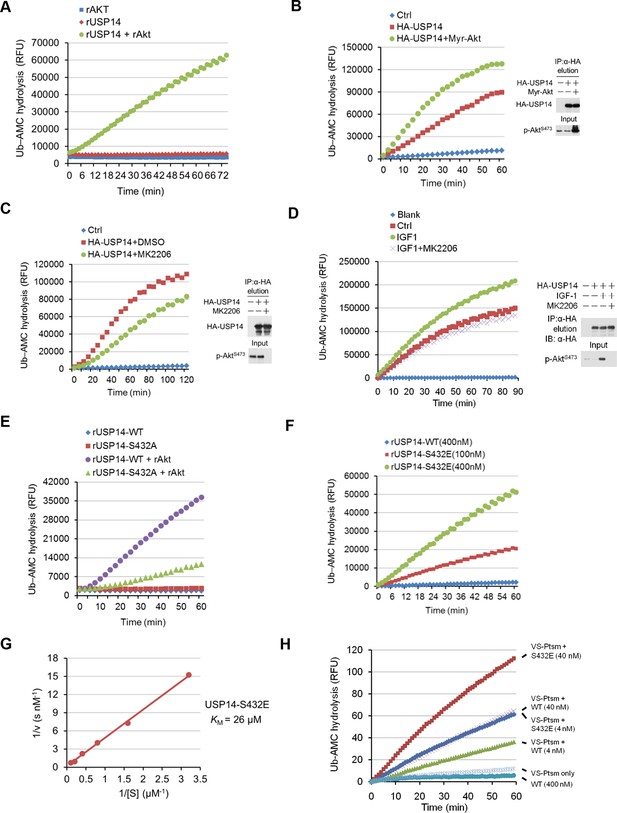
Phosphorylation of ubiquitin-specific protease-14 (USP14) by Akt activates USP14 DUB activity.
(A) Akt activates USP14 DUB activity in vitro. USP14 protein (1 μg) was incubated with or without active Akt (1 μg) in kinase assay buffer in a total volume of 50 μL for 1 hr at 30°C, then the reaction mixtures were subjected to Ub-AMC assay. RFU, relative fluorescence units. (B, C) Akt activates USP14 in cells. USP14 was immunoprecipitated from HEK293T cells coexpressed with activated Akt (B) or treated with 10 μM MK2206 for 4 hr (C) and then eluted with HA-peptide following Ub-AMC hydrolysis assay. (D) Activation of USP14 by stimulating cells with IGF-1. HEK293T cells were serum-starved and pretreated with or without Akt inhibitor MK2206 (1 μM) for 30 min before stimulation with IGF-1 (100 ng/mL) for 30 min. USP14 was then immunoprecipitated and eluted with HA-peptide. The activity of USP14 was determined using Ub-AMC hydrolysis assay. (E) USP14 activation by Akt is blocked by S432A mutation. Ub-AMC hydrolysis assay of wildde type USP14 or S432A mutant in the presence or absence of active Akt. (F) Ub-AMC hydrolysis assay of bacterially expressed and purified wild type USP14 or S432E mutant. (G) Lineweaver–Burk analysis of USP14 S432E, obtained by measuring the initial rates at varying Ub-AMC concentrations (see Figure 3—figure supplement 2E for reference). (H) The activity of phospho-mimetic USP14 mutant can be further stimulated by the presence of proteasome. Ub-AMC hydrolysis assay of wild type USP14 or S432E mutant in the presence or absence of Ub-VS-treated human proteasome (VS-proteasome (see Lee et al., 2010); 1 nM). Ptsm, 26S proteasome.
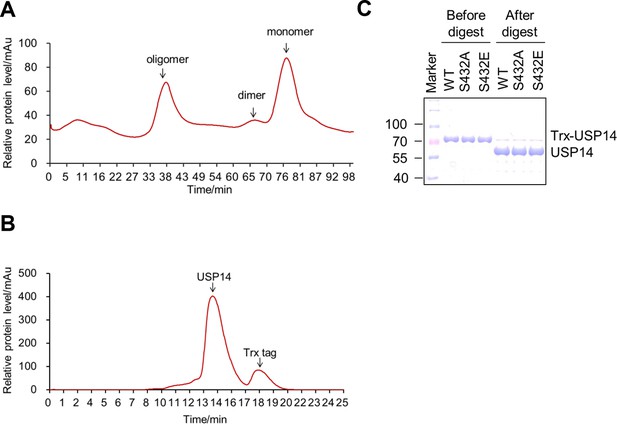
Purification of ubiquitin-specific protease-14 (USP14) recombinant protein.
(A, B) A representative curve of USP14 recombinant protein purification before (A) and after (B) cleavage by 3C protease on size-exclusion chromatography. Bacterially expressed USP14 forms oligomer and dimer, only monomer was used for further experiments. Trx-tag was removed to get tag-free USP14 protein for in vitro kinase assay or Ub-AMC and ubiquitin cleavage assay. (C) One dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie Brilliant Blue (CBB) staining of purified USP14 protein before and after cleavage by 3C protease.
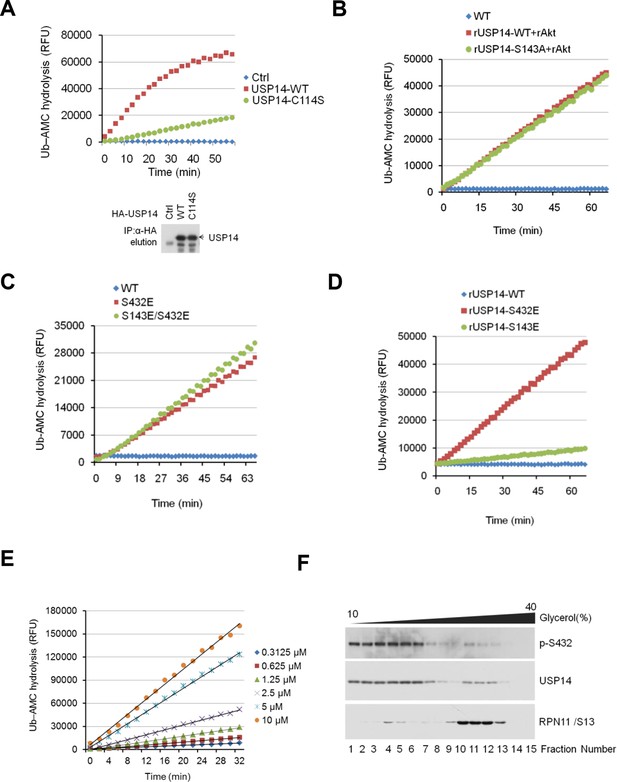
Phosphorylation of USP14 by Akt activates USP14 DUB activity.
(A) Validation of Ub-AMC assay on immunoprecipitated USP14 from HEK293T cells. Wild type or inactive mutant USP14 was immunoprecipitated from transfected HEK293T cells and then eluted with HA-peptide following Ub-AMC hydrolysis assay. (B–D) Ser143 phosphorylation has limited effect on USP14 activity. S143A mutant had similar levels of hydrolyzing activity as that of WT USP14 in the presence of active Akt (B). Double E mutant (S143E/S432E) showed similar levels of hydrolyzing activity as that of S432E single mutant (C). S143E mutant has minor effect on USP14 activity compared with S432E mutant (D). (E) Linear kinetics (R2 > 0.99) of Ub-AMC hydrolysis. (F) Distribution of total and phosphorylated USP14 specific in glycerol gradient centrifugation. Soluble lysates of Pten–/– mouse embryonic fibroblast (MEF) cells, prepared as described in Materials and methods, were subjected to glycerol density gradient centrifugation. Gradient fractions were collected and subjected to western blotting with the indicated antibodies. Anti-RPN11 was used as a control for proteasome.
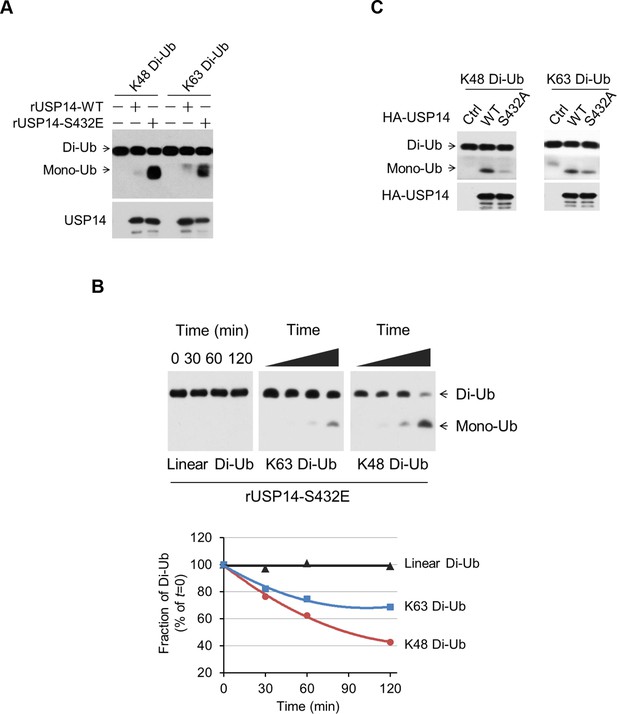
Phosphorylation stimulates ubiquitin-specific protease-14 (USP14) activity towards both K48 and K63 ubiquitination.
(A) Dimeric Ub cleavage assay. USP14 S432E cleavage of Lys 48 and Lys 63 Ub chain linkages was analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). (B) Cleavage of Lys 48, Lys 63 and linear dimeric Ub chain types in the presence of USP14 S432E was measured over time and analyzed using SDS-PAGE. Quantification of the amount of dimer remaining from the data was shown below. (C) Dimeric Ub cleavage analysis of immunoprecipitates derived from HEK293T cells transfected with wild type HA-USP14 or S432A mutant plasmids.
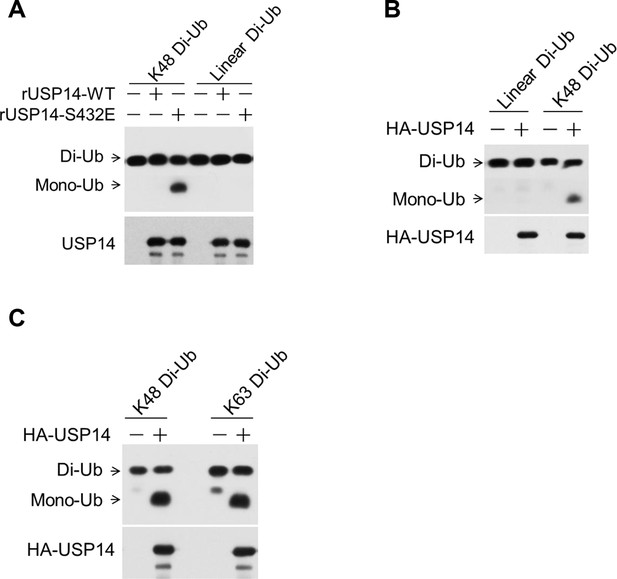
Phosphorylation of ubiquitin-specific protease-14 (USP14) promotes both K48 and K63 deubiquitination activity.
(A) USP14 S432E does not cleave linear di-Ub. USP14 S432E cleavage of linear dimeric Ub chain types and analyzed using SDS-PAGE. Cleavage of Lys48 dimeric Ub chain types was used as a positive control. (B, C) USP14 immunoprecipitated from transfected HEK293T cells and eluted with HA-peptide has high deubiquitination activity towards both K48 and K63 di-Ub but not linear di-Ub.
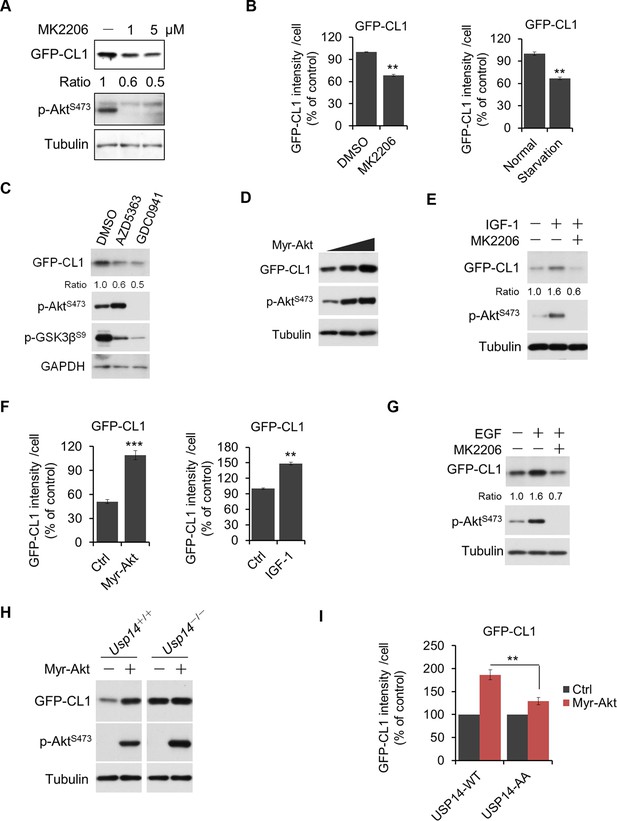
Akt regulates ubiquitin–proteasome system (UPS) function through phosphorylation of ubiquitin-specific protease-14 (USP14).
(A) Inhibition of Akt promotes UPS function. H4-GFP-CL1 cells were treated with different concentrations of MK2206 as indicated for 4 hr. The cells were then harvested and subjected to western blotting analysis using indicated antibodies. (B) H4-GFP-CL1 cells were treated as in (A) and Figure 5—figure supplement 1D. Images of the cells were collected using an ArrayScan HCS 4.0 Reader. The average GFP intensity in 2,000 cells from each indicated sample was determined. Data are displayed as mean ± SD of the GFP intensity per cell. **p<0.01, ***p<0.001 (C) Inhibition of Akt or phosphoinositide 3-kinase (PI3K) promotes UPS function. H4-GFP-CL1 cells were treated with Akt inhibitor AZD5363 (1 μM) or PI3K inhibitor GDC0941 (1 μM) as indicated for 4 hr. The cells were then harvested and subjected to western blotting analysis using indicated antibodies. p-GSK3β(S9) was blotted to indicate the inhibition of Akt. (D) Activation of Akt inhibits UPS. H4-GFP-CL1 cells were transfected with Myr-Akt for 24 hr. The cells were then harvested and subjected to western blotting analysis using indicated antibodies. (E) IGF-1 stimulation inhibits UPS function. H4-GFP-CL1 cells were serum-starved and pretreated with Akt inhibitor MK2206 (1 μM) for 30 min before stimulating with IGF-1 (100 ng/mL) for 30 min. The cells were then imaged and quantified as in (B). (F) H4-GFP-CL1 cells were treated as in (D) and Figure 5—figure supplement 1E. Then cells were imaged and quantified as in (B). (G) EGF stimulation inhibits UPS function. H4-GFP-CL1 cells were serum-starved and pretreated with Akt inhibitor MK2206 (1 μM) for 30 min before stimulation with EGF (100 ng/mL) for 1 h. The cells were then harvested and subjected to western blotting analysis using indicated antibodies. (H) Akt regulates UPS function through USP14. Myr-Akt was transfected into either wild type or Usp14–/– H4 cells stably expressing GFP-CL1 for 24 hr. The cells were then harvested and subjected to western blotting analysis using indicated antibodies. (I) Akt regulates UPS function through phosphorylation of USP14. Myr-Akt was transfected into either wild type USP14 or USP14 AA reconstitution cell lines stably expressing GFP-CL1 for 24 hr. The cells were then imaged and quantified as in (B).
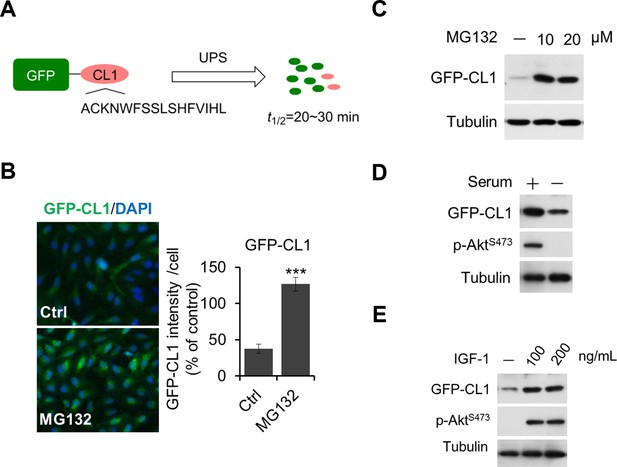
Regulation of UPS by Akt.
(A, B, C) Validation of GFP-CL1 assay. A schematic representation of GFP-CL1 assay. CL1 degron (ACKNWFSSLSHFVIHL) was added to the C-terminal of GFP as a UPS activity reporter (A). H4-GFP-CL1 cells were treated with 10 μM MG132 for 4 hr. Images of the cells were collected using an ArrayScan HCS 4.0 Reader. The average GFP intensity in 2,000 cells from each indicated sample was determined. The data are displayed as means ± SD of the GFP intensity per cell (B). H4-GFP-CL1 cells were treated with different concentration of MG132 as indicated for 4 hr, and then cells were harvested and subjected to western blotting analysis using indicated antibodies (C). (D) H4-GFP-CL1 cells were serum-starved overnight and harvested and subjected to western blotting analysis using indicated antibodies. (E) H4-GFP-CL1 cells were serum-starved overnight before stimulation with IGF-1 for 30 min. Then the cells were harvested and subjected to western blotting analysis using indicated antibodies.
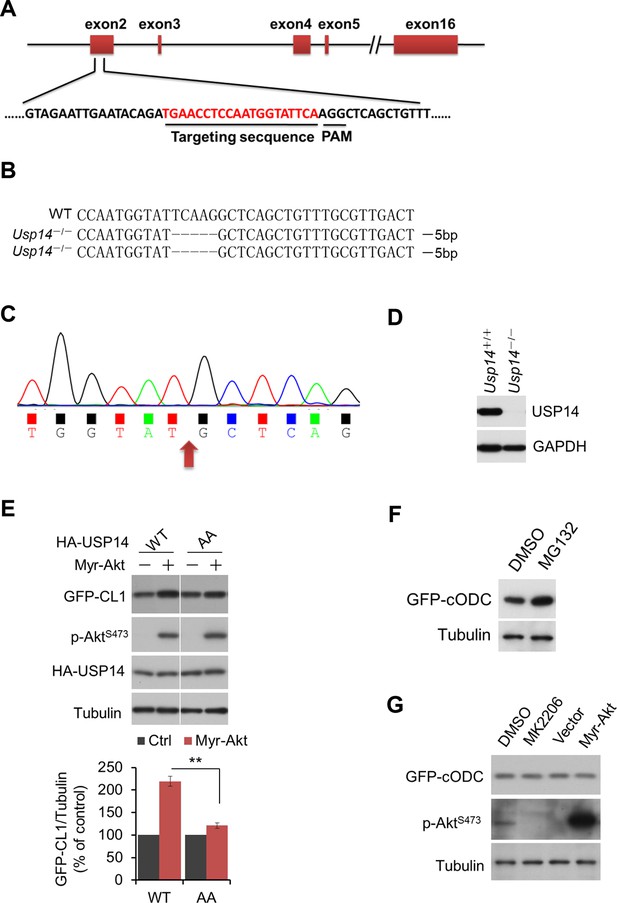
Regulation of UPS by Akt depends on phosphorylation of USP14.
(A, B, C, D) Generation of USP14 knockout H4 cell line using CRISPR/Cas9 system. A schematic of the Cas9/sgRNA/oligo targeting site in the exon2 of Usp14. The sgRNA coding sequence is underlined and labeled in red. The protospacer-adjacent motif (PAM) sequence is underlined (A). The deleted sequences in the Usp14–/– cell lines are presented. The number of sequences analyzed is indicated right (B). Sequencing analysis of Usp14–/– cell lines. The arrow indicates the missing sequences (TCAAG) (C). Western blotting analysis of USP14 expression in WT cells and Usp14–/– cells (D). (E) Akt regulates UPS function through phosphorylation of USP14. An expression vector for Myr-Akt was transfected into either wild type USP14 or USP14 AA reconstitution cell lines stably expressing GFP-CL1 and incubated for 24 hr. Then the cell lysates were harvested and subjected to western blotting analysis using indicated antibodies. (F) GFP-cODC assay was verified. H4-GFP-cODC cells were treated with 10 μM MG132 for 4 hr, and then the cell lysates were harvested and subjected to western blotting analysis using indicated antibodies. (G) H4-GFP-cODC cells were treated with 10 μM MK2206 for 4 hr or transfected with an expression vector for Myr-Akt as indicated for 24 hr. Then the cell lysates were harvested and subjected to western blotting analysis using indicated antibodies.
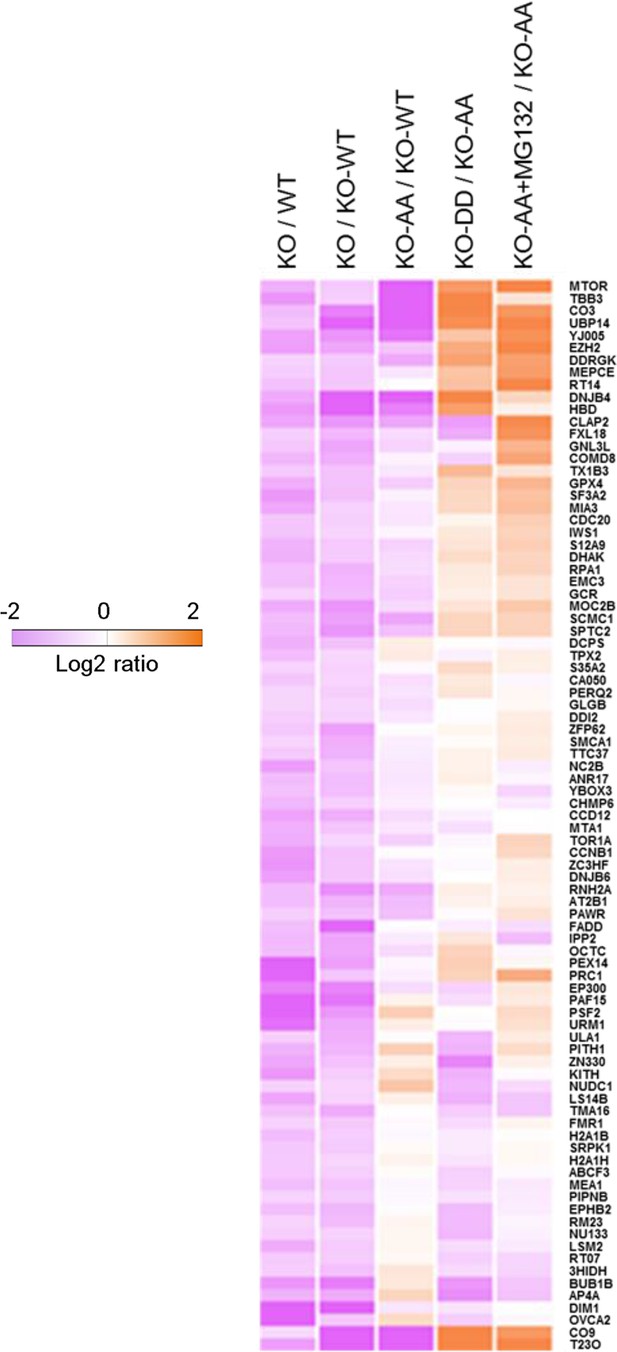
Phosphorylation of ubiquitin-specific protease-14 (USP14) regulates global protein degradation.
The quantitative analysis of proteome change in USP14 knockout or USP14 mutant cells were performed by tandem mass tag (TMT)-isobaric labeling followed by shotgun analysis. The heat map was plotted based on the set of 87 proteins that are down-regulated greater than or equal to 1.2-fold in H4 KO cells compared to H4 WT cells or to H4 KO cells complemented with WT USP14 (KO-WT). The log base 2 of average ratios was plotted as indicated.
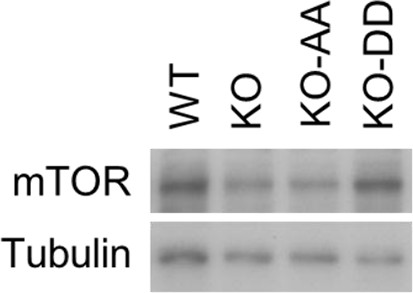
Western blotting analysis of mTOR expression in WT cells and USP14 mutant-reconstituted cells.
The cell lysates were harvested and subjected to western blotting analysis using indicated antibodies.





