Photo-switchable tweezers illuminate pore-opening motions of an ATP-gated P2X ion channel
Figures
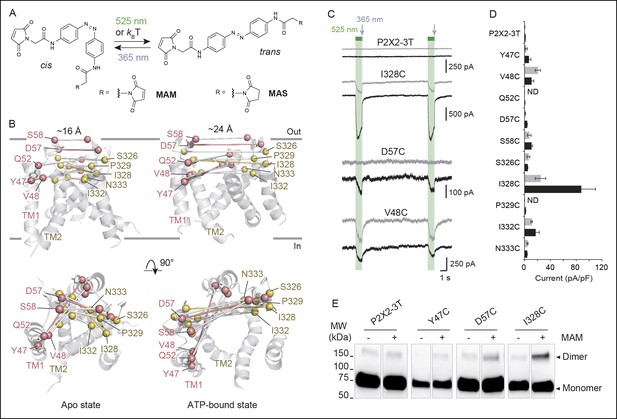
Lateral expansion between TM1 and TM2 helices drives channel opening.
(A) Chemical structures of MAM and MAS in the cis and trans states. (B) Cartoon representation of the TMD of a P2X2 homology model viewed parallel (upper) and perpendicular (lower) to the membrane plane in an apo (left) and ATP-bound state (right). Cβ atoms of residues selected for cysteine substitutions are shown as red and yellow spheres in TM1 and TM2 helices, respectively. Indicated values are the average distances separating pairwise β-atoms from two adjacent subunits (grey bridges). Highlighted bridges indicate actual MAM cross-linking. (C) Whole-cell currents recorded during and after illumination at 525 nm (green bars, 1 s) and 365 nm (violet arrows, 80 ms) in HEK cells expressing the P2X2-3T receptor or the indicated cysteine-substituted mutants after treatment with MAM (black traces) or MAS (gray traces). Just before recordings, cells were irradiated for 85 ms with a light pulse of 365 nm. (D) Screening for all constructs showing light-gated currents following MAM (filled bars) or MAS (gray bars) treatment. All light-gated mutants were activated at 525 nm and inactivated at 365 nm, except for N333C, which responded in the opposite sense to these wavelengths. ND stands for not determined (n = 4–5 cells; mean ± s.e.m.). (E) Western blot analysis of cell-surface cross-linking of the indicated P2X2-3T constructs expressed in TSA-201 cells after treatment (+) or without treatment (-) with MAM. Monomer and dimer are indicated. Uncut gel image is shown in Figure 1—figure supplement 2B. MAM: 4,4´-bis(maleimido-glycine)azobenzene; MAS: 4-(maleimido-glycine)-4'-(succimido-glycine)azobenzene; MW: Molecular weight; TMD: Transmembrane domain.
-
Figure 1—source data 1
Interatomic distances between pairwise residues.
- https://doi.org/10.7554/eLife.11050.004
-
Figure 1—source data 2
Estimated EC50 and Hill coefficients for ATP activation.
- https://doi.org/10.7554/eLife.11050.005
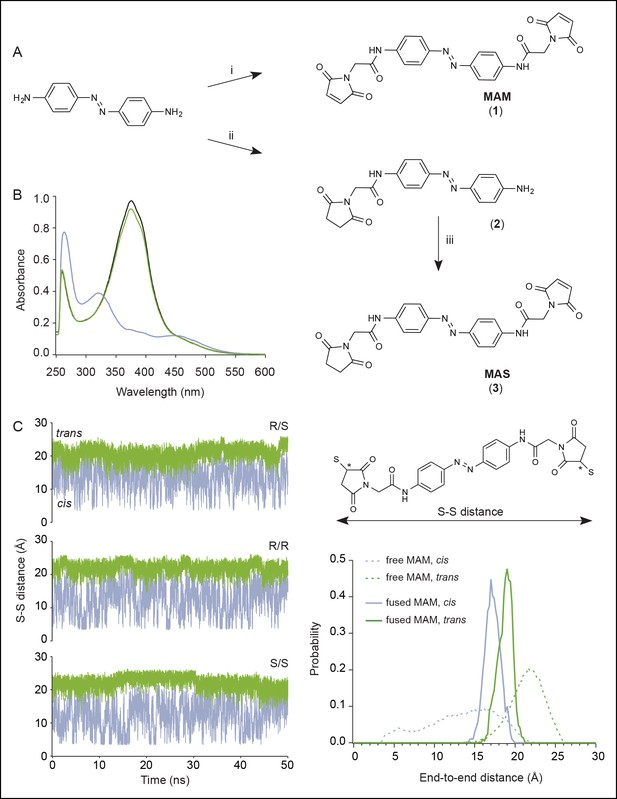
Chemical synthesis and physico-chemical properties of azobenzene derivatives.
(A) Synthesis of MAM (1) and MAS (3); i) HATU, DIEA, 2-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)acetic acid, ACN/DMF, 19 h, RT, ρ = 55%; ii) TBTU, Et3N, 2-(2,5-dioxopyrrolidium-1-yl)acetic acid, ACN/DMF, 19 h, RT, ρ = 27%; iii) HATU, DIEA, 2-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)acetic acid, ACN/DMF, 19 h, RT, ρ = 60%. (B) UV/visible spectrum of MAM (30 μM) in DMSO in the dark (black trace), after illumination at 365 nm (violet trace) and subsequently at 525 nm (green trace). (C) Left, time series of the end-to-end distance of free MAM in bulk water determined from six 50 ns-long MD simulations in either cis (violet) or trans configuration (green). Shown is the S–S distance computed from two sulfur atoms of the three stereoisomers (R/S, R/R, and S/S) following reaction with maleimides as shown in the top right. Asterisks indicate stereocenters. Bottom right, normalized probability distributions for the end-to-end distance of MAM either free in solution (thin dashed lines, S–S distance) or fused to the protein in the new model of the open state (thick lines, Cβ–Cβ distance) in either trans (one horizontal MAM) or cis (three vertical MAM photo-linkers) configurations. MAM: 4,4´-bis(maleimido-glycine)azobenzene. MAS: 4-(maleimido-glycine)-4'-(succimido-glycine)azobenzene.
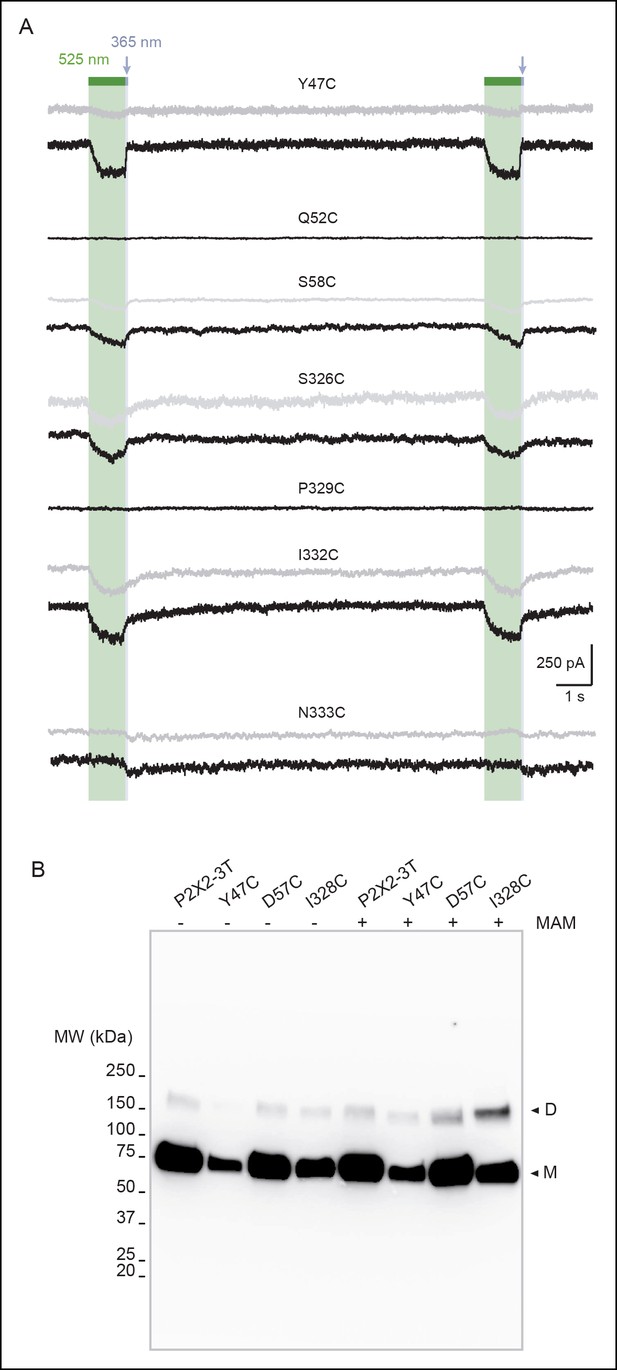
Horizontal screening confirms an outward expansion of the TM helices.
(A) Whole-cell currents evoked by light at the indicated wavelengths in cells expressing the indicated cysteine-substituted mutants after treatment with MAM (black traces) or MAS (grey traces). Just before recordings, cells were briefly irradiated with a light pulse of 365 nm, except for N333C mutant. (B) Uncut gel image of cross-linked P2X2-3T subunits from Figure 1E. MAM: 4,4´-bis(maleimido-glycine)azobenzene; MAS: 4-(maleimido-glycine)-4'-(succimido-glycine)azobenzene; TM: Transmembrane.
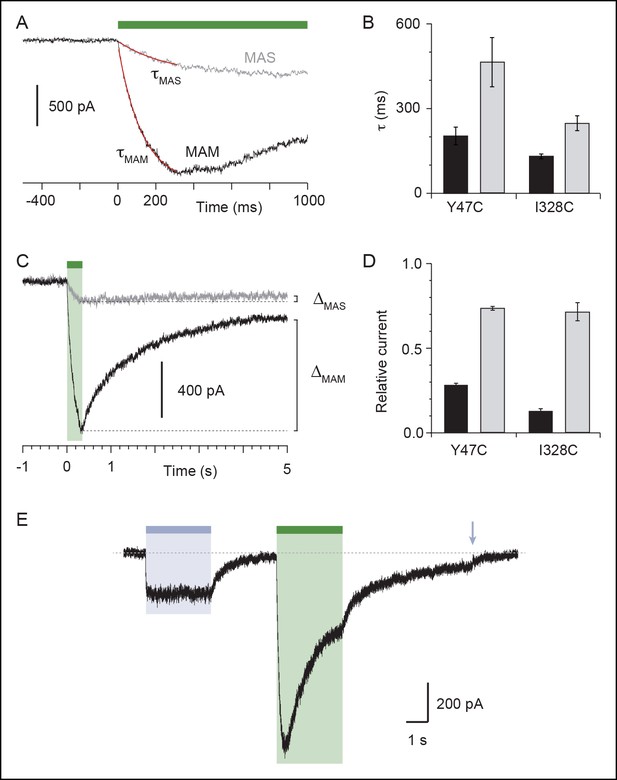
Characterization of currents induced by the isomerization of azobenzene compounds attached at the I328C or Y47C mutant.
(A) Light-induced whole-cell currents in I328C mutant treated with MAS (grey trace) or MAM (black trace). Just before recording, cells were irradiated with a light pulse of 365 nm. Data were best fit with a single exponential function (red traces) giving the time constant (τ) of receptor activation. (B) Bar plot showing time constants of activation during illumination at 525 nm for Y47C and I328C receptors treated with MAS (grey bars) or MAM (black bars) (n = 4–8 cells; mean ± s.e.m.). (C) Light-induced whole-cell currents in I328C mutant treated by MAS (grey trace) or MAM (black trace) shown on a different time scale. ΔMAS and ΔMAS indicate current stability, which was defined as the ratio of currents measured 5 s after 525 nm illumination and currents measured at the peak. (D) Bar plot showing the current stability for Y47C or I328C mutant treated with MAS (grey bars) or MAM (black bars) (n = 4–8 cells; mean ± s.e.m.). (E) Whole-cell current evoked by 365 nm and 525 nm illumination recorded from a cell expressing the I328C mutant. At the end of the recording, channels were turned off by a brief illumination at 365 nm (arrow). Before recording, the cell was briefly irradiated at 365 nm.
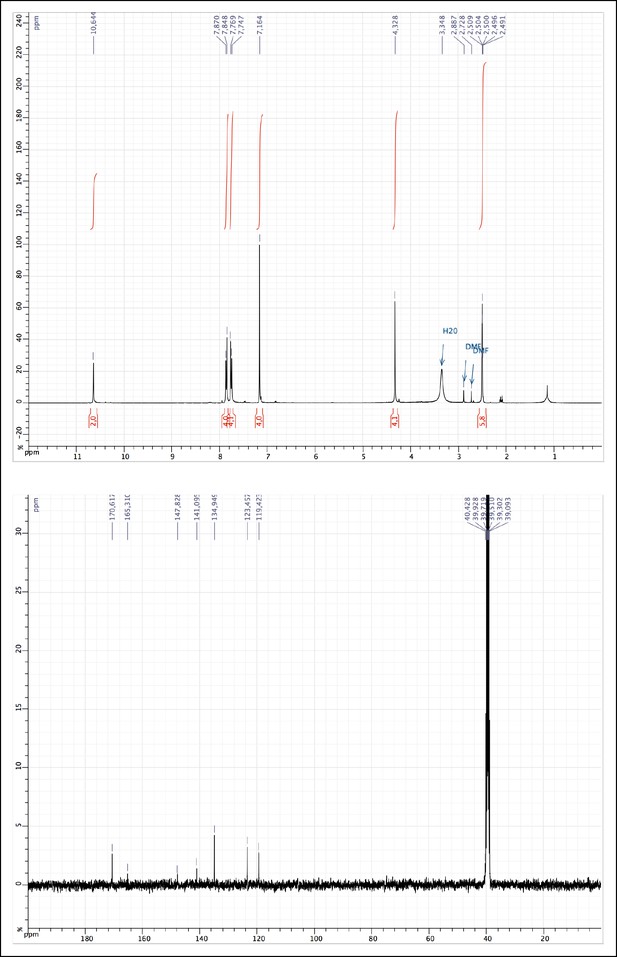
1H and 13C NMR of MAM (1).
https://doi.org/10.7554/eLife.11050.009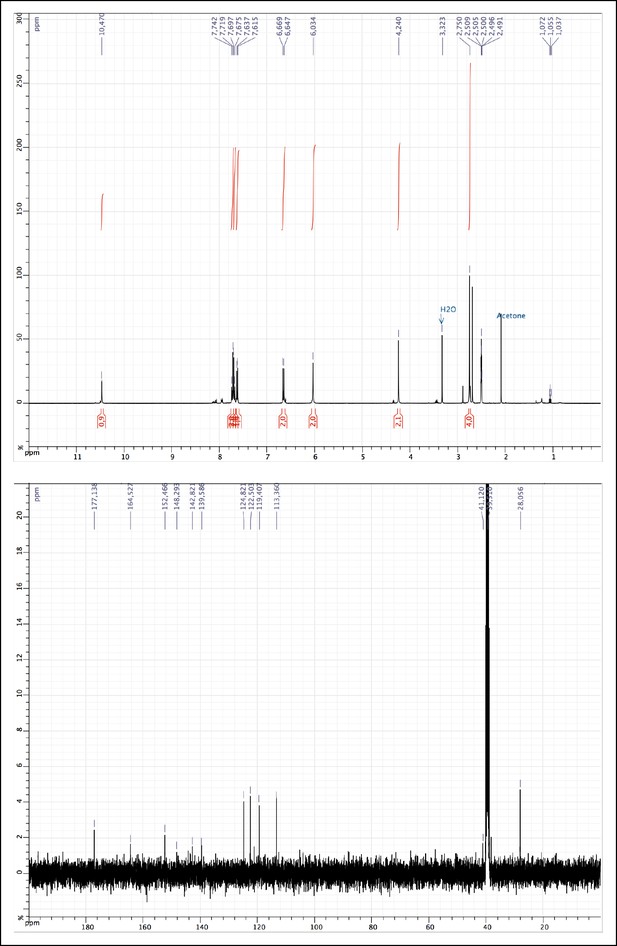
1H and 13C NMR of 2.
https://doi.org/10.7554/eLife.11050.010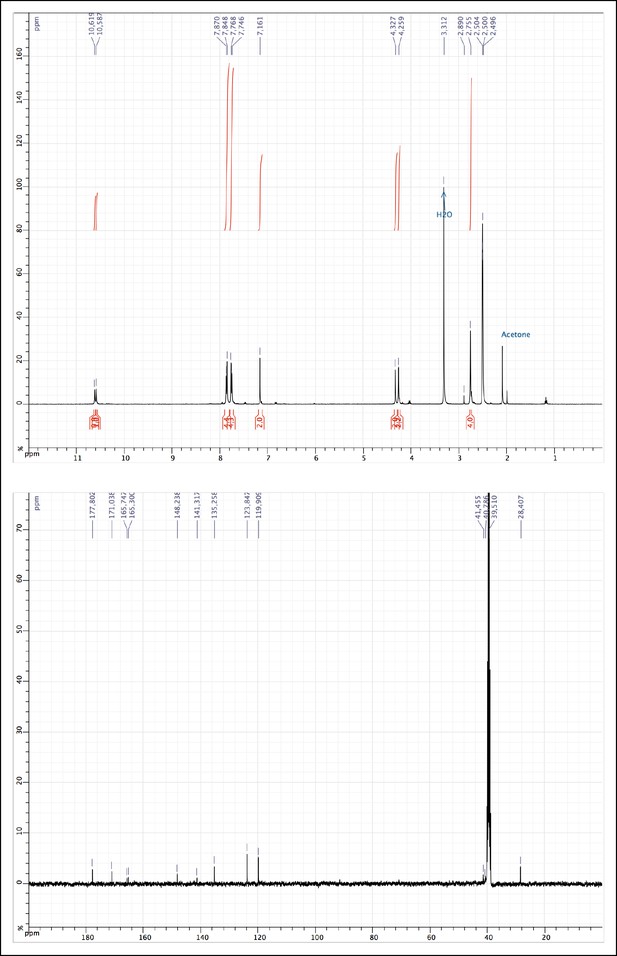
1H and 13C NMR of MAS (3).
https://doi.org/10.7554/eLife.11050.011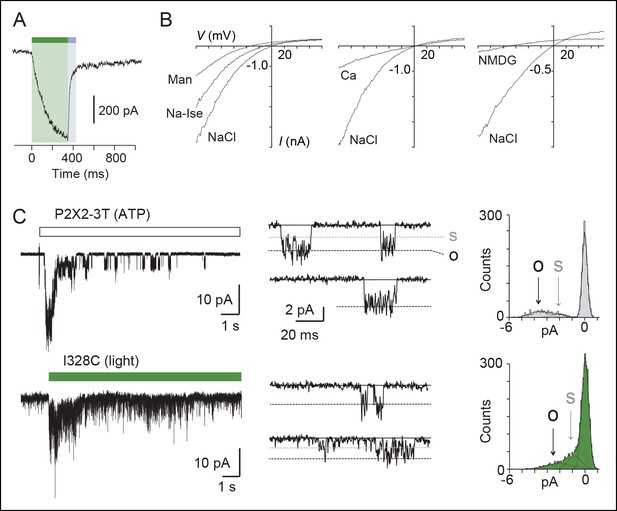
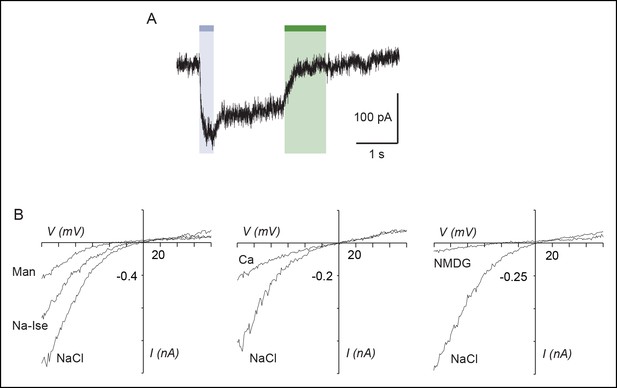
Light-driven open states are similar to those induced by ATP in the I328C mutant.
(A) Optimized illumination times at 525 nm (green bar, 350 ms, 4.1 mW/mm2) and 365 nm (violet bar, 80 ms, 8.1 mW/mm2) of I328C mutant treated with MAM to observe maximal opening and closing. (B) Current-voltage curves recorded in different extracellular solutions (Man, mannitol; Na-Ise, sodium isethionate; Ca, calcium; NaCl, symmetrical NaCl external solution; NMDG, N-methyl-D-glucamine). Shown are light-gated currents obtained after subtracting peak photocurrents recorded at 525 nm light to those obtained in the dark after switching to 365 nm light. (C) Left, single-channel currents recorded from outside-out patches at -120 mV in response to ATP for the P2X2-3T (10 μM, upper panel) or to 525 nm illumination for I328C mutant treated with MAM (4.1 mW/mm2, lower panel). In these conditions, both ATP- and light-gated currents correspond to ~30% of a maximal ATP response. Middle, unitary currents shown on an expanded scale. Full (O) and sublevel (S) openings are indicated by dashed black and gray lines, respectively. Black lines indicate closed channels. Right, corresponding all-points histograms, fitted to the sum of three Gaussians. Full and sublevel openings are also indicated.
-
Figure 2—source data 1
Relative ion permeability for chloride.
- https://doi.org/10.7554/eLife.11050.014
-
Figure 2—source data 2
Relative ion permeability for calcium.
- https://doi.org/10.7554/eLife.11050.015
-
Figure 2—source data 3
Relative ion permeability for NMDG.
- https://doi.org/10.7554/eLife.11050.016
-
Figure 2—source data 4
Single-channel properties of light-gated and ATP-gated receptors.
- https://doi.org/10.7554/eLife.11050.017
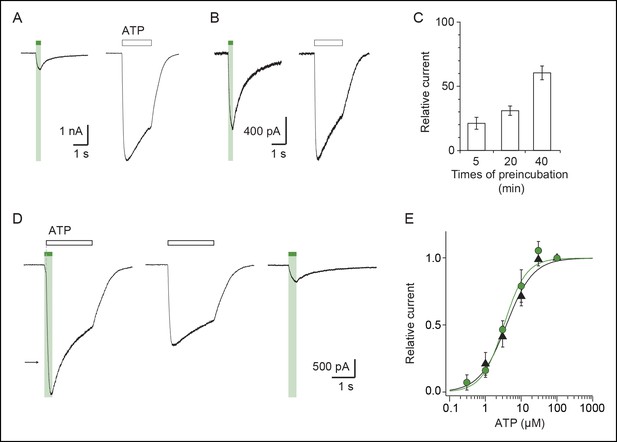
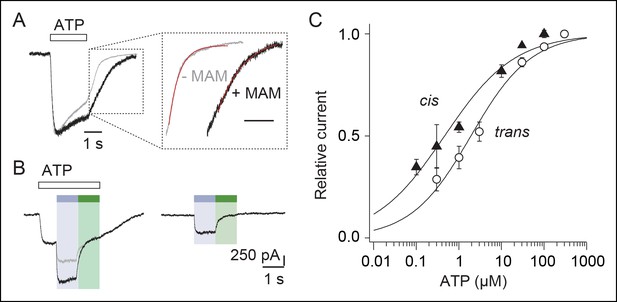
Kinetics of MAM labeling and effect of light on ATP currents in cells expressing the I328C mutant.
Whole-cell currents evoked by illumination at 525 nm light or by a saturating concentration of ATP (100 μM) recorded from the same cells that were preincubated with MAM for 5 (A) or 40 min (B). (C) Bar plot showing the relative current defined as the ratio of light-gated currents to ATP-gated currents for different times of MAM preincubation (n = 7–9 cells). (D) Left, whole-cell current evoked by a saturating concentration of ATP (100 μM) is potentiated by a short (350 ms) visible light irradiation that slightly precedes ATP application. Control currents evoked by 100 μM ATP (middle) or 525 nm light irradiation alone (right) are shown from the same cell. Preincubation time with MAM was 20 min. The arrow indicates the predicted current if ATP-gated and light-gated currents at 525 nm were additive. (E) Concentration–response relationships for ATP at 525 nm (green circles, time irradiation: 350 ms) or in the dark after illumination at 365 nm (filled triangles) from cells treated with MAM for 20 min (n = 4 cells; mean ± s.e.m.). Currents were normalized to 100 μM ATP. The Hill equation was fit to the data.
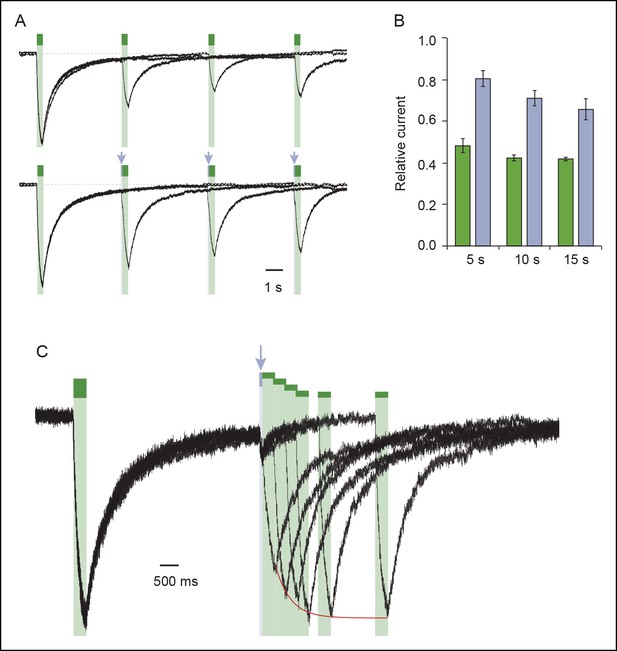
Exploration of desensitization and resensitization of the I328C mutant treated with MAM.
(A) Superimposed light-gated currents from the same cell desensitize as monitored by subsequent activation at different waiting times (upper panel). Desensitization was less pronounced if a very short pulse of UV light (80 ms) is delivered just before activation (indicated by arrows, bottom panel). In each case, cells were briefly shone at 365 nm before recordings to resensitize receptors. (B) Bar plot showing the ratio of maximal current recorded in the second to first irradiation with (violet bars) or without (green bars) the short pulse at 365 nm (n = 4 cells; mean ± s.e.m.) at different waiting times. (C) Superimposed light-gated currents from the same cell slowly recover from desensitization after a short UV light (arrow) as monitored by subsequent activations performed at different times. The red line depicts the recovery time course (τ = 396 ± 27 ms, n = 3; mean ± s.e.m.). MAM: 4,4´-bis(maleimido-glycine)azobenzene.
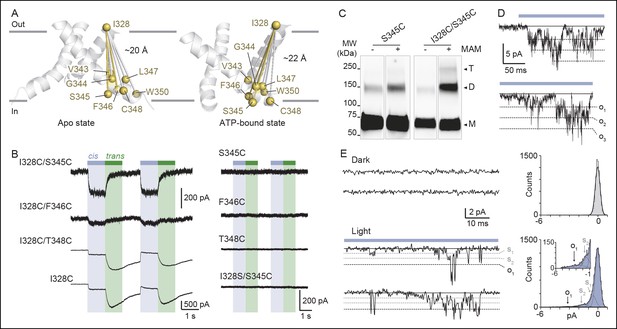
Shortening of the vertical distance separating adjacent TM2 ends drives channel openings.
(A) Side views of TM2 helices of a P2X2 homology model in the apo state (left) and ATP-bound state (right). The β-atoms of residues selected for cysteine substitutions are shown as yellow spheres. Indicated values are the average distances separating pairwise β-atoms from two adjacent TM2 helices (grey bridges). Highlighted bridges between residues indicate actual MAM cross-linking. For clarity, TM1 helices are omitted. (B) Whole-cell currents evoked by light at 365 nm (cis) or 525 nm (trans) in HEK cells expressing the indicated cysteine-substituted mutants treated with MAM. (C) Western blot analysis of cell-surface cross-linking of the indicated mutated subunits expressed in TSA-201 cells after treatment (+) or without treatment (-) with MAM. Monomer (M), dimer (D), and trimer (T) are indicated. Uncut gel image is shown in Figure 3—figure supplement 1C. MW, Molecular weight. (D) Single-channel currents recorded from an outside-out patch expressing the I328C/S345C mutant at -120 mV in response to 365 nm illumination. Three simultaneous openings (O) indicated by dashed black lines were detected. Black lines indicate closed channels. (E) Unitary currents (left) and corresponding all-points histograms (right) recorded before (upper) and during 365 nm illumination (lower) from the same patch expressing the I328C/S345C mutant. Sublevel openings (S1 and S2) are indicated by dotted gray lines. Inset shows expanded scale. All-points histograms were fitted to one Gaussian (upper) or to the sum of four Gaussians (lower).
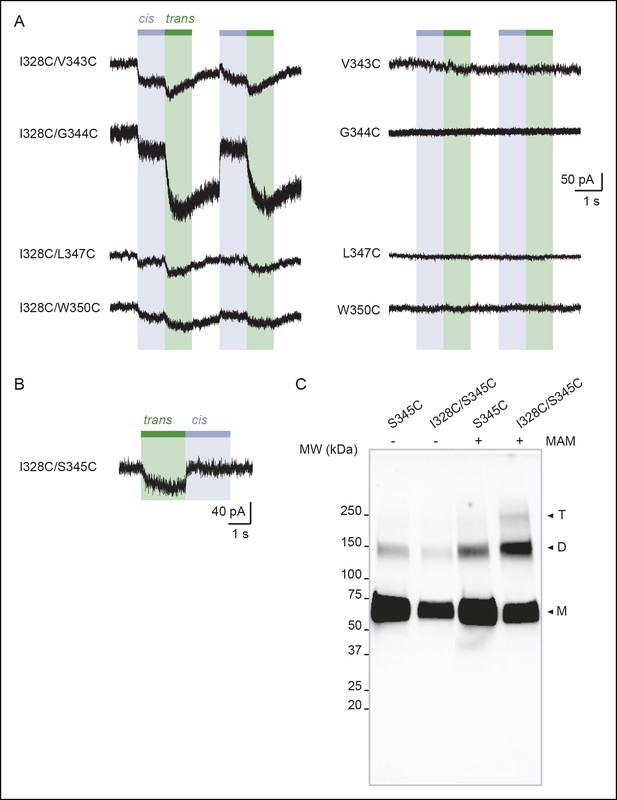
Vertical screening identifies a shortening of the distance separating adjacent TM2 ends during activation.
(A) Whole-cell currents evoked by light at 365 nm (cis) or 525 nm (trans) in cells expressing the indicated cysteine-substituted mutants treated with MAM. (B) Whole-cell current evoked by light at 525 nm (trans) in a cell expressing the indicated cysteine-substituted mutant treated with MAS. (C) Uncut gel image of cross-linked P2X2-3T subunits from Figure 3B. MAM: 4,4´-bis(maleimido-glycine)azobenzene. MAS: 4-(maleimido-glycine)-4'-(succimido-glycine)azobenzene.
Biophysical properties of the I328C/S345C mutant.
(A) Whole-cell current recorded in the dark after illumination at 365 nm and 525 nm in a cell expressing the I328C/S345C mutant. (B) Current-voltage curves recorded in different extracellular solutions (Man, mannitol; Na-Ise, sodium isethionate; Ca, calcium; NaCl symmetrical NaCl external solution; NMDG, N-methyl-D-glucamine). Light-gated currents were obtained after subtracting photocurrents recorded at 365 nm light to those obtained in the dark after switching to 525 nm light.
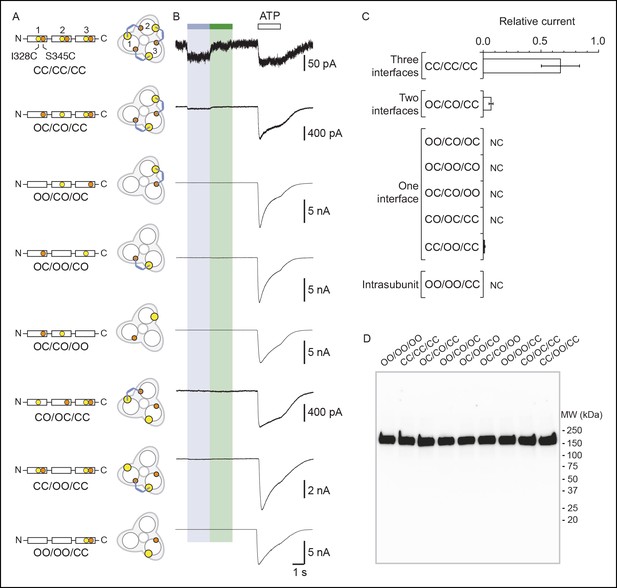
Concatenated P2X2-3T receptors are gated by UV light with only two cross-linked MAM.
(A) Schematic representation of the trimeric P2X2-3T concatemers containing a wild-type subunit and/or a mutated subunit at I328 (yellow spheres) and/or at S345 (orange spheres). C and O indicate cysteine mutation and wild-type residue, respectively. The expected locations of cis-MAM cross-linking (colored stick) within the concatenated trimeric receptor are also indicated. (B) Whole-cell currents recorded from TSA-201 cells expressing the concatenated trimeric P2X2-3T receptors (indicated in panel A) following light switching and ATP application (100 μM, saturating as determined from controls in which current amplitudes evoked by 300 μM ATP were similar to those evoked by 100 μM ATP). Actual light-gated current amplitudes were 3.0 ± 1.5 for CC/CC/CC and 3.8 ± 1.5 pA/pF for OC/CO/CC (n = 4–6 cells). (C) Bar plot summarizing the ratio between light (365 nm)-gated currents and ATP-gated currents (n = 4–6 cells; mean ± s.e.m.) for the indicated concatemers. NC stands for no current. (D) Western blot analysis from SDS/PAGE of concatenated P2X2-T receptors shows the presence of a predominant protein expressed at the surface of TSA-201 cells that had a molecular weight corresponding to that of a trimer. Concatemer that contained wild-type residues in the first, second and third subunits is depicted OO/OO/OO. MW, Molecular weight; MAM: 4,4´-bis(maleimido-glycine)azobenzene.
Vertical motions induced by cis-MAM at the I328C/S345C mutant increase ATP function.
(A) Normalized whole-cell currents evoked by a saturating concentration of ATP (100 μM) recorded before (gray trace) and after (black trace) treatment with MAM. Inset highlights part of the currents upon ATP washout, fitted to single exponential decay functions (red traces). (B) Whole-cell light-gated currents recorded from the same cell in the presence (left) or absence (right) of ATP (100 μM). Gray trace indicates the predicted current if ATP-gated and light-gated currents at 365 nm were additive. (C) Concentration–response relationships for ATP at 525 nm (open circle, trans state) or in the dark immediately after illumination at 365 nm (filled triangle, cis state) (n = 4–8 cells; mean ± s.e.m.). Currents were normalized to 100 μM ATP at 365 nm and 300 μM ATP at 525 nm. The Hill equation was fit to the data.
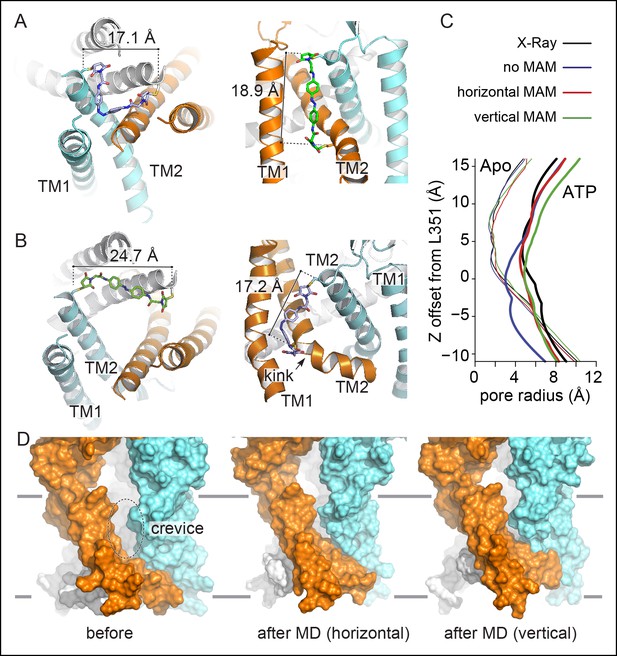
Molecular dynamics of zfP2X4 open-channel state cross-linked by MAM reduce inter-subunit interface in the TMD.
(A) Cartoon representation of the TMD of zfP2X4 receptor simulated in the closed state after MD, in which MAM (in stick representation) is conjugated horizontally between two I336C (cis configuration, left) or vertically between I336C and N353C (trans configuration, right). For clarity, only one MAM is shown vertically. Distances separating Cβ atoms of engineered cysteines are 17.1 ± 0.5 Å (n = 800) and 18.9 ± 0.9 Å (n = 2400). (B) Same views of the TMD simulated in the open state. Distances separating Cβ atoms are 24.7 ± 0.6 Å (n = 800) and 17.2 ± 1.0 Å (n = 2400). TM1 and TM2 helices and the location of a kink in one of the three TM2 helices are also shown. (C) Transmembrane pore radius along the axis of the ion channel for the apo (thin lines) and ATP-bound (thick lines) states. The profiles were calculated considering the backbone atoms only (see Materials and methods) and were derived from the X-ray structures (black) or models obtained after MD relaxation computed with (red and green) or without MAM (blue) as indicated. In the absence of MAM, the open state rapidly closes in MD simulations near L351 (rP2X2: V343). (D) Lateral view of the channel displayed in surface representation before (left) and after MD following MAM attachment between two I336C (middle) or between I336C and N353C (right). MAM: 4,4´-bis(maleimido-glycine)azobenzene; MD: Molecular dynamics; TMD: Transmembrane domain.
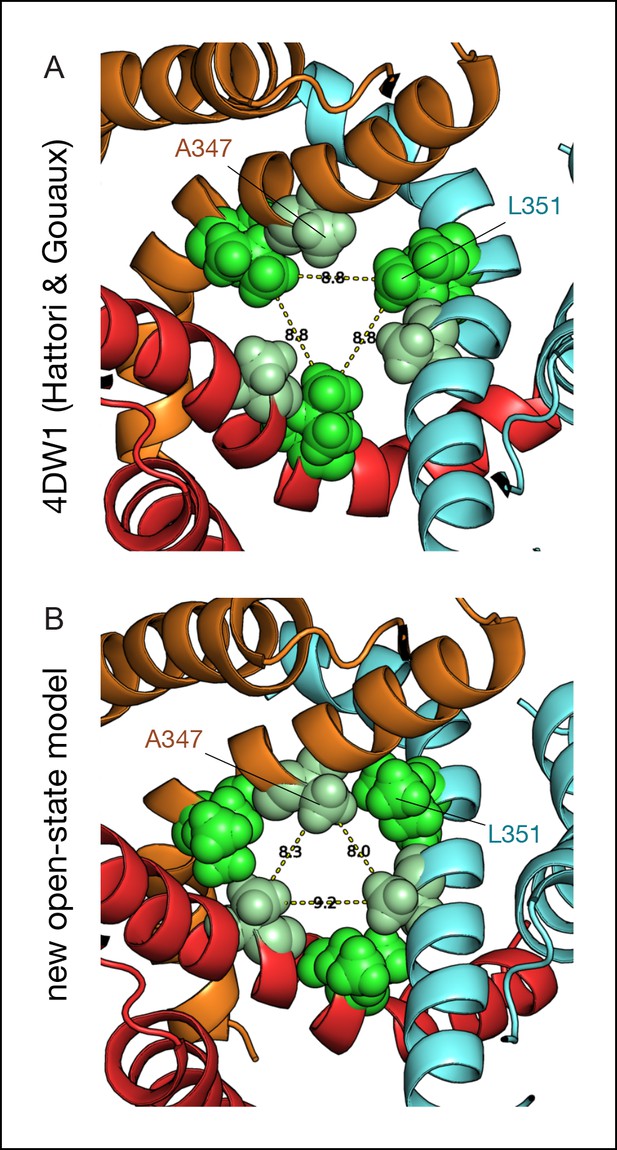
Comparison between the ATP-bound crystal structure and the new model of the open state.
Comparison of the TMD in the open-state conformation between the ATP-bound X-ray structure of zfP2X4 (A) and the new model of the open state produced by MD simulations with MAM cross-linked vertically to I336C/N353C (B). View from the extracellular region. A space-filling representation of the interfacial residues A347 (pale green) and L351 (light green) shows a striking change at the TM2-TM2 interface and the constriction point, which moves from L351 to A347 in the new model of the open state. MD: Molecular dynamics; TMD: Transmembrane domain.
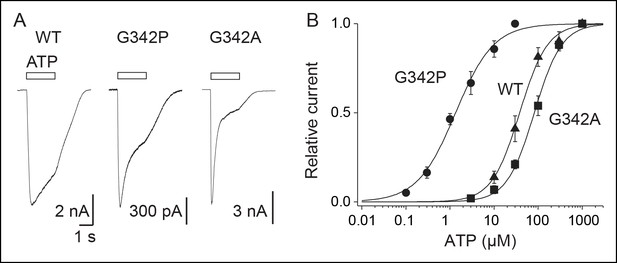
Proline mutation supports bending of the TM2 helices during P2X activation.
(A) Whole-cell currents evoked by ATP (at saturating concentrations) from HEK cells expressing the wild-type (WT) P2X2 receptor (1000 μM), G342P (100 μM) or G324A (1000 μM) mutant. (B) Concentration-response relationships for ATP at WT P2X2 receptor and mutant receptors, as indicated (n = 4–6 cells; mean ± s.e.m.). The Hill equation was fit to the normalized data.
Tables
Comparison of the Cβ–Cβ distances (in Å) in the crystal structures of the closed and open states and after MD relaxation with fused MAM, along with the end-to-end distances for the free MAM in solutiona.
| Horizontal cross-linking | Vertical cross-linking | |||
|---|---|---|---|---|
| State/isomer | closed/cis | open/trans | closed/trans | open/cis |
| X-Ray | 16.1 | 27.7 | 20.8 | 23.2 |
| Free MAM MD △ from X-Ray | 16.0 ± 4.6 -0.1b | 21.7 ± 2.0 -6.0 | 21.7 ± 2.0 0.9 | 16.0 ± 4.6 -7.2 |
| Fused MAM MD △ from X-Ray | 17.1 ± 0.5 +1.0 | 24.7 ± 0.6 -3.0 | 18.9 ± 0.9 -1.9 | 17.2 ± 1.0 -6.0 |
-
aThe distances are measured between the Cβ atoms of the native or cysteine-cross-linked residues, that is, I336(C) for horizontal cross-linking and I336(C)/N353(C) for vertical cross-linking, or between the S atoms for the free MAM. bNote that in this case, ∆ gives only an estimate of the difference because it was determined from the S-S distances of free MAM and Cβ–Cβ distances of the X-ray data.
-
MD: Molecular dynamics; MAM: 4,4´-bis(maleimido-glycine)azobenzene.





