A dystonia-like movement disorder with brain and spinal neuronal defects is caused by mutation of the mouse laminin β1 subunit, Lamb1
Figures
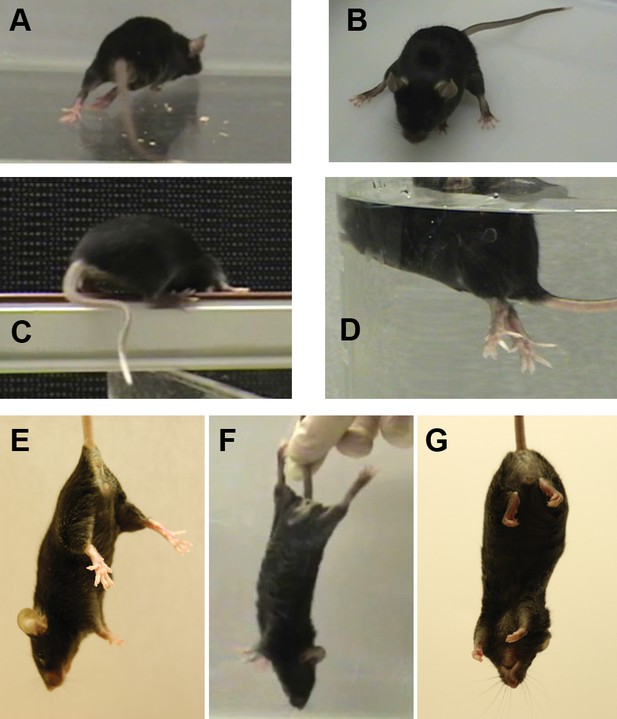
The mutant mouse had intermittent dystonic behaviors affecting hindlimbs and tail.
(A) During ambulation, hyperextension could affect either hindlimb or both, sometimes with inversion of the foot. When hyperextension was unilateral, some mice had a preferred side et al. switched sides. Hyperextension of hindlimbs was seen at the youngest age during locomotion, but by 4 months of age was sometimes seen at rest and sometimes was bilateral. A bilateral, maximally extended posture is within a WT mouse’s normal repertoire because it is shown by nursing dams straddling a large litter. (B) Hyperextension often continued when the animals sat. (C) Briefly curved tail was sometimes the first symptom in weanlings but was seen in older adults mainly when stressed. The curvature, in the plane of the floor, utilizes lateral muscle groups, and Straub tail was seldom if ever seen. (D) While WT mice sometimes have brief periods of rigidity and tilting when dropped in water, the mutant mice adopted an upright posture with extreme hyperextension and spread toes. They soon recovered and swam. (E) The normal WT reflex when suspended by the tail. (F) Mutants exhibited caudal hyperextensions involving one or both hindlimbs. This is also within the normal repertoire because WT exhibit a hindlimb posture like this when suspended just out of reach of an object and reaching with the forelimbs. (G) The mutants also exhibited transient hyperflexions of one or both hindlimbs. This was not a coordinated 'clasped' posture (limbs held together at the midline). Vibration stimulation of the knee joint in awake, hand-held mutant mice sometimes elicited strong dystonic movements when mice were released (not shown).
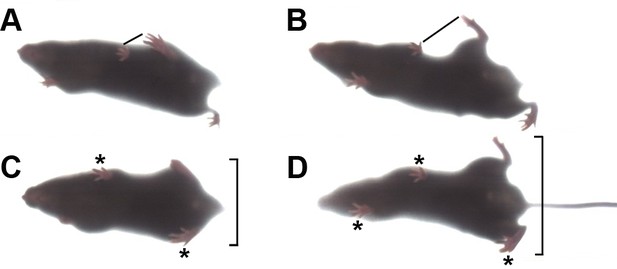
Gait abnormality in lamb1t mice.
They were filmed ventrally from below the DigiGait transparent belt. The plane of focus was shallow, so feet that are seen clearly are in contact with the belt, while feet and tail that are more than a few mm above the belt are distorted or not resolved. (A) Treadmill-running WT mice placed their hindlimbs immediately behind the forelimbs in the alternating step pattern, and the hindlimb made contact close to the ipsilateral forelimb, as marked with a line. (B) In contrast, when mutant mice ran, the pattern was still alternating, but accuracy varied. In this example, the hindlimbs swung wide and did not get close to the forelimb. (C) Stance in mid-stride. In the WT the swinging hindlimb stayed close to the body, and mice had only two feet in contact with the belt (*). (D) The mutant's hindlimbs were both splayed out, and three feet were in contact with the belt. Utilizing both front feet may have compensated for deficient hindlimb mechanics. WT had three feet in contact only when breaking stride to rest briefly. The mice studied ranged in age from 63 to 132 days old, mean 109, n = 9 per group.
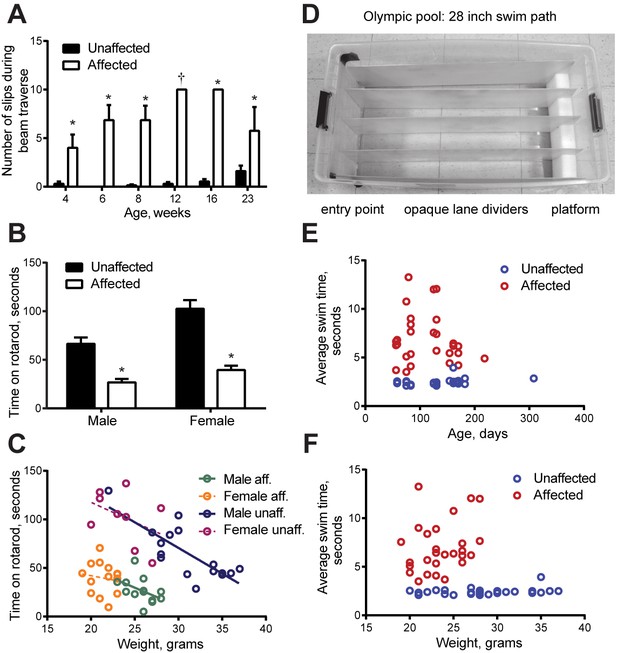
Quantitative measures of impairment.
The mice (all on the C57Bl/6N background) were designated affected and unaffected before discovery of the gene. Bar graphs show mean ± SEM and two-way ANOVA was applied. (A) Crossing an elevated beam to return to the home cage was tested with one cohort repeatedly at the ages shown (n = 14 WT, 10 males, 4 females, and n = 7 lamb1t, 4 males, 3 females). Symptoms normally appeared between 3 and 4 weeks of age. Young mutant mice (1–2 months old) tended to exhibit only slips, while mature mutant mice (3–6 months old) tended to have a mix of foot slips, hyperextension, and full control, and often could not stay on the beam. Traverse time and foot slip data from trials where a mouse fell were not included in the calculations, and the mouse was given another trial. (Numbers completing the task: at 4 weeks, WT 13, mutant 7; 6 and 8 weeks, WT 14, mutant 7; 12 weeks, WT 13, mutant 1; 16 weeks, WT 13, mutant 3; 23 weeks, WT 10, mutant 4.) There was no significant difference between WT and mutant in time to cross at any age (p ranged from 0.28 to 0.76). However, the data showed a significant difference in the number of foot slips during beam traverse. At 4 weeks, p = 0.0021; 6 weeks, p = 3.7 x 10−6; 8 weeks, p = 3.1 x 10−6; 12 weeks, n.a.; 16 weeks, p = 3.9x10−11; 23 weeks, p = 0.034 for main genotype effect (*), and Bonferroni’s test confirmed significance. At 12 weeks, only one mutant mouse was able to complete the task, and the bar (†) was a single data point. (B) In the accelerating rotarod task, both male and female mutant mice showed substantially shorter latencies to falling off. After 2 days of training, two trials on the third day were averaged (WT, n = 17 male and 9 female mice; mutant, 13 males and 14 females; p = 3.4 x 10−5 for males and 6.5 x 10−7 for females, followed by Tukey’s test). In multiple comparisons, all differences were significant except male affected vs. female affected mice. Ages ranged from 60 to 180 days (averages WT 125 days ± 47.5, SD; mutant 114 ± 44.5), and there was no trend with age. (C) Weight gain was initially normal in lamb1t mice, but they plateaued at 3–4 months, likely due to the metabolic demands of elevated muscle activity. Weight as a confounding variable is not often considered in rotarod testing. Plotting the rotarod data against weight showed it to be a continuous independent variable in WT males (linear regression for WT males had a significant slope [R square = 0.6299, F = 24.99, p = 0.0002]; slopes in the other groups tested non-significant). However, the main effect of genotype dominated the results even though male lamb1t mice weighed less. (D) The Olympic pool (empty). Mice swam down a lane to a submerged platform. (E) Swimming speed results as a function of age, and (F) as a function of weight. Each symbol is the average of two trials for one mouse. There was little overlap between genotypes, and no significant deterioration with age or weight was found by linear regression. SD, standard deviation; SEM, standard error of the mean; WT, wild type.
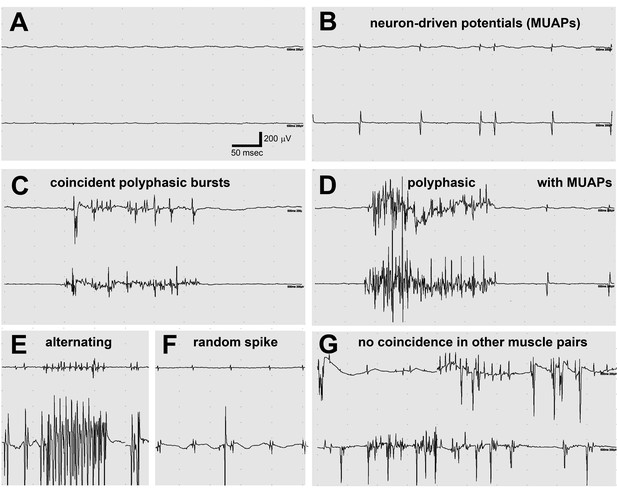
Electromyography (EMG) in the lamb1t mouse showed co-contraction.
(A–F) Electrodes were in opposing hindlimb muscle pairs (anterior rectus femoris and posterior biceps femoris). Simultaneous activity in opposing muscles is a cardinal feature of dystonia. (A) Because young mice stopped showing symptoms when they warmed up after awakening, EMG was used to test for myotonia, a muscle channelopathy where a warm-up phenomenon is well-known, but results were negative. (B,D,F) Semi-rhythmic MUAPs (motor unit action potentials) typical of voluntary movement occurred simultaneously at 10–20 Hz under anesthesia in lamb1t mice. Sometimes recruitment of a second MUAP could be seen (as occurs with increasing force), but there was less recruitment than normal. Vigorous coincident bursts of action potentials occurred either without MUAPs (C), or with MUAPs (D). (E) An uncommon complex repetitive discharge at 120 Hz with antagonist muscle group alternation, a spinal discharge pattern usually associated with locomotion. (F) Spontaneous large single spikes were random and not seen in the opposing muscle. (G) Example of electrodes in the same or opposing muscles in different legs: no co-contraction or synchronization. Images are representative of n = 6 lamb1t mice. Silent recordings of littermate controls (n = 2 WT) are not shown. The mice ranged in age from 43 to 113 days, mean 59.
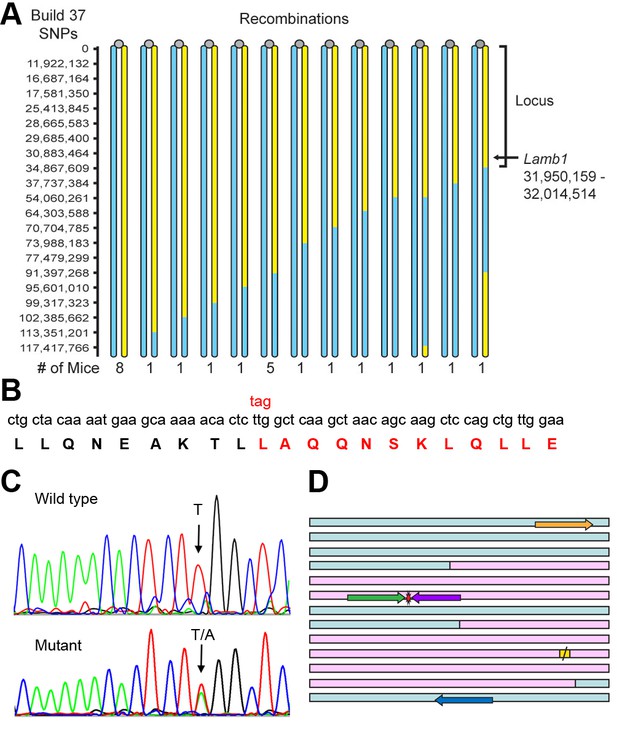
Identification of the Lamb1 mutation.
(A) SNP locus mapping summary for chromosome 12 in B6/FVB hybrids. The mutation is necessarily in B6 DNA (yellow); FVB DNA is blue. Mouse centromeres are at the top (gray ovals). There were no informative SNPs between base 0 and 11,922,132. (B) Exome sequencing result. Nucleotide and protein sequence for Lamb1 (laminin β1) amino acids 1721 to 1741 flanking the mutation. Mutation at a single nucleotide generated a stop codon, TAG, and the sequence in red and beyond (amino acids 1730 to 1786) was truncated. Eight other variants identified by exome sequencing in the locus were in exon-flanking intron sequence or 3'UTR and not predicted to be damaging. (C) We validated the mutation by Sanger sequencing. The identified causative Lamb1 mutation was not a reported variant in dbSNP, the Mouse Phenome Database, or the Sanger1 database. To date, the mutation has been verified in 33 symptomatic mice. (D) Allele-specific PCR design. Pink blocks are exons 32 and 33, the red symbol is the mutation, and the yellow square is the normal stop codon. The forward allele-specific primers (green) were longer than the reverse allele-specific primers (violet) because of high AT content. Each set of otherwise-identical internal primers ended with either T or A. If the mismatch is sufficiently destabilizing, priming will be absent or very low. If the mismatch is not sufficiently destabilizing, another base 5’ of the mutation can be changed to reduce stability and improve selectivity; the reverse WT allele-specific primer also had a substitution of G for C at –2. Forward and reverse outside primers (gold and blue) were predicted in the flanking DNA at convenient distances from the mutation based on melting temperatures matching the allele-specific primers. Diagnostic PCR was done with the gold/violet pair with the mutation, while the gold/blue pair served as a positive control.
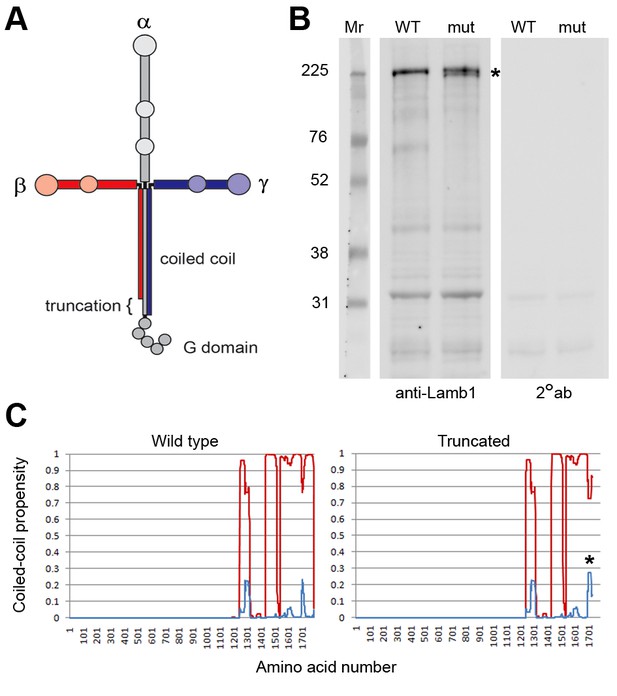
Laminin β1 protein structure.
(A) Diagram of the laminin trimer of α (gray), β (red), and γ (blue) subunits. Circles are major globular domains and the largest ones are binding sites for each other and other extracellular matrix components. The G domain repeating globular domains are sites of attachment to cell surfaces through integrin and dystroglycan. The rod-like coiled-coil trimer domain, a quaternary structure, is altered by truncation of the last 57 amino acids in the β1 subunit in lamb1t. Coiled-coils are composed of alpha-helices tightly wound together, and the absence of a portion should destabilize the α and γ segments at that site. (B) SDS gel electrophoresis (NuPage 4–12% polyacrylamide gradient MES gels run 50% longer than normal) followed by immunoblot with laminin β1-specific antibody. The doublet resolved in the mutant (*) is assumed to be the proteins produced from WT and mutant alleles. (C) We used MultiCoil software to calculate the propensity of protein sequence to form two-stranded or three-stranded coiled coils (http://groups.csail.mit.edu/cb/multicoil/cgi-bin/multicoil.cgi). Laminin β1 is strongly predicted to form three-stranded coiled coils. Just upstream of the truncation, there was a slight decrease in triple-stranded coil propensity (red) and slight increase in double-strand coil propensity (*, blue) for 27 amino acids, but no change for the rest.
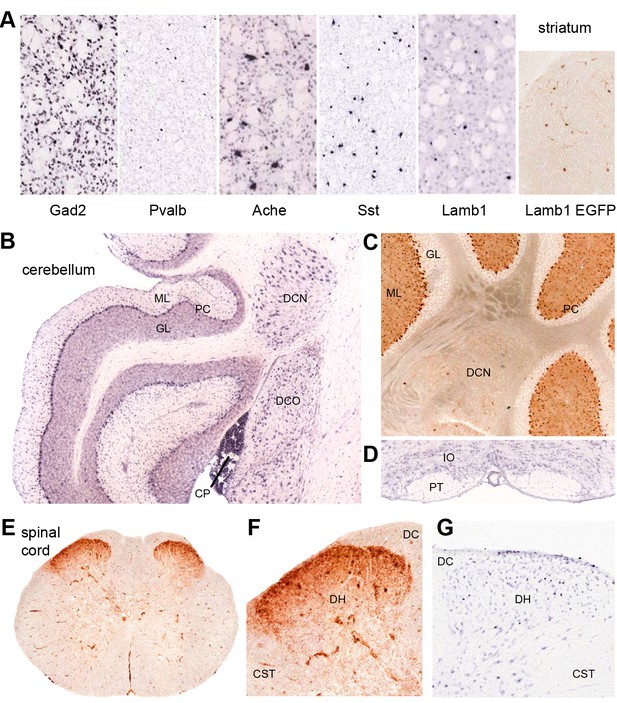
Discrete nervous system expression of Lamb1.
Images are reproduced from the Allen Brain Atlas (Allen Institute for Brain Science) (A,B,D,G), or GENSAT (C,E,F). (A) In situ hybridization in striatum on lightly counter-stained mouse sections. Gad2 signal marks the abundant medium spiny neurons. Pvalb, Ache, and Sst are markers of different interneuron populations. Cholinergic neurons (Ache) are very large. Lamb1-positive cells are apparently in an interneuron population, but unlikely to be cholinergic because of their small size. EGFP expression confirms the in situ hybridization signal for Lamb1. (B) Cerebellum. Lamb1 in situ hybridization is high in choroid plexus (CP) and Purkinje cells (PC), and expressed at a lower level in the deep cerebellar nuclei (DCN), including all three, dentate, interpositus and fastigial. Label in dorsal cochlear nucleus (DCO) is also present. (ML) molecular layer where Purkinje cells arborize. There are strongly stained interneurons scattered in the molecular layer. (GL) granular layer, where the granule cells, the abundant excitatory inputs to Purkinje cells, do not express Lamb1. There are sparse labeled cells, however. (C) EGFP expression supports the findings. (D) Another major excitatory input to Purkinje cells, the climbing fibers, come from the inferior olivary nucleus (IO), which was not labeled for Lamb1. (PT) pyramidal tract. (E) Lumbar spinal cord. EGFP expression was in a diffuse band in lamina 1 of the spinal cord, and in strongly labeled scattered cells in lamina 2 or 3. There was little or no label in other structures other than blood vessels. (F) Higher magnification with dorsal horn (DH), dorsal sensory column (DC), and corticospinal tracts (CST) indicated. (G) Available in situ hybridization in spinal cord was faint, but a similar subpopulation of cells in lamina 2 or 3 as well as some cells at the surface were labeled. Reproduced with permission.
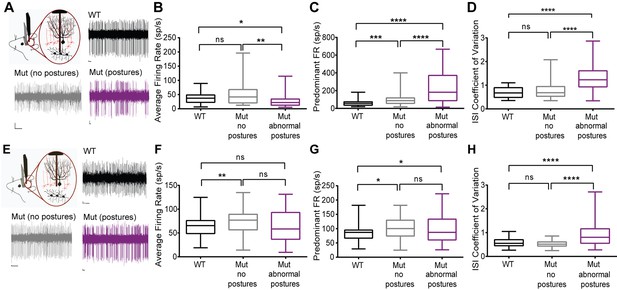
Cerebellar neurons in lamb1t mice during abnormal postures exhibited high-frequency bursts.
Extracellular recordings were performed in vivoin awake head-restrained wild-type and mutant mice. (A) Representative raw traces of spontaneous single-unit recordings in cells of the DCN show abnormal burst firing of cells in the mutant during abnormal postures (magenta) when compared to periods where the mouse did not exhibit abnormal postures (gray) and also compared to the WT (black). (B) Even though there was no significant difference in the average firing rate of cells in the DCN of WT compared to the mutant without postures, during abnormal postures the average firing rate decreased significantly. (*p<0.05, **p<0.01, mean ± SEM) (C) The data were binned to construct a histogram of the interspike intervals (ISI) from which the peak value (the mode of the distribution) was determined for each cell. The predominant firing rate was calculated as the reciprocal of the mode of the ISI. During abnormal postures in the mutant animal, the predominant firing rate of cells in the DCN was more than 3-fold higher compared to the WT and twofold higher compared to conditions when the mutant was not displaying abnormal postures (****p<0.0001, ***p<0.001) (D) The coefficient of variation of the ISI (CV ISI) was significantly higher in the mutant during abnormal postures (****p<0.0001), whereas during conditions of no postures the CV ISI was similar to the WT (E) Raw traces showing irregular firing of Purkinje cells in the mutant when compared to the WT. (F) When compared to the WT, the average firing rate was statistically higher in the mutant with no postures (**p<0.01) but not significant in the mutant during abnormal postures. However, there was no significant difference between mutants with and without abnormal postures. (G) Predominant firing rate was significantly higher in the mutant with and without postures (*p<0.05) when compared to the WT. (H) Similar to cells in the DCN, the CV ISI was significantly increased in the mutant Purkinje cells during abnormal postures compared to normal postures and to WT (****p<0.0001). Scale bars in raw traces: X-axis: 300 ms and Y-axis: 20 µV. WT, wild type.
Videos
Symptoms.
Lamb1t mice displaying dystonic symptoms. 1) In a tray (novel environment) a mouse walked with kicks, adopted a wide-based sitting stance, then abruptly overcame symptoms to sit and groom. 2) A mouse with extreme hindlimb hyperextension on an elevated rack, where it also displayed curvy tail. 3) A mouse on an elevated beam traversed it with rigid hindlimbs by pulling itself across with the forelimbs. On a second trial the mouse recovered the ability to perform almost normal walking. In the home cage, mice often resumed normal motor control. Representative of many observations.
Running.
Lamb1t mice performed well when relaxed and poorly when stressed. 1) Lamb1t mice given a running wheel in the home cage ran voluntarily as soon as the lights turned off, and ran for hours as detected by a meter. This was filmed in the dark with an infrared camera. In the example shown the mouse ran smoothly but a single hindlimb hyperextension terminated the run. A magnet attached to the disk activated the meter; the viewer can use it to count rotations. Representative of n = 4. 2) Forced running on a treadmill moving at fixed speed, in contrast, was stressful and running success varied from trial to trial. 3) Slow motion ventral plane videography (DigiGait) of WT and lamb1t mice, representative of n = 9 each. WT, wild type.
Sleep and anesthesia.
Sleep and anesthesia disinhibit abnormal spinal activity. 1) A WT female (left) and lamb1t male (right) sleeping in an igloo were filmed from underneath the cage. The mutant slept with both hindlimbs extended to different degrees. 2) Spontaneous twitching activity of another lamb1t mouse sleeping in the home cage. 3) Lamb1t mice (hybrids with different unlinked coat color gene combinations) lying anesthetized in an O2/isoflurane vapor chamber. Representative of many observations.
EMG.
A continuous reading of motor unit activity recorded by EMG from opposing muscles in a lamb1t mouse. Coincident timing of both MUAPs and polyphasic bursts can be seen. Representative of n = 6.
Spinal transection.
Under continuous anesthesia delivered by nose cone, two examples of hindlimb activity before and after spinal transection are shown. In the first case, rigidity and twitching increased. In the second case, gentle stimulation appeared to elicit hyperreflexia. Representative of n = 6.
Tables
Cerebellar firing patterns.
| WT | lamb1t normal | p, norm. vs. WT | lamb1t abnormal | p, abnorm. vs. WT | p, norm vs. abnorm. | |
|---|---|---|---|---|---|---|
| Deep cerebellar nuclei neurons | ||||||
| # of cells | 26 | 42 | 56 | |||
| average f.r. | 37.4 ± 3.7 | 50.3 ± 5.9 | 0.3499 | 26.7 ± 2.9 | 0.0109 | 0.0029 |
| predominant f.r. | 61.0 ± 6.9 | 110.1 ± 13.8 | 0.0004 | 221.9 ± 20.9 | <0.0001 | 0.0001 |
| CV ISI | 0.677 ± 0.05 | 0.751 ± 0.05 | 0.6542 | 1.272 ± 0.07 | <0.0001 | <0.0001 |
| Purkinje neurons | ||||||
| # of cells | 61 | 76 | 45 | |||
| average f.r. | 62.9 ± 2.8 | 73.6 ± 2.8 | 0.0052 | 62.7 ± 5.0 | 0.3400 | 0.0619 |
| predominant f.r. | 83.2 ± 3.4 | 98.7 ± 3.6 | 0.0141 | 103.8 ± 8.1 | 0.0128 | 0.4462 |
| CV ISI | 0.578 ± 0.02 | 0.531 ± 0.01 | 0.3036 | 0.919 ± 0.07 | <0.0001 | <0.0001 |
-
Means and significance of firing rates and coefficients of variation. Definitions: f.r., firing rate. CV ISI, coefficient of variation of the interspike interval; that is, variability. The data are means ± SEM, and the p values were determined by the Kolmogorov-Smirnov test for non-parametric distributions, which can be seen in Figure 8. N = 3 WT and N = 4 lamb1t mice; n for each class of cell recording is given in the table.





