De novo centriole formation in human cells is error-prone and does not require SAS-6 self-assembly
Figures
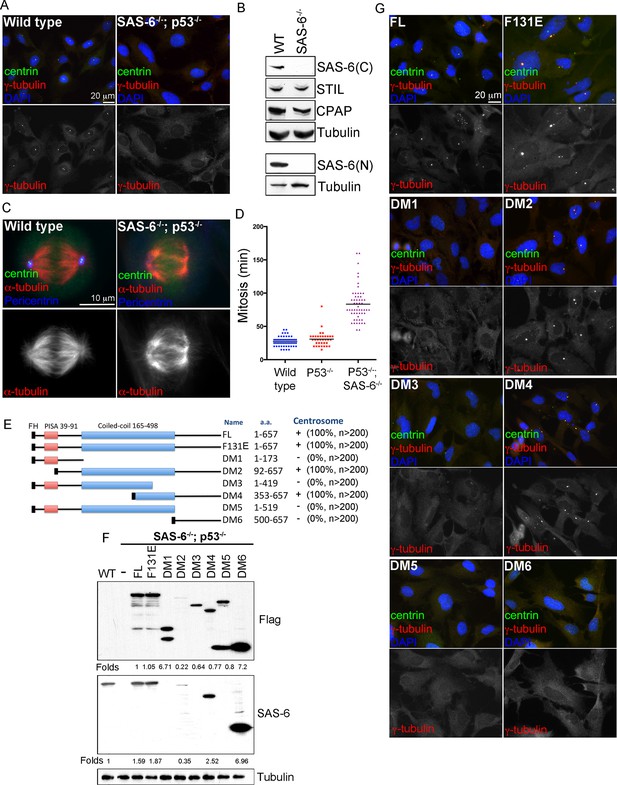
De novo centrosome formation in the absence of SAS-6 self-oligomerization.
(A) Wild-type (WT) or acentrosomal (p53-/-; SAS-6 -/-; clone #1) RPE1 cells were stained with anti-centrin (green) and γ-tubulin (red). DNA (DAPI, blue). (B) Western blot analysis of WT or SAS-6-/- cells with indicated antibodies, including both N- and C-terminal SAS-6 antibodies (SAS-6N; SAS-6C). (C) WT or acentrosomal cells in mitosis were stained with anti-centrin (green), α-tubulin (red), and pericentrin (blue). (D) The duration of mitosis in WT or SAS-6-/- cells was measured through time-lapse imaging of live cells. (E) A schematic diagram showing various SAS-6 mutants tagged with Flag and HA (FH). Clone #1 p53-/- SAS-6-/- cells were infected with lentiviruses carrying various of SAS-6 constructs, and the ability of each construct in rescuing centrosome formation was indicated and quantified in infected cells expressing detectable HA-tagged SAS-6. n, number of infected cells examined. (F) Isogenic SAS-6-/-; p53-/- cells carrying indicated SAS-6 constructs (see Methods) were induced for SAS-6 expression for 3 days and then analyzed by western blot with Flag antibodies or C-terminal SAS-6 antibodies (SAS-6C). For analyses with N-terminal SAS-6 antibodies (SAS-6N), please see Figure 1—figure supplement 1D. Note that in Flag staining, DM6 leaked to the lane of DM5. The expression level of each SAS-6 construct, indicated as fold changes (Folds) relative to the endogenous SAS-6 or SAS-6FL, is shown. (G) Isogenic SAS-6-/-; p53-/- cells carrying indicated SAS-6 constructs were treated as (F) and analyzed by immunofluorescence microscopy using indicated antibodies (Scale bar: 20 μm in A and G, 10 μm in C).
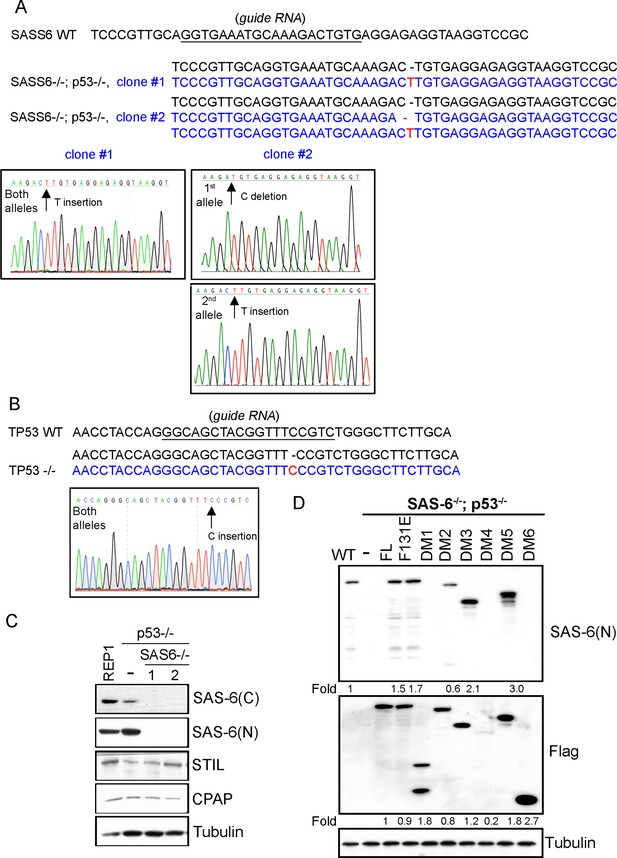
Characterization of SAS6-/-; p53-/- cell lines (clone #1 and #2).
(A, B) Sequence analyses of SAS-6 and p53 alleles in clone #1 and clone #2 as indicated. The sequence of wild-type and edited alleles are in black and blue, respectively. The lesion (deletion or insertion) is shown in red. (C) Western blot analysis of WT, SAS6-/-; p53-/- clone #1, and SAS6-/-; p53-/- clone #2 cells with indicated antibodies. SAS-6C and SAS-6N are antibodies recognizing the N-terminus and C-terminus of SAS-6, respectively. (D) Isogenic SAS6-/-; p53-/- cells carrying indicated SAS-6 constructs (see ‘Methods’) were induced to express indicated SAS-6 mutants for 3 days as in Figure 1F, and then processed for Western blot with N-terminal SAS-6 antibodies (SAS-6N), Flag antibodies, and tubulin antibodies as the loading control. The expression level of each SAS-6 construct, indicated as fold changes (Folds) relative to the endogenous SAS-6 or SAS-6FL, is shown.
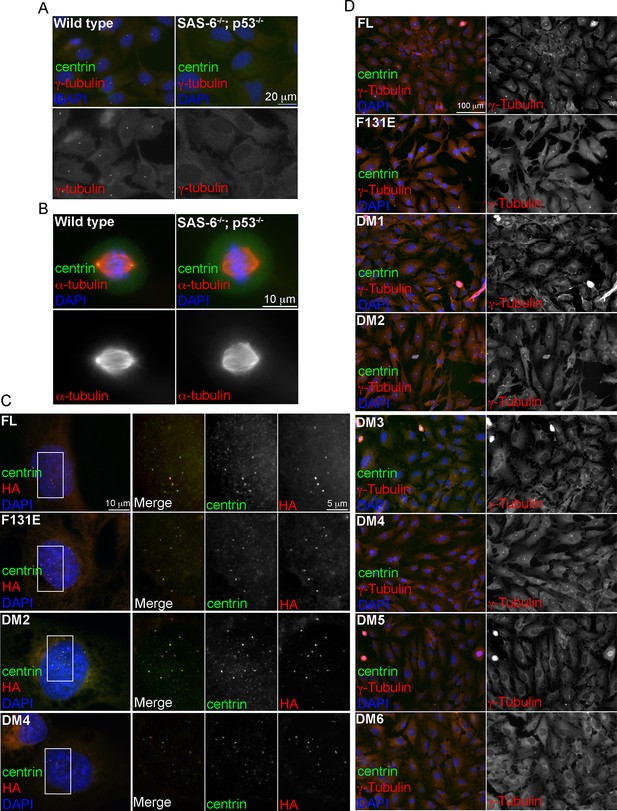
Induction of de novo centrosome formation in clone #2 SAS6-/-; p53-/- cells in the absence of SAS-6 self-oligomerization.
(A, B) Wild-type (WT) or clone #2 acentrosomal (p53-/-; SAS-6 -/-) RPE1 cells were stained with indicated antibodies during interphase (A) and mitosis (B). DNA (DAPI, blue). (C, D) Clone #2 acentrosomal cells were infected with lentiviruses, and induced to express indicated SAS-6 constructs for 16h (C) or 3 days (D). The ability of each SAS-6 mutant in rescuing de novo centriole (C) or centrosome (D) formation was examined with indicated antibodies. HA staining marks SAS-6.
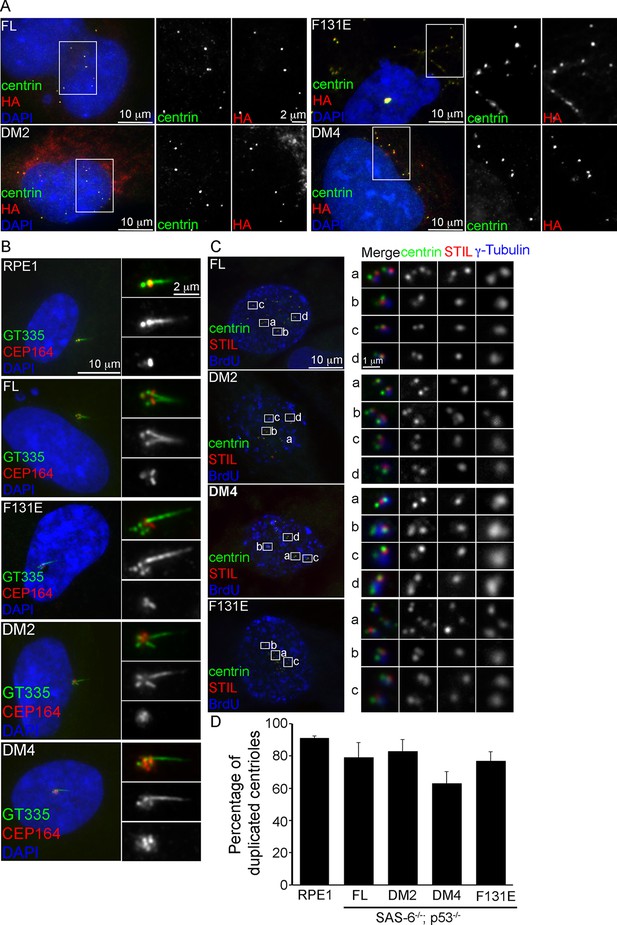
De novo centrioles formed in the absence of SAS-6 self-oligomerization can ciliate and duplicate.
(A) Isogenic SAS-6-/-; p53-/- cells carrying indicated SAS-6 constructs (see ‘Methods’) were arrested in S phase and induced to express indicated SAS-6 mutants for 16 hr and then immunostained with indicated antibodies to visualize de novo centrioles (centrin, green; SAS-6/HA, red). DNA (DAPI, blue). (B) Isogenic SAS-6-/-; p53-/- cells carrying indicated SAS-6 constructs (see ‘Methods’) were induced to express indicated SAS-6 mutants for 3 days, arrested in G1 for additional 36 hr, and then processed for immunofluorescence microscopy to visualize cilia (GT335, green) and distal appendage (CEP164, red). DNA (DAPI, blue). (C) Isogenic SAS-6-/-; p53-/- cells carrying indicated SAS-6 constructs (see ‘Methods’) were induced to express indicated SAS-6 mutants for 3 days, and then processed for BrdU pulse-chase before fixation for immunofluorescence. Centriole duplication was revealed with indicated antibodies (centrin, green; daughter centriole marker, STIL, red; PCM marker, γ-tubulin, blue). S-phase cells labeled with BrdU were shown (blue). (D) Quantification of the centriole duplication efficiency from (C). S-phase cells were identified (BrdU+) and their centrioles were analyzed by immunofluorescence with centrin, STIL, and γ-tubulin antibodies. Error bars represent standard error of the mean (SEM); n > 150, N = 3.
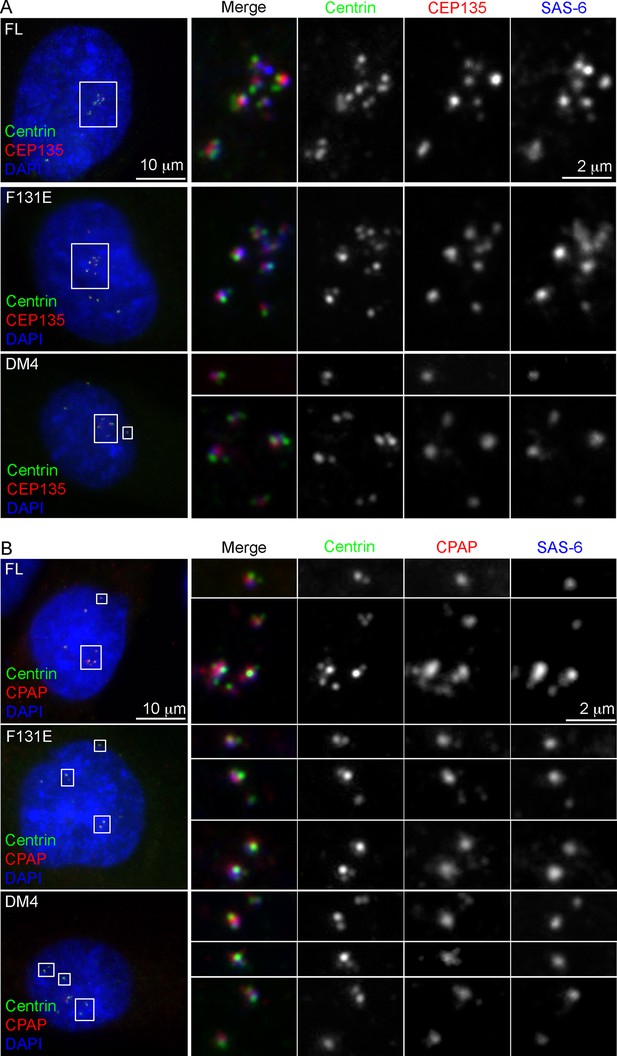
Characterization of de novo centrioles formed in the absence of SAS-6 self-oligomerization.
p53-/-; SAS-6 -/- cells (clone #1) exogenously expressing full-length SAS-6 (FL) or various SAS-6 mutants as indicated were analyzed by immunofluorescence with indicated antibodies.
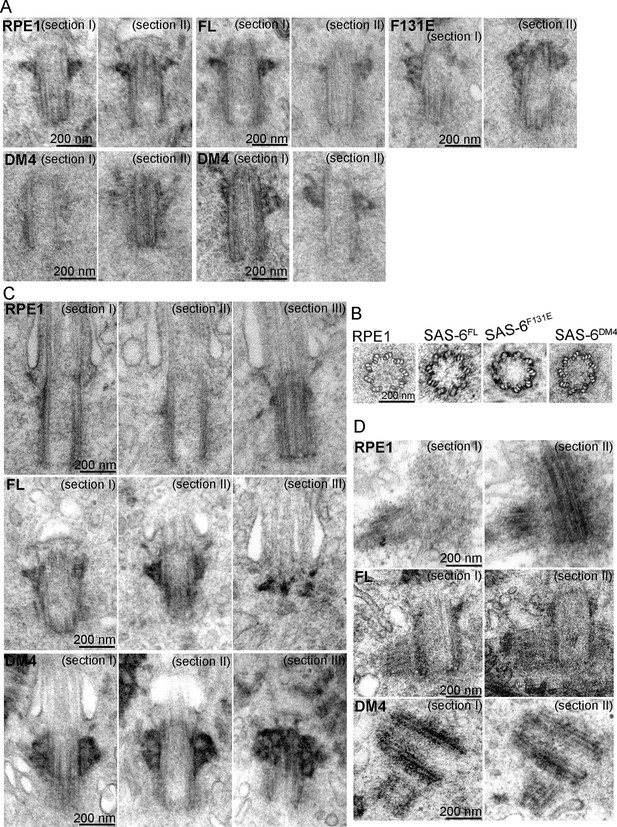
SAS-6 self-assembly is not essential for the ninefold symmetry of centrioles.
Isogenic SAS-6-/-; p53-/- cells carrying indicated SAS-6 constructs (see ‘Methods’) were induced to express indicated SAS-6 mutants for 3 days, and then processed for serial sectioning electron microscopy. (A,B) Mature canonical centrioles in WT RPE1 cells, or de novo centrioles formed in SAS6-/- cells expressing full-length (FL) or mutant SAS-6 as indicated are shown in longitudinal (A) or cross sectional (B) views. Note that these centrioles were mature and have acquired appendages. (C) Ciliated centrioles from indicated cell types were serially sectioned and examined by EM. (D) Duplicated, engaged centriole pairs from indicated cell types were serially sectioned and examined by EM. Scale bar: 200 nm.
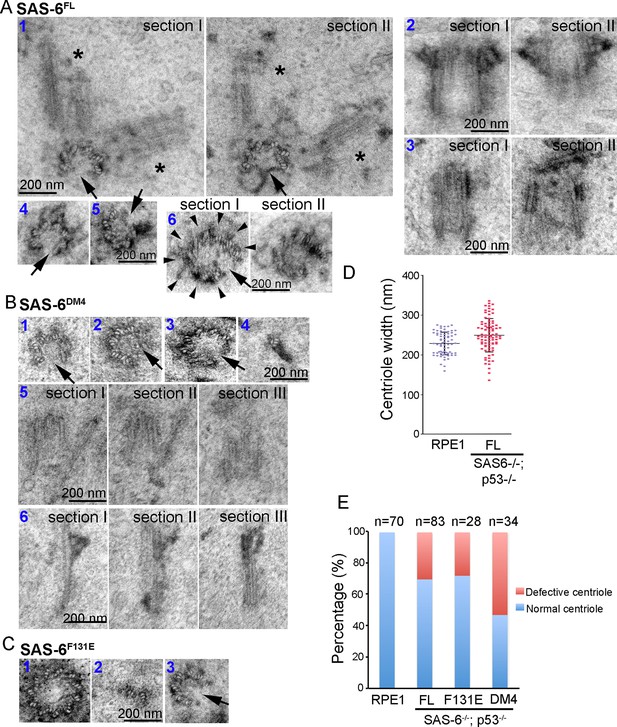
Centrioles formed through de novo assembly are error-prone.
(A–C) Isogenic SAS-6-/-; p53-/- cells carrying indicated SAS-6 constructs (see ‘Methods’) were induced to express indicated SAS-6 mutants for 3 days, and then processed for serial sectioning electron microscopy. (A1) A mature, SAS-6FL-derived centriole missed three MT triplets but was able to duplicate, producing daughter centrioles also made of an incomplete set of MT triplets (see stars in sections I and II). It appears that two daughter centrioles were formed at the same time. (A2) A mature, SAS-6FL-derived centriole 33% wider than normal centrioles was able to acquire appendages. (A3) A SAS-6FL-derived centriole made of disorganized MTs. (A4&5) Cross-sectional views of SAS-6FL-derived, abnormal centrioles missing MT triplets. (A6) A SAS-6FL-derived centriole carrying 9 MT triplets (arrow heads) but existing as a distorted open cylinder (arrow). (B) Abnormal centrioles derived from SAS-6DM4 were shown in cross-sectional or longitudinal views. (C) Abnormal centrioles derived from SAS-6F131E were shown in cross-sectional views. Note that a larger centriole made of 11 MT triplets was shown (C1). (D) The outer diameter of centrioles was quantified for both canonical centrioles (in normal RPE1 cells), and SAS-6FL-derived de novo centrioles. (E) Quantifications showing the error rates of de novo and canonical centrioles. Sample size (n) is indicated. Note that arrows in all images indicate the missing of MT triplets.
Videos
EM tomography of a normal centriole from SAS-6DM4 expressing cells (related to Figure 3).
Note that a centriole on the right in cross-sectional views contains nine MT triplets.
EM tomography of an abnormal centriole from SAS-6DM4 expressing cells (related to Figure 4).
Note that a centriole on the right in cross-sectional views contains eight MT triplets.





