Local mechanical forces promote polarized junctional assembly and axis elongation in Drosophila
Figures
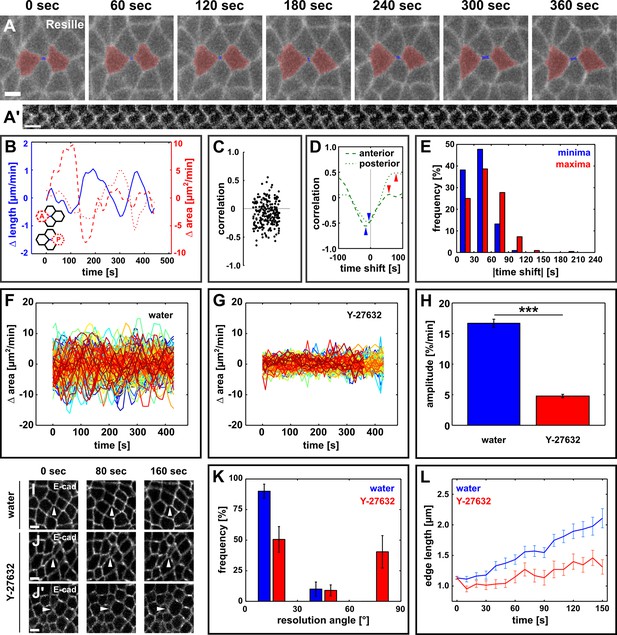
Directional assembly of new interfaces during vertex resolution is associated with pulsatile apical contractions and requires contractile activity.
(A) Vertex resolution during axis elongation in an embryo expressing Resille:GFP. Blue indicates the new DV interface, red labels the anterior and posterior cells. (A’) Kymograph illustrating the elongation of the DV interface shown in (A). Scale bar, 10 s. The interface is rotated by 90° with respect to (A). Anterior down, dorsal left. (B) Rates of change for edge length (blue, solid line), anterior cell area (red, dashed line), and posterior cell area (red, dotted line) during the neighbour exchange event shown in (A). Rate of change was calculated with respect to t + 60 s. (C) Correlation coefficients between changes in edge length and changes in anterior or posterior cell area (n = 220 pairs in 110 neighbour exchange events in 13 embryos). (D) Changes in correlation between edge length and anterior (dashed) or posterior (dotted) cell area during the neighbour exchange event shown in (A) when the edge length signal was shifted in time in 10-s increments. Arrowheads indicate the correlation minima (blue) or maxima (red) closest to 0-s shift. (E) Distribution of time shifts (absolute value) required to obtain the minimum (blue) and maximum (red) correlations in all 220 signal pairs shown in (C). (F, G) Rate of change in cell area in embryos injected with water (F, n = 122 cells in 3 embryos) or 100 mM Y-27632 (G, n = 99 cells in 3 embryos). Each line represents a single cell. (H) Oscillation amplitude for changes in cell area in embryos injected with water (blue) or 100 mM Y-27632 (red). Asterisks indicate p < 0.001. (I–J') Vertex resolution during axis elongation in embryos expressing E-cadherin:GFP and injected with water (I) or with 100 mM Y-27632 (J, J’). Arrowheads indicate nascent DV interfaces. (K) Distribution of vertex resolution angles relative to the AP axis in embryos injected with water (blue, n = 28 vertices in 3 embryos) or 100 mM Y-27632 (red, n = 25 interfaces in 3 embryos). Angles were measured 150 s after the onset of vertex resolution. An angle of 90° with respect to the AP axis corresponds to the DV axis. (L) Length of new DV interfaces forming within 30° of the AP axis in embryos injected with water (blue, n = 25 interfaces in 3 embryos) or 100 mM Y-27632 (red, n = 11 interfaces in 3 embryos). (A, I–J’) Anterior left, dorsal up. Scale bars, 5 µm. (B, F, G, L) Time is with respect to the onset of vertex resolution, defined as the first time point in which the length of the nascent interface exceeded 1 µm. (H, K, L) Error bars, s.e.m. AP, anterior-posterior; DV, dorsal-ventral.
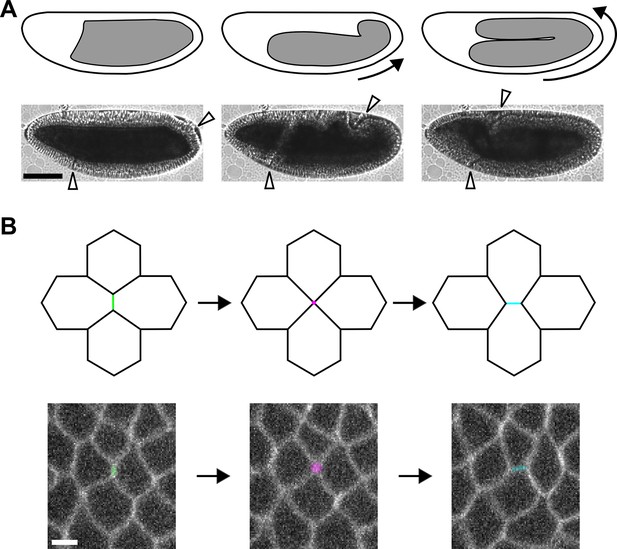
Axis elongation in Drosophila is driven by neighbour exchange events.
(A) Germband position at the beginning (left), during (centre), and at late stages of axis elongation (right). Arrows indicate the direction of cell movement. White arrowheads delimit the germband. Scale bar, 100 µm. (B) Diagram (top) and germband cells (bottom) showing a neighbour exchange event. An AP interface contracts (left, green), forming a vertex where four cells meet (centre, magenta). The vertex resolves through the assembly of a new DV interface (right, cyan). Scale bar, 5 µm. (A, B) Anterior left, dorsal up. AP, anterior-posterior; DV, dorsal-ventral.
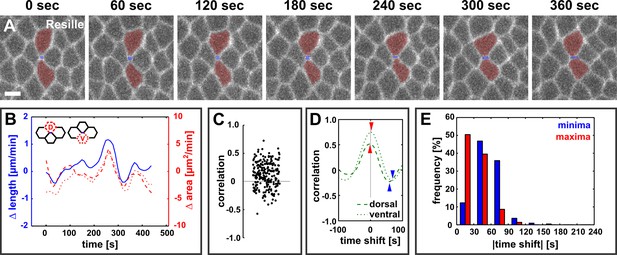
Dorsal and ventral cells oscillate with new DV interfaces.
(A) Vertex resolution during axis elongation in an embryo expressing Resille:GFP. Blue indicates the new DV interface, red labels the dorsal and ventral cells. Anterior left, dorsal up. Scale bar, 5 µm. (B) Rates of change for edge length (blue, solid line), dorsal cell area (red, dashed line), and ventral cell area (red, dotted line) during the neighbour exchange event shown in (A). Rate of change was calculated with respect to t + 60 s. (C) Correlation coefficients between changes in edge length and changes in dorsal or ventral cell area (n = 220 pairs in 110 neighbour exchange events in 13 embryos). (D) Changes in correlation between edge length and dorsal (dashed) or ventral (dotted) cell area during the neighbour exchange event shown in (A), when the edge length signal was shifted in time in 10-second increments. Arrowheads indicate the correlation minima (blue) or maxima (red) closest to 0 s shift. (E) Distribution of time shifts (absolute value) required to obtain the minimum (blue) and maximum (red) correlations in all 220 signal pairs shown in (C). DV, dorsal-ventral.
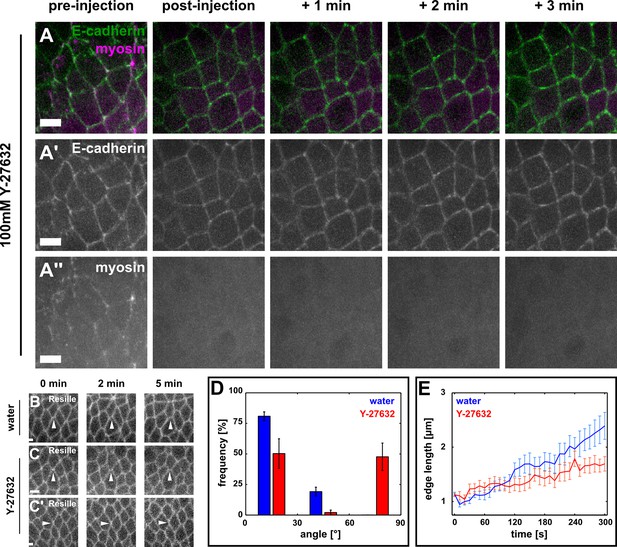
Directional assembly of new DV interfaces during vertex resolution requires actomyosin contractility.
(A–A’’) Germband cells expressing E-cadherin:GFP (green, A’) and myosin:mCherry (magenta, A’’), before (pre-injection) and at different times after injection with 100 mM Y-27632. Anterior left, dorsal up. Scale bars, 5 µm. (B–C') Vertex resolution during axis elongation in embryos expressing Resille:GFP and injected with water (B) or with 100 mM Y-27632 (C, C’). Arrowheads indicate nascent DV interfaces. Anterior left, dorsal up. Scale bars, 5 µm. (D) Distribution of vertex resolution angles relative to the AP axis in embryos injected with water (blue, n = 26 vertices in 3 embryos) or 100 mM Y-27632 (red, n = 43 interfaces in 7 embryos). Angles were measured 150 s after the onset of vertex resolution. An angle of 90° with respect to the AP axis corresponds to the DV axis. Error bars, s.e.m. (E) Length of new DV interfaces forming within 30° of the AP axis in embryos injected with water (blue, n = 21 interfaces in 3 embryos) or 100 mM Y-27632 (red, n = 19 interfaces in 7 embryos). Time is with respect to the onset of vertex resolution, defined as the first time point in which the length of the nascent interface exceeded 1 µm. Error bars, s.e.m. AP, anterior-posterior; DV, dorsal-ventral.
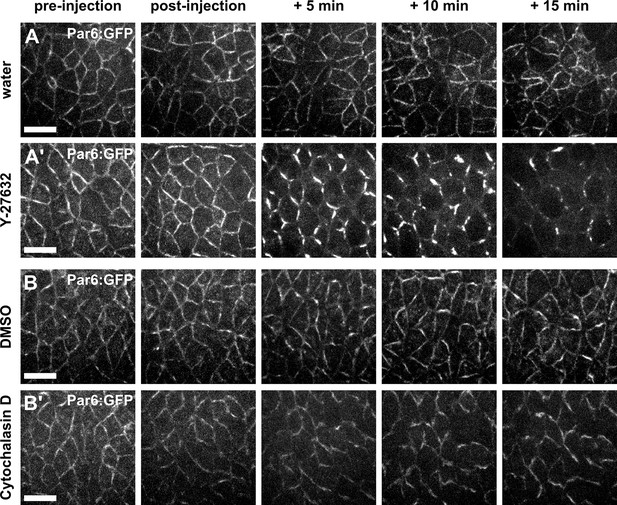
Par complex localization is affected by Y-27632, but not by Cytochalasin D.
(A–B') Germband cells expressing Par-6:GFP at endogenous levels and injected with water (A), 100 mM Y-27632 in water (A’), 50% DMSO (B), or 5 mM Cytochalasin D in 50% DMSO (B’). Anterior left, dorsal up. Scale bars, 10 μm.
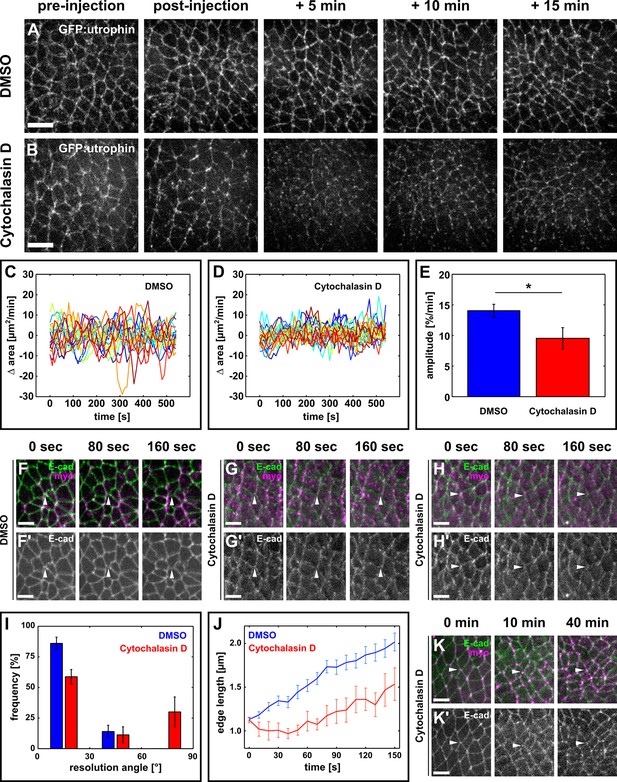
Oriented assembly of new DV interfaces requires actin-based contraction.
(A, B) Germband cells expressing GFP:utrophin in embryos injected with DMSO (A) or with 5 mM Cytochalasin D (B). Scale bars, 10 µm. (C, D) Rate of change in cell area in DMSO (C) or Cytochalasin D-injected embryos (D). Each line represents a single cell (n = 20 cells in 4 embryos in both C and D). (E) Oscillation amplitude for changes in cell area in embryos injected with DMSO (blue) or 5 mM Cytochalasin D (red). Asterisk indicates p < 0.05. Error bars, s.e.m. (F–H) Vertex resolution during axis elongation in embryos expressing E-cadherin:GFP (green, top; grayscale, bottom) and myosin:mCherry (magenta, top) and injected with DMSO (F) or with 5 mM Cytochalasin D (G-H'). Arrowheads indicate nascent DV interfaces. (I) Distribution of vertex resolution angles relative to the AP axis in embryos injected with DMSO (blue, n = 50 vertices in 5 embryos) or 5 mM Cytochalasin D (red, n = 15 vertices in 4 embryos). Angles were measured 150 s after the onset of vertex resolution. An angle of 90° with respect to the AP axis corresponds to the DV axis. (J) Length of new DV interfaces forming within 30° of the AP axis in embryos injected with DMSO (blue, n = 43 interfaces in 5 embryos) or 5 mM Cytochalasin D (red, n = 9 interfaces in 4 embryos). Time is with respect to the onset of vertex resolution, defined as the first time point in which the length of the nascent interface exceeded 1 µm. (I, J) Error bars, s.e.m. (K) Non-resolving vertex in an embryo expressing E-cadherin:GFP (green, top; greyscale, bottom) and myosin:mCherry (magenta, top), and injected with 5 mM Cytochalasin D. Arrowheads indicate the vertex. (A, B, F–H', K) Anterior left, dorsal up. (F–H', K, K') Scale bars, 5 µm. AP, anterior-posterior; DV, dorsal-ventral.
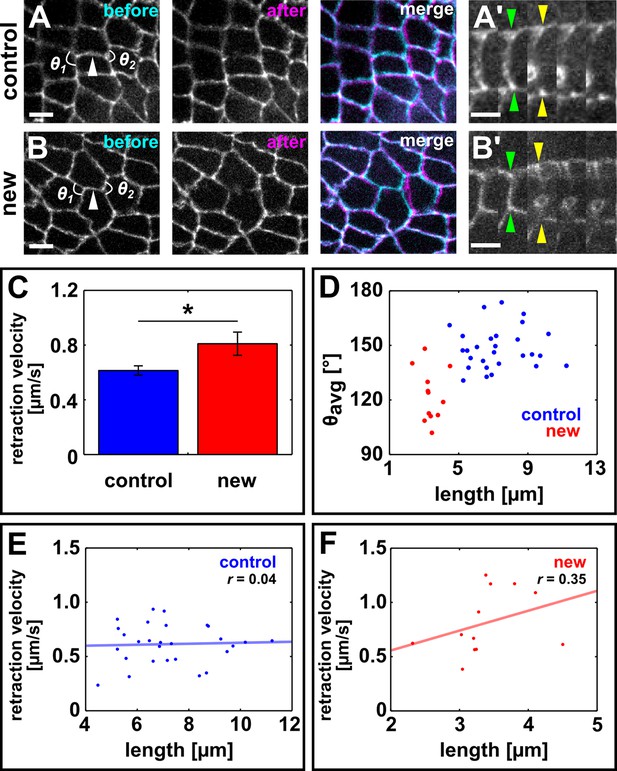
Resolving edges sustain increased mechanical tension during axis elongation.
(A, B) Germband cells expressing E-cadherin:GFP before and after ablation of a control DV edge (A) or a newly forming DV edge (B). White arrowheads point to the ablated interface. θ1 and θ2 indicate the angles between the junctions anterior and posterior to the ablated interface, respectively. Anterior left, dorsal up. Scale bars, 5 µm. (A’, B’) Kymographs showing the vertex displacement caused by laser ablation of the edges shown in (A, B). Arrowheads indicate vertex position prior to ablation (green) or immediately after (yellow). Interfaces are rotated by 90° with respect to (A, B) Anterior down, dorsal left. Scale bar, 3 s. (C) Retraction velocity after laser ablation in control (blue, n = 28) and new (red, n = 12) DV interfaces. Asterisk indicates p = 0.05. Error bars, s.e.m. (D) Scatterplot showing interface length vs. average junction angle at the anterior and posterior ends (θavg = (θ1 +θ2)/2). (E, F) Scatterplots showing interface length vs. retraction velocity after laser ablation for control (E) and new (F) DV interfaces. Solid lines are best-fit lines. DV, dorsal-ventral.
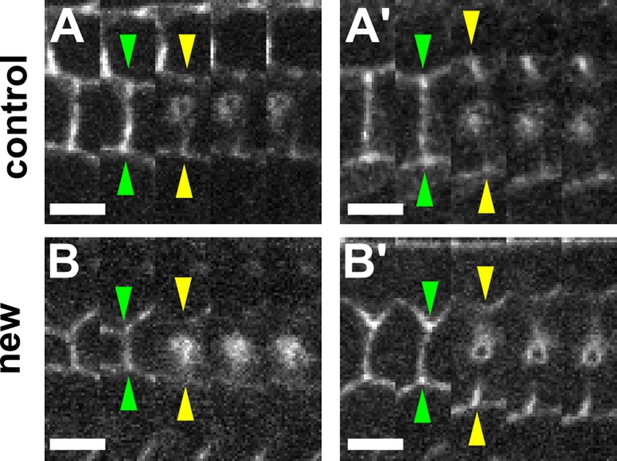
The retraction velocity after ablation of new and control DV edges is not anti-correlated with their length.
(A–B') Kymographs showing the vertex displacements caused by laser ablation of relatively short (A, B) and long (A’, B’) control (A) or new (B) DV edges. Arrowheads indicate vertex position prior (green) or immediately after (yellow) ablation. Anterior down, dorsal left. Scale bars, 3 s. DV, dorsal-ventral.
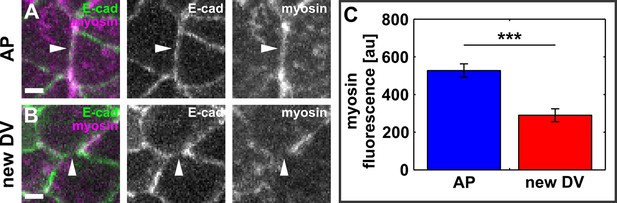
New DV edges do not display a significant myosin accumulation.
(A, B) AP- (A) and newly forming DV- (B) oriented interfaces in embryos expressing E-cadherin:GFP (green) and myosin:mCherry (magenta). Arrowheads indicate the interfaces. Scale bars, 2 μm. Anterior left, dorsal up. (C) Myosin:mCherry fluorescence in AP and newly forming DV interfaces. Asterisks indicate p < 0.001. Error bars, s.e.m. DV, dorsal-ventral.
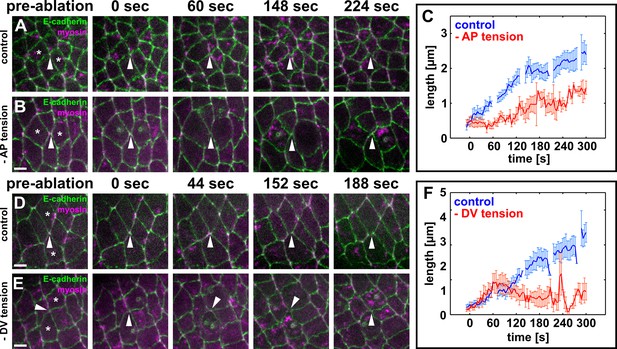
Local actomyosin contractility is necessary for vertex resolution and new DV interface assembly.
(A, B, D, E) Cells expressing E-cadherin:GFP (green) and myosin:mCherry (magenta) in sham-irradiated controls (A, D) or when UV irradiation was used to reduce local tension (B, E). White arrowheads indicate resolving interfaces. Asterisks show the targeted cells. Time is with respect to the first laser irradiation. Anterior left, dorsal up. Scale bars, 5 µm. (C, F) Length of resolving DV interfaces over time in controls (blue, n = 10 interfaces in C and F), under reduced AP tension (red, n = 7 interfaces in C), or under reduced DV tension (red, n = 7 interfaces in F). Discontinuities in the blue lines indicate times at which cells were targeted with the attenuated UV laser in all experiments. Error bars, s.e.m. DV, dorsal-ventral.
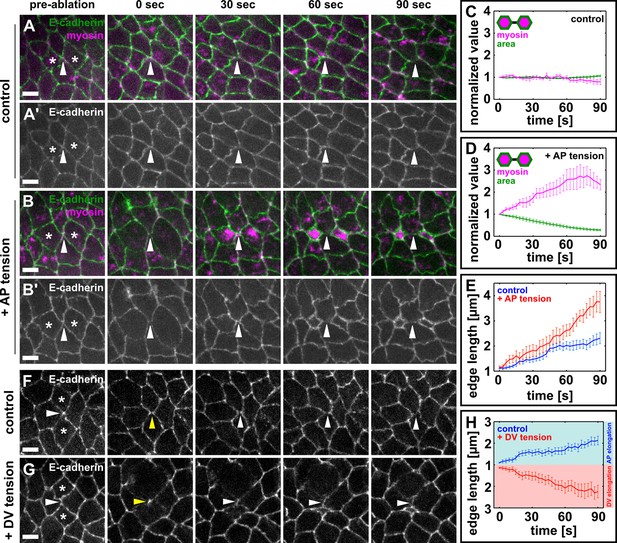
Local mechanical tension is sufficient to promote and orient new interface assembly during vertex resolution.
(A–B') Cells expressing E-cadherin:GFP (green) and myosin:mCherry (magenta) in sham (A) or UV-irradiated (B) embryos. (C, D) Medial myosin intensity (magenta) and cell area (green) in sham (C, n = 22 cells in 11 embryos) and UV-irradiated embryos (D, n = 16 cells in 8 embryos). (E) Length of resolving DV interfaces over time in controls (blue, n = 11 interfaces) and under increased tension along the AP axis (red, n = 8 interfaces). (F, G) Cells expressing E-cadherin:GFP in sham (F) or UV-irradiated (G) embryos. Asterisks show the cells around a four-cell vertex (white arrowheads) that were irradiated. Yellow arrowheads indicate the formation of a four-cell vertex. (A, B, F, G) Anterior left, dorsal up. Scale bars, 5 µm. (H) Length of resolving interfaces over time in controls (blue, n = 12) and under increased tension along the DV axis (red, n = 13). Turquoise indicates elongation parallel to the AP axis, pink denotes DV elongation. (C–E, H) Time is with respect to the time point when the nascent DV interface first exceeded 1 µm in length. Error bars, s.e.m. (C, D) Normalization is with respect to the value at 0 s. AP, anterior-posterior; DV, dorsal-ventral.
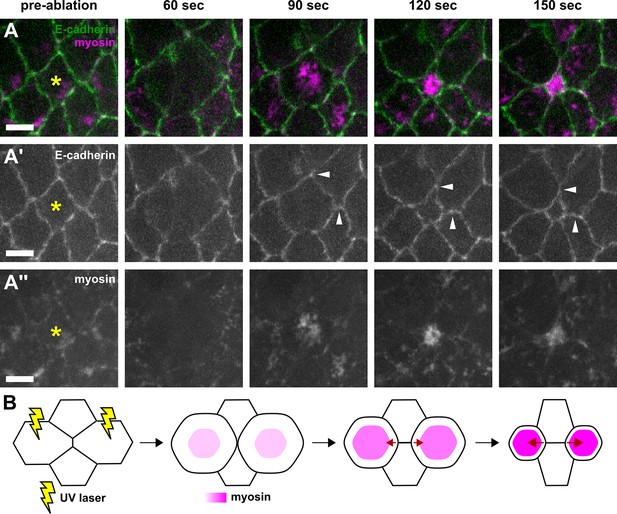
Wounded cells undergo apical constriction and induce ectopic tension on adjacent cell-cell junctions.
(A–A’’) Germband cells expressing E-cadherin:GFP (green, A’) and myosin:mCherry (magenta, A’’), before (pre-ablation) and at different times after UV-irradiation of the cell denoted by the yellow asterisk. White arrowheads indicate neighbouring junctions under ectopic strain when the wounded cell constricts apically. Time after wounding is shown. Anterior left, dorsal up. Scale bars, 5 μm. (B) Schematic representation of a method to induce ectopic AP-oriented tension (red arrows) on a vertex by wounding (yellow rays) the neighbouring anterior and posterior cells. AP, anterior-posterior.
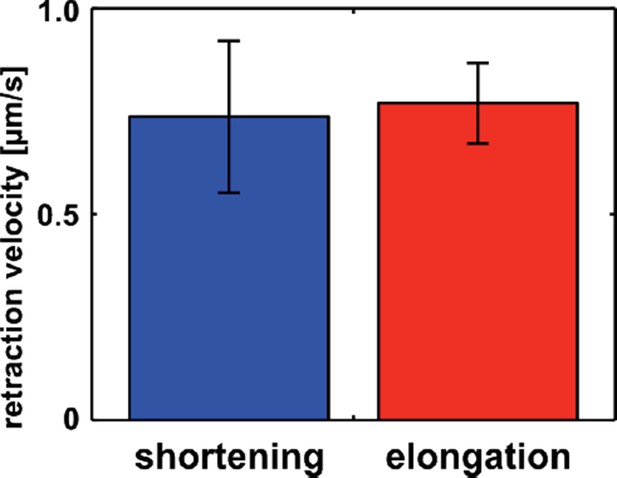
New DV edges sustain similar tension during elongation and shortening.
Retraction velocity after laser ablation of new DV edges at the shortening (blue, n = 4 interfaces in 4 embryos) and elongation (red, n = 10 interfaces in 10 embryos) phases of the new edge assembly cycle.
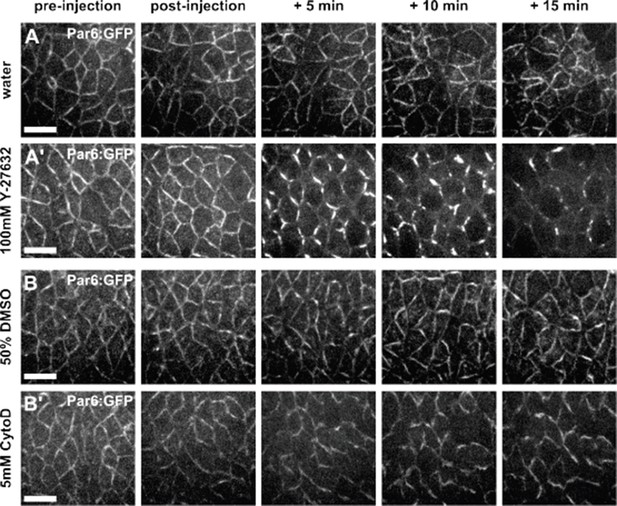
Par complex localization is affected by Y-27632, but not by Cytochalasin D.
(A-B) Germband cells expressing Par-6:GFP at endogenous levels and injected with water (A), 100 mM Y- 27632 in water (A’), 50% DMSO (B) or 5 mM Cytochalasin D in 50% DMSO (B’). Anterior left, dorsal up. Scale bars, 10 µm.
Videos
Polarized cell rearrangements drive Drosophila axis elongation.
Germband cells expressing Resille:GFP during germband extension. A stack was acquired every 10 s. Time is indicated as min:s. Anterior left, dorsal up. This video relates to Figure 1—figure supplement 1.
Actomyosin contractility is required for directional vertex resolution.
Germband cells expressing E-cadherin:GFP in embryos injected with water (left) or 100 mM Y-27632 (centre and right). A stack was acquired every 10 s. Time is indicated as min:s. Anterior left, dorsal up. This video relates to Figure 1.
Stabilization of actin filaments impairs directional vertex resolution.
Germband cells expressing E-cadherin:GFP in embryos injected with 50% DMSO (left) or 5 mM Cytochalasin D (centre and right). A stack was acquired every 10 s. Time is indicated as min:s. Anterior left, dorsal up. This video relates to Figure 1—figure supplement 5.
Mechanical tension promotes rapid elongation of new DV interfaces.
Germband cells in embryos expressing E-cadherin:GFP (green) and myosin:mCherry (magenta) under sham irradiation (left) or upon wounding and apical constriction of the cells anterior and posterior to a multicellular vertex (right). Arrows indicate resolving multicellular vertices. A stack was acquired every 3 s. Time is indicated as min:s. Anterior left, dorsal up. This video relates to Figure 4. DV, dorsal-ventral.





