The ATPases of cohesin interface with regulators to modulate cohesin-mediated DNA tethering
Figures
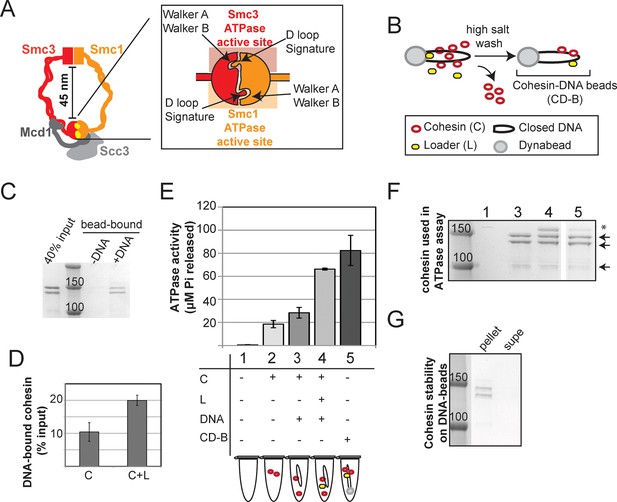
Stable DNA binding does not suppress ATPase activity of cohesin.
(A) Cartoon representation of the cohesin complex. Inset shows a model of the ABC ATPase domain of cohesin complex, based on the X-ray crystal structure of Rad50 in the presence of ATP (Hopfner et al., 2000), and of Smc1 head domain in the presence of ATP and Mcd1 C-terminus (Haering et al., 2004). This model has not been experimentally verified for the cohesin complex heterodimeric ATPase head domain. (B) Schematic showing in vitro assembly of stable cohesin-DNA bead complexes. DNA bearing CARC1 sequence was attached to dynabeads via biotin-streptavidin interaction at both ends. Cohesin was incubated with bead-bound DNA and loader in buffer containing 25 mM KCl and 25 mM NaCl, then washed in 500 mM KCl to wash off salt-sensitive cohesin. The remaining DNA-bound cohesin (and small amount of loader) is referred to as cohesin-DNA-beads (CD-B). (C) Cohesin assembly on DNA-beads. S. pombe cohesin and loader complexes were purified from Y4443 and Y4483, respectively. Purified cohesin and loader were incubated with dynabeads-DNA or dynabeads alone for 1 hour at 30°C, then cohesin was washed off as described in B. Cohesin bound DNA-beads (DNA) but failed to bind beads lacking DNA (-). (D) Cohesin binding to DNA is stimulated by the loader complex. DNA binding was done as described in B & C, except loader was omitted in one sample. Percent cohesin bound was calculated by quantifying bands on Coomassie-stained SDS-PAGE. Data from two independent experiments, error bars represent standard deviation. (E) Effect of stable DNA binding on cohesin ATPase activity. ATPase activity of cohesin alone (2) was compared to cohesin with DNA (3), cohesin in presence of loader complex and DNA (4), and cohesin in stable cohesin-DNA complexes (CD-B, 5). Proteins were incubated in ATPase buffer 2 spiked with hot ATP for 1 hour at 30°C. Released Pi was calculated and plotted as described in Methods. Error bars represent standard deviation from two independent experiments. (F) Equal concentrations of cohesin were used in the ATPase reactions. Arrows point to S. pombe homologs of cohesin subunits, Smc1, Smc3 (Psm1 and Psm3 in S. pombe, ~150 kD), Mcd1 and Scc3 (Rad21 and Psc3 in S. pombe, ~100 kD). Asterisks mark subunits of the S. pombe homolog of the loader complex, Scc2/Scc4 (Mis4/Ssl3 in S. pombe). Due to the lower ability of the loader complex to bind to DNA under these conditions, there is less loader complex present in sample 5 than in sample 4. (G) Stably DNA bound cohesin remained bound to DNA-beads throughout the course of the ATPase experiment. Cohesin was incubated with DNA-beads in the presence of loader and ATP as described before and washed in 0.5 M KCl, then resuspended in ATPase buffer. Samples were incubated for 1 hour at 30°C. Supernatant and beads were separated and visualized on SDS-PAGE. Protein gels are representative of at least 2 independent experiments. Bands in some panels were spliced from the same gel for representation purposes. Please see Figure supplements 1–4 for further characterization of cohesin’s ATPase activity while stably bound to DNA.
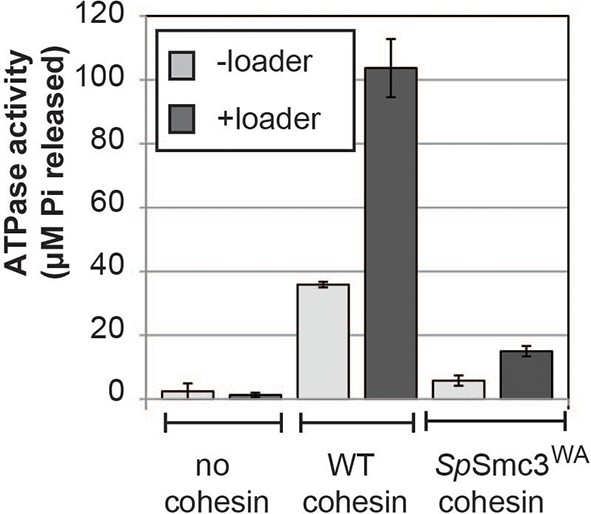
The ATPase activity of cohesin is stimulated by the loader and abolished by a walker-A (K38I) mutation in Psm3 (S. pombe Smc3 homolog).
Equal amounts of wild type or mutant cohesin was incubated in the presence of loader and DNA in reaction buffer spiked with hot ATP for 1 hour at 30°C. Released Pi was calculated and plotted as described in Methods. Error bars represent standard deviation from two independent experiments.
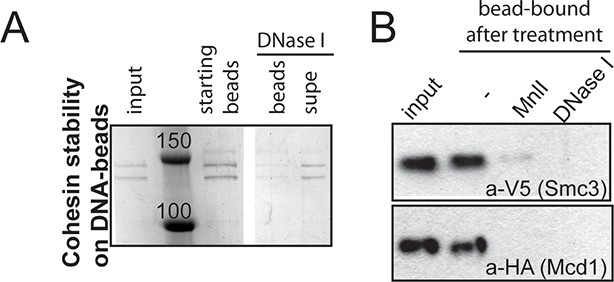
Stably DNA-bound cohesin (CD-B) can be eluted off the DNA-beads by a DNase or restriction enzyme (Mnl I) digest.
(A) CD-B assembled as described in Figure 1B was resuspended in CL1 buffer containing DNase. Beads were separated from the supernatant and proteins were visualized by SDS-PAGE. (B) CD-B assembled as described in Figure 1B was resuspended in buffer CL1 buffer containing DNase or Mnl I. Beads were separated from the supernatant and proteins were visualized by SDS-PAGE, followed by Western blotting.
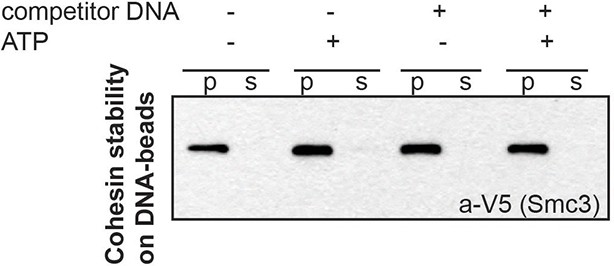
Stably DNA-bound cohesin does not come off the DNA-beads after incubation with competitor DNA.
CD-B assembled as described in Figure 1B was resuspended in 20 μL CL1 buffer, in the presence or absence of 0.5 mM ATP and 2.5 μg plasmid DNA (5x excess in mass compared to CD-B). Supernatant and pellets were separated at the end of 30-minute incubation at 30°C. Cohesin in supernatant and pellet fractions was visualized by Western blotting against the V5-tagged Smc3 homolog of S. pombe.
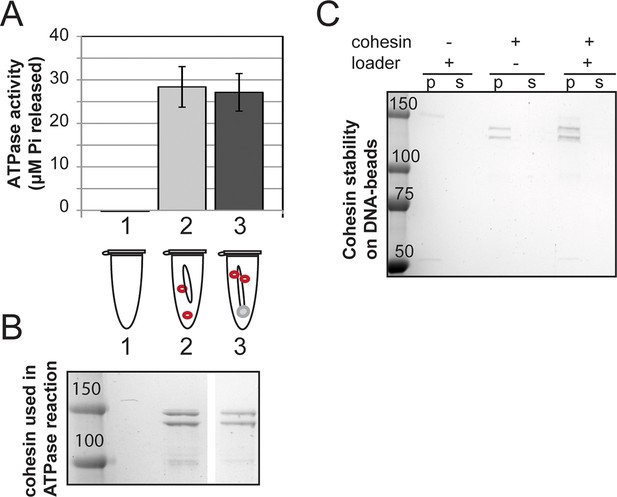
Cohesin stably assembled on DNA in the absence of loader has at least as high ATPase activity as cohesin +DNA.
(A) Cohesin + DNA in solution (sample 1) and CD-B assembled in the absence of loader (sample 2) were incubated in reaction buffer spiked with hot ATP for 1 hour at 30°C. Released Pi was calculated and plotted as described in Methods. Error bars represent standard deviation from two independent experiments. (B) SDS-PAGE showing cohesin used in A. (C) Stability of cohesin on DNA, similar to Figure 1D. Cohesin that is assembled in the absence of the loader also can bind DNA stably, although the assembly is less efficient. Protein gels are representative of at least 2 independent experiments. Bands in some panels were spliced from the same gel for representation purposes.
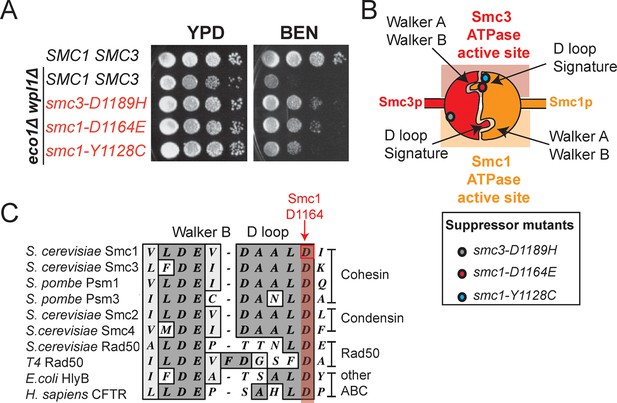
smc1-D1164E and smc1-Y1128C mutations suppress the benomyl sensitivity of eco1△ wpl1△ cells and are part of the Smc3 ATPase active site.
(A) Assessing effects of smc1-D1164E and smc1-Y1128C on eco1∆ wpl1∆ benomyl sensitivity. Haploid wild-type cells (VG3460-2A), and eco1∆ wpl1∆ cells alone (VG3502-1A) or containing smc1-D1164E (VG3574-5A), smc1-Y1128C (VG3576-1C) or smc3-D1189H (VG3547-3B) were grown to saturation in YPD at 23°C, plated as 10-fold serial dilutions on YPD alone, or containing benomyl at 12.5 μg/mL (BEN) then incubated at 23°C for 3 days. (B) Cartoon depicting the Smc1 and Smc3 ATPase active sites along with the position of three suppressor mutations shown in A. All three suppressor mutations are in the vicinity of the Smc3 ATPase active site. Note that the Smc1 D-loop and signature motifs form part of the Smc3 ATPase active site. (C) The conservation of residues around the D-loop in distant ABC ATPases.
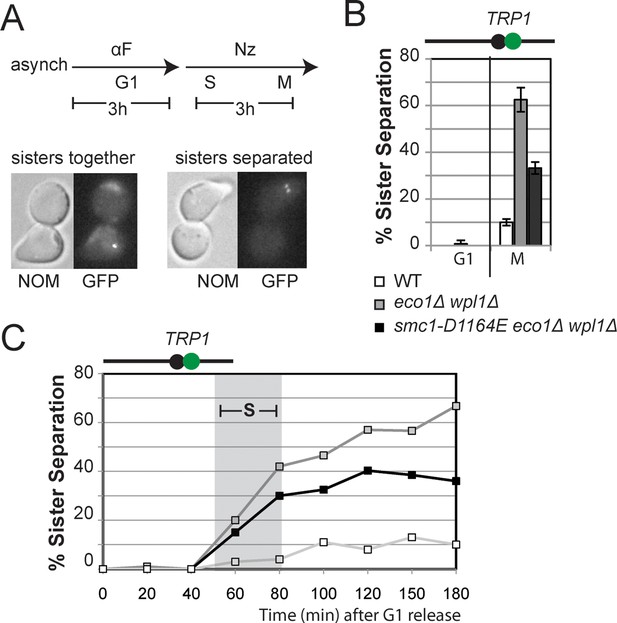
smc1-D1164E allele restores cohesion in the absence of both Eco1 and Wpl1.
(A) Regimen used to assess sister chromatid cohesion in cells. Mid-log phase cultures of asynchronously growing cells at 23°C were arrested in G1 with alpha factor for 3 hours, then released into media containing nocodazole for 3 hours to arrest cells in M phase. Representative images of M phase arrested cells are shown with cells being visualized by Nomarski (NOM) and cohesion (GFP), which marks a CEN-proximal TRP1 locus. Left side shows a cell where cohesion exists (one GFP spot.) Right side shows a cell where sisters have separated (2 GFP spots). (B,C) smc1-D1164E partially restored cohesion in eco1△ wpl1△ cells at the CEN-proximal TRP1 locus. Haploid wild type (WT, VG3460-2A), eco1∆ wpl1∆ (VG3502-2A) and smc1-D1164E eco1∆ wpl1∆ (VG3574-5A) cells were released from G1 and arrested in M phase using nocodazole as described in A. The percentage of cells with two GFP spots was plotted. (B) Cohesion loss at a CEN-proximal locus (TRP1) in M phase arrested cells. DNA content of these cells is shown in Figure 3—figure supplement 1, panel A. (C) Time course to assess kinetics of cohesion loss at a CEN-proximal locus (TRP1). Cell aliquots were fixed in G1 and at 20-minute intervals after release. Grey box shows S phase (based on DNA content shown in Figure 3—figure supplement 1, panel B). Please see Figure supplements 1–6 for further characterization of cohesin activator mutants.
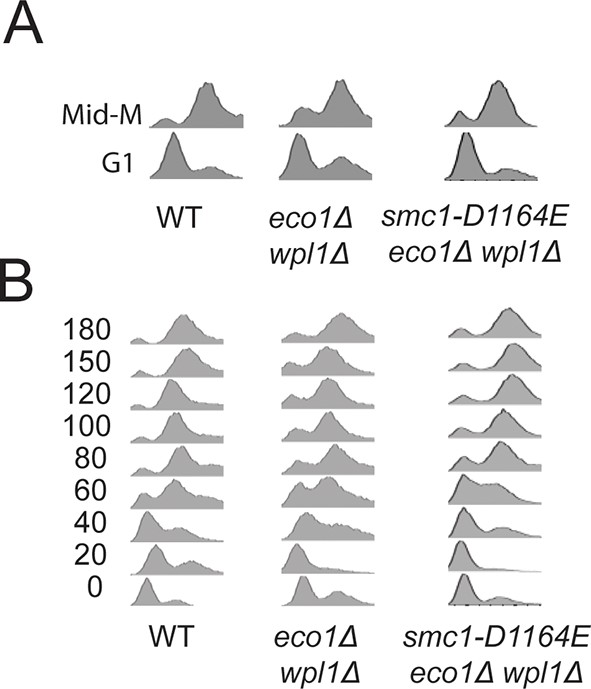
DNA content analysis of cells from Figure 3B(A) and Figure 3C(B).
https://doi.org/10.7554/eLife.11315.010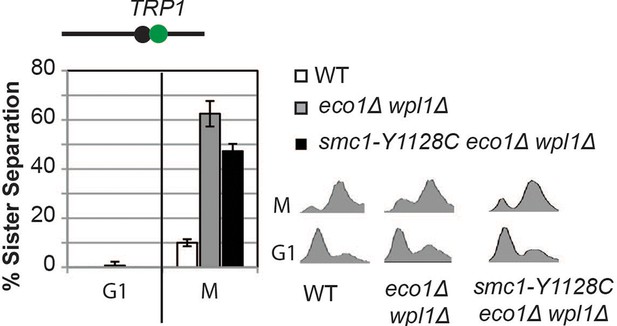
Cohesion loss in smc1-Y1128C at CEN-proximal (TRP1) locus.
Haploid wild type (WT, VG3460-2A), eco1∆ wpl1∆ (VG3502-2A) and smc1-Y1128C eco1∆ wpl1∆ (VG3576-1C) were arrested in G1 then released into M phase arrest as described in Figure 3A. Cohesion (left) and DNA content (right).
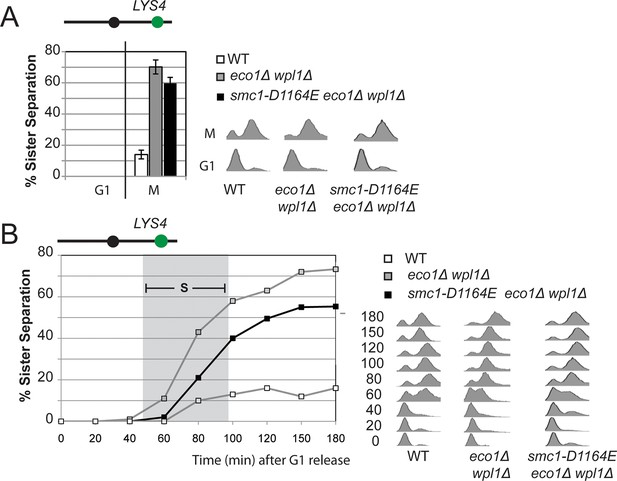
Cohesion loss at a CEN-distal locus (LYS4) in smc1-D1164E cells.
Haploid wild type (VG3349-1B), eco1∆ wpl1∆ (VG3503-4A) and smc1-D1164E eco1∆ wpl1∆(VG3575-2C) were arrested in G1 then released into M phase arrest as described in Figure 3A. (A) Cohesion loss in M phase arrested cells. Cohesion (left) and DNA content (right). (B) Time course to assess kinetics of cohesion loss. Cohesion (left) and DNA content (right). Grey box shows S phase.
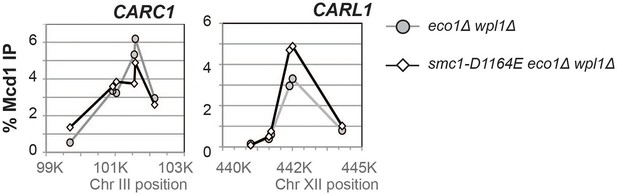
Effect of the smc1-D1164E on cohesin (Mcd1) binding in M phase arrested eco1∆ wpl1∆ cells.
Haploid eco1∆ wpl1∆(VG3502-1A) and smc1-D1164E eco1∆ wpl1∆ (VG3574-5A) cells arrested in M phase as described in Figure 3A were fixed and processed for ChIP using Mcd1 antibodies. Mcd1p binding was assessed by qPCR. Data is presented as percentage of total DNA assayed using the same primer pairs at each site. Mcd1 ChIP at Chromosome III peri-centric CARC1 (left side). Six primer pairs used to assay Mcd1 binding at loci spanning a ~2.6 kb region including CARC1 of chr. III. Mcd1 ChIP at chromosome XII CEN-distal CARL1 (right side). Six primer pairs used to assay Mcd1p binding at loci spanning a ~4.5 kb region including CARL1 of chr. XII. eco1∆ wpl1∆ (grey line, grey circles) and smc1-D1164E eco1∆ wpl1∆ (black line, open diamonds).
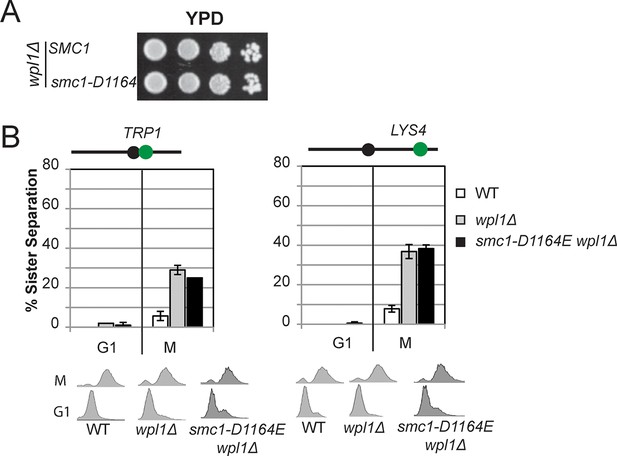
The Smc3 ATPase active site D-loop cohesion activator mutation smc1-D1164E cannot suppress a wpl1∆.
(A) Viability of smc1-D1164E in wpl1△background. Haploid wpl1△ (VG3623-2A) and smc1-D1164E wpl1△ (VG3625-2C) strains were grown then dilution plated onto YPD as described in Figure 2A. (B) Cohesion loss in M phase arrested smc1-D1164E in wpl1△cells. Cells were grown and arrested in M phase as described in Figure 3A. Cohesion loss at the CEN-proximal TRP1 locus (left side). Haploid WT (VG3460-2A), wpl1△ (VG3623-2A) and smc1-D1164E wpl1△ (VG3625-2C). Cohesion at the CEN-distal LYS4 locus. Haploid WT (VG3557-2A), wpl1△ (VG3601-8B) and smc1-D1164E wpl1△ (VG3603-3D). DNA content analysis of cells (bottom).
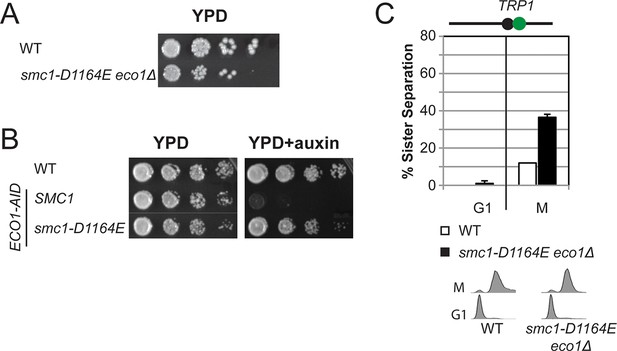
The Smc3 ATPase active site D-loop cohesion activator mutation smc1-D1164E partially suppresses the requirement for Eco1.
(A) Viability of smc1-D1164E in eco1△background. Haploid WT (VG3596-6A) and smc1-D1164E eco1△(VG3779-1E) cells were grown and dilution plated on YPD as described in Figure 2A. (B) Viability of smc1-D1164E in ECO1-AID background. Haploid WT (VG3349-1B), SMC1 ECO1-AID (VG3646-1A) and smc1-D1164E ECO1-AID (VG3648-2C) cells were grown as described Figure 2A then serial dilution (10-fold) plated on YPD alone or YPD + Auxin and incubated 2 days at 23°C. Auxin induces depletion of ECO1-AID allowing assessment of ability to bypass the essential ECO1 function. All strains were analyzed on the same plate but rearranged for illustration purposes. (C) Cohesion loss at the CEN-proximal TRP1 locus in M phase arrested smc1-D1164E eco1△ cells. Strains in C were assayed for cohesion in M phase arrested cells (top panel) as described in Figure 3A and for DNA content (bottom panel).
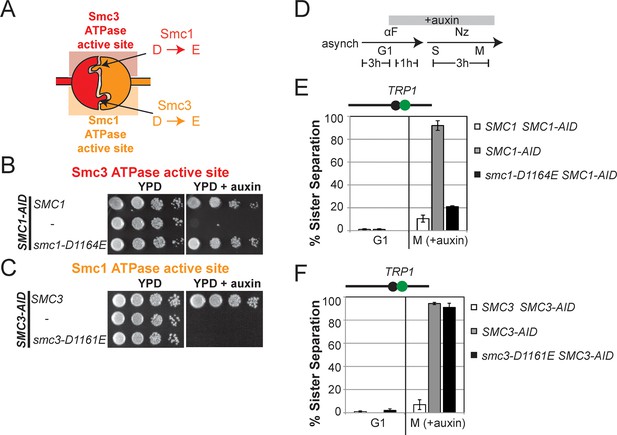
D-loop mutations in Smc1 and Smc3 ATPase active sites reveal that the two sites are not functionally equivalent.
(A) Cartoon representation of cohesin’s ATPases. The Smc1-encoded D-loop is part of the Smc3 ATPase active site and the Smc3-encoded D-loop is part of the Smc1 ATPase active site. (B) Assessing whether the Smc3 ATPase active site D-loop mutant smc1-D1164E promotes cell viability. Haploid SMC1 SMC1-AID (VG3764-3A), SMC1-AID (VG3711-5D), and smc1-D1164E SMC1-AID (VG3765-3D) cells were grown and plated on as described in Figure 2A onto YPD alone or YPD + auxin and incubated 3 days at 23°C. SMC1-AID was depleted in media containing auxin, which allows assessment of whether smc1-D1164E promotes viability. (C) Assessing whether the Smc1 ATPase active site D-loop mutant smc3-D1161E promotes cell viability. Haploid SMC3 SMC3-AID(VG3771-10C), SMC3-AID (VG3651-3D), and smc3-D1161E SMC3-AID (VG3773-16D) were grown and plated dilution as described in C. (D) Regimen used to assess sister chromatid cohesion in cells containing AID tagged proteins. Asynchronous cells were arrested in G1, depleted for AID tagged proteins by the addition of auxin, then released from G1 and arrested in M phase in the presence of auxin. (E,F) Cohesion loss at the CEN-proximal locus (TRP1) in M phase cells depleted for AID tagged proteins. SMC1-AID or SMC3-AID was depleted in strains from G1 through M phase as described in D. The percentage of cells with two GFP spots (sister separation) is plotted. (E) smc1-D1164E promotes cohesion in SMC1-AID depleted cells. Haploid SMC1 SMC1-AID (VG3794-2E), SMC1-AID (VG3711-5D) and smc1-D1164E SMC1-AID (VG3795-2C) assayed for cohesion. DNA content analysis of these cells can be seen in Figure 4—figure supplement 4. (F) smc3-D1161E fails to promote cohesion in to promote cohesion SMC1-AID depleted cells. Haploid SMC3 SMC3-AID (VG3797-1A), SMC3-AID (VG3651-3D) and smc3-D1161E SMC3-AID (VG3799-3C) strains assayed for cohesion. DNA content analysis of these cells can be seen in Figure 4—figure supplement 4. Please see Figure supplements 1–6 for further characterization of smc1-D1164E and smc3-D1161E alleles.
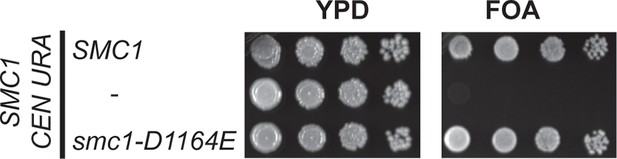
Viability of smc1-D1164E allele as sole source of SMC1.
Plasmid shuffle assay showing that the smc1-D1164E allele supports viability as sole SMC1 source. Haploid SMC1 shuffle strain (VG3568-8A) has the endogenous SMC1 deleted but kept viable by plasmid pTH2 (SMC1 URA3 CEN). This shuffle strain alone or containing SMC1 or smc1-D1164E integrated at LEU2 were grown to saturation in YPD at 23°C. Cells were plated as 10-fold serial dilutions on YPD or on media containing 5-fluoroorotic acid (FOA) then grown 3d at 23°C. FOA selectively kills URA3 cells so selects for Ura- cells that have lost plasmid pTH2, thereby revealing whether integrated SMC1 or smc1-D1164E alleles can support viability as the sole SMC1.
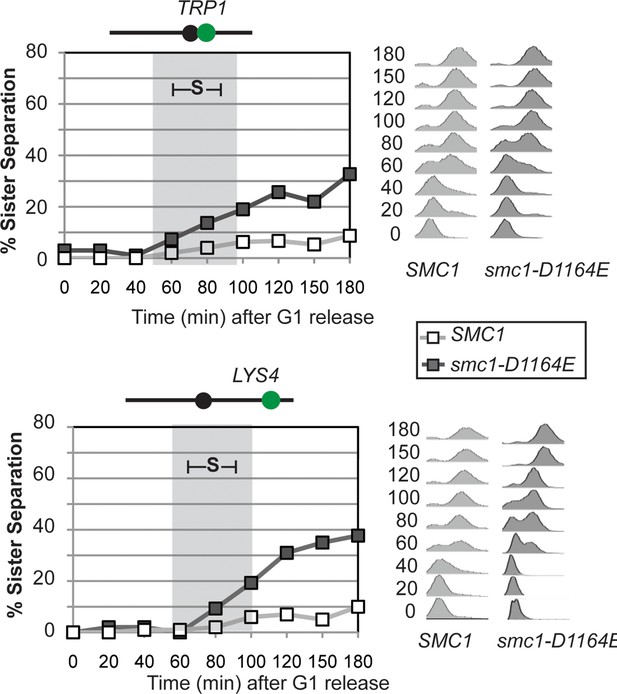
Time course assessing kinetics of cohesion loss in SMC1 and smc1-D1164E cells.
Strains were released from G1 and arrested in M phase as described in Figure 3A. Cell aliquots were fixed in G1 and at 20-minute intervals after release then scored to assess cohesion. Cohesion (left) and DNA content (right). Grey box shows S phase. Top panel: Cohesion loss at the CEN-proximal (TRP1) locus. Haploid SMC1 (VG3460-2A) and smc1-D1164E (VG3598-8A) strains were used for this experiment. Bottom panel: Cohesion loss at the CEN-distal (LYS4) locus. Haploid SMC1 (VG3557-2A) and smc1-D1164E (VG3581-8B) strains were used for this experiment.
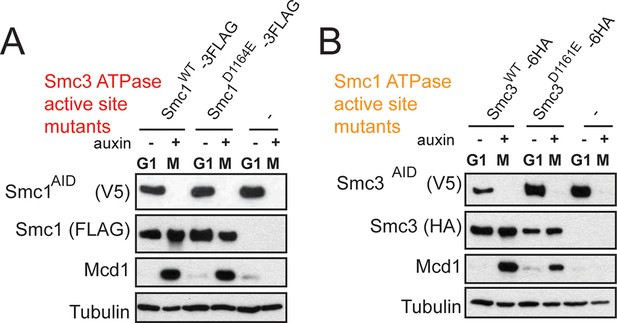
Assessing SMC-AID depletion and Mcd1 levels after depletion.
(A) Haploid cells expressing Smc1AID-3V5 Smc1FLAG (VG3764-3A), Smc1AID-3V5 (VG3311-5D), and Smc1AID-3V5 Smc1D1164E-3FLAG (VG3565-3D) were arrested in G1 and were depleted for Smc1AID-3V5 from G1 through M arrest as described in Figure 4D. Trichloroacetic acid extracted total cell proteins were prepared from G1 cells before auxin addition and from M phase cells under depletion of conditions then analyzed by Western Blot. Depletion of Smc1AID-3V5 protein is monitored using aV5 antibodies (Top panel). Smc1FLAG and Smc1D1164E-3FLAG is monitored using αFLAG antibodies (second panel). Mcd1 was monitored using αMcd1 antibodies and αTUB antibodies were used as a loading control (bottom panel). Mcd1 is absent from G1 extracts due to proteolytic destruction. Mcd1 forms a trimer with Smc1 and Smc3 from S phase through M phase cells, which protects Mcd1 from destruction. When either Smc1 or Smc3 is destroyed (Smc1AID-3V5 only in M phase + auxin), Mcd1 is also destroyed as it cannot be protected. Therefore, Mcd1 presence is a proxy for trimer formation. When either Smc13FLAG or Smc1D1164E-3FLAG are in Smc1AID-3V5 M phase + Auxin cells, Mcd1 is still present. Therefore, Smc1 and Smc1D1164E form the cohesin trimer (i.e support cohesin complex assembly). (B) Haploid cells expressing Smc3AID-3V5 Smc36HA (VG3726-6A), Smc3AID-3V5 (VG3711-5D), and Smc3AID-3V5 Smc3D1161E-6HA (VG3728-2A) were arrested in G1 and were depleted for Smc3AID-3V5 from G1 through M arrest, as in (E). The only difference is that αHA antibodies were used to monitor Smc36HA and Smc3D1161E-6HA. Both Smc3 and Smc3D1161E form the cohesin trimer (support cohesin complex assembly) as Mcd1 is present in M phase + auxin cells.
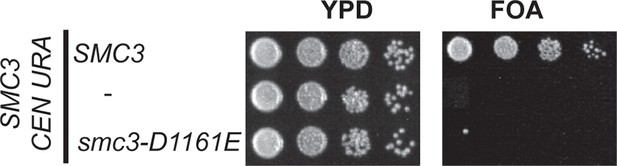
Inviability of smc3-D1161E allele as sole source of SMC3.
Plasmid shuffle assay showing smc3-D1161E cannot support viability as sole SMC3 source. Haploid SMC3 shuffle strain (VG3464-16C) has the endogenous SMC3 deleted but is kept viable by plasmid pEU42 (SMC3 URA3 CEN). This SMC3 shuffle strain alone or with either SMC3 or smc3-D1161E integrated at LEU2 were grown and plated on YPD or FOA plates as described in Figure 4–Figure supplement 1 to assess the ability of smc3-D1161E to support viability as the sole SMC3 after pEU42 loss.
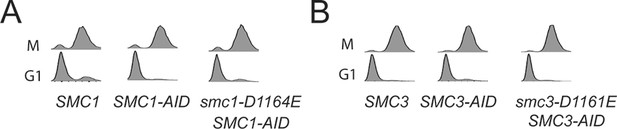
DNA content analysis of cells used in Figure 4E,F.
https://doi.org/10.7554/eLife.11315.021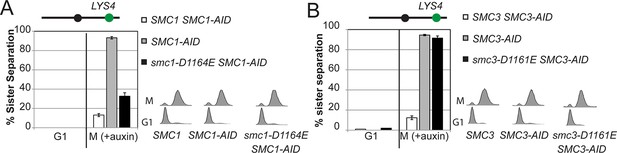
Cohesion assay of smc1-D1164E and smc3-D1161E at a CEN-distal locus.
(A) smc1-D1164E has robust cohesion at the CEN-distal LYS4 locus. Haploid strains from Figure 4B were depleted from Smc1AID-3V5 with auxin, released from G1 and arrested in M phase using nocodazole in continued presence of auxin as described in Figure 4D. The percentage of cells with 2-GFP signals (sister separation) is plotted. The lack of G1 cells with 2-GFP spots demonstrates absence of pre-existing aneuploidy. (B) smc3-D1161E cells are severely defective in cohesion at the CEN-distal LYS4 locus. Haploid strains from Figure 4C were assayed for cohesion as described in A.
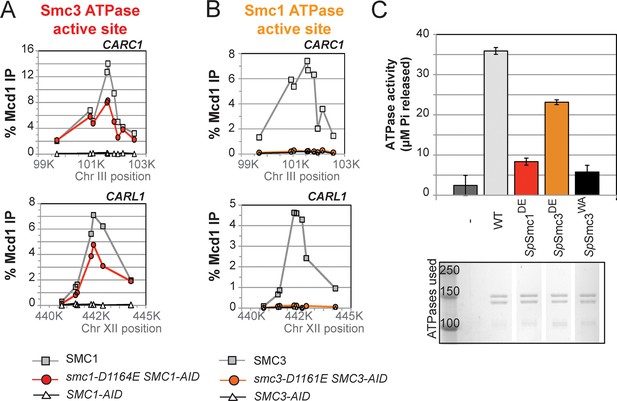
D-to-E mutations in Smc1 and Smc3 ATPase active sites uncouple the level of ATPase activity from chromosome binding.
(A,B) ChIP of Mcd1 in M phase cells depleted for AID tagged proteins. G1 cells were depleted for AID tagged proteins then released under depletion condition and arrested in M phase as described in Figure 4D. M phase cells were fixed and processed for ChIP using Mcd1 antibodies, and the% Mcd1 binding plotted as described in Figure 3—figure supplement 4. (A) ChIP of Mcd1 in smc1-D1164E cells at centromere-proximal CARC1 (top panel) and centromere-distal CARL1 (bottom panel). Haploid M phase cells from Figure 4B, expressing Smc13FLAG Smc1AID (SMC1; light grey line, light grey squares), Smc1-D1164E3FLAG Smc1AID (smc1-D1164E SMC1-AID; red line, red circles), and Smc1AID alone (SMC1-AID; black line, open triangles) were used for ChIP under conditions in which AID-tagged proteins were degraded. (B) ChIP of Mcd1 in M phase in smc3-D1161E cells at centromere-proximal CARC1 (top panel) and centromere-distal CARL1 (bottom panel). Haploid M phase cells from Figure 4C expressing Smc36HA Smc3AID (SMC3; light grey line, light grey squares), Smc3-D1161E6HA Smc3AID (smc3-D1161E SMC3-AID; orange line, orange circles), and Smc3AID alone (SMC3-AID; black line, open triangles) were used for ChIP under conditions in which AID-tagged proteins were degraded. (C) ATPase activity of purified S. pombe cohesin bearing D-loop mutations Psm1-D1167E or Psm3-D1132E, analogous to smc1-D1164E or smc3-D1161E, respectively. Same amount of cohesin was used in ATPase experiments (lower panel). Cohesin complexes were purified from cells overexpressing wild type S. pombe cohesin (WT) or S. pombe cohesin with mutations analogous with Smc3 ATPase active site D-loop-E mutation (SpSmc1DE) or Smc1 ATPase active site D-loop mutation (SpSmc3DE). ATPase assays were carried out in ATPase buffer 1 for 2 hours at 30°C. Cohesin with a K-to-I mutation in the Walker A motif of Smc3 ATPase active site (SpSmc3WA, Psm3 K38I in S. pombe) abolished most, if not all, ATPase activity. Coomassie-stained protein bands were spliced from the same gel for representation purposes. Please see figure supplement 1 for further characterization of chromosomal association of cohesin in smc1-D1164E or smc3-D1161E. Figure supplement 2 shows ATPase activity of Rad50 protein when the D-loop residue is mutated to an E or an A.
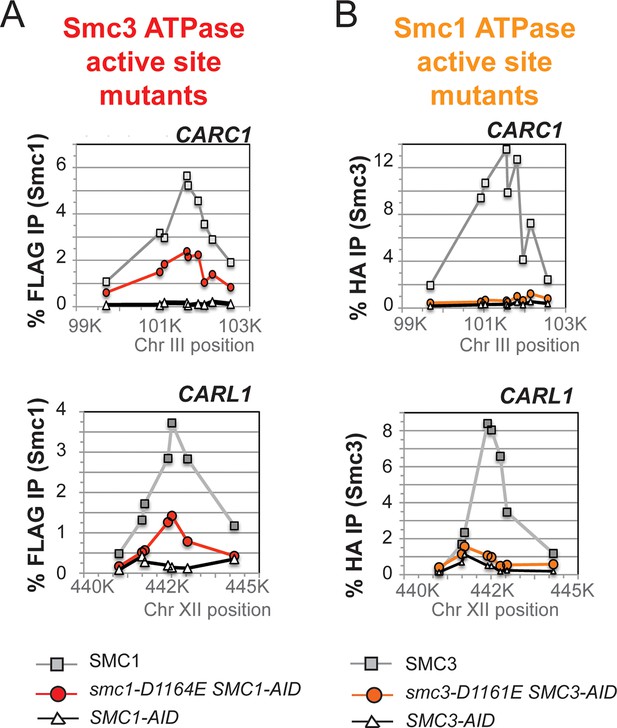
Chromosomal binding of cohesin in smc1-D1164E and smc3-D1161E mutants assayed by antibodies against tagged Smc1 and Smc3 subunits.
(A) ChIP of 3Flag tagged Smc1 in M phase cells depleted for Smc1-AID. Cells from Figure 5A were processed for ChIP using anti-FLAG antibodies and% Flag tagged Smc1 binding plotted as described for Mcd1 in Suppl. Figure 2F. ChIP of Smc13FLAG and smc1-D1164E3FLAG at chr. III centromere-proximal CARC1 (top panel) and chr. XII centromere-distal CARL1 (bottom panel). Smc13FLAG Smc1AID (SMC1; light grey line, open squares), Smc1-D1164E3FLAG Smc1AID (smc1-D1164E SMC1-AID; dark grey line, grey circles), and Smc1AID alone (SMC1-AID; black line, open triangles). (B) ChIP of 6HA tagged Smc3 in M phase cells depleted for Smc3-AID. Cells from Figure 5B were processed for ChIP using anti-HA antibodies and% HA tagged Smc3 binding plotted as described for Mcd1 in Figure 3—figure supplement 4. ChIP of Smc36HA and smc3-D1161E6HA at chr. III centromere-proximal CARC1 (top panel) and chr. XII centromere-distal CARL1 (bottom panel). Smc36HA Smc3AID (SMC3; light grey line, open squares), Smc3-D1161E6HA Smc3AID (smc3-D1161E SMC3-AID; dark grey line, grey circles), and Smc3AID alone (SMC3-AID; black line, open triangles).
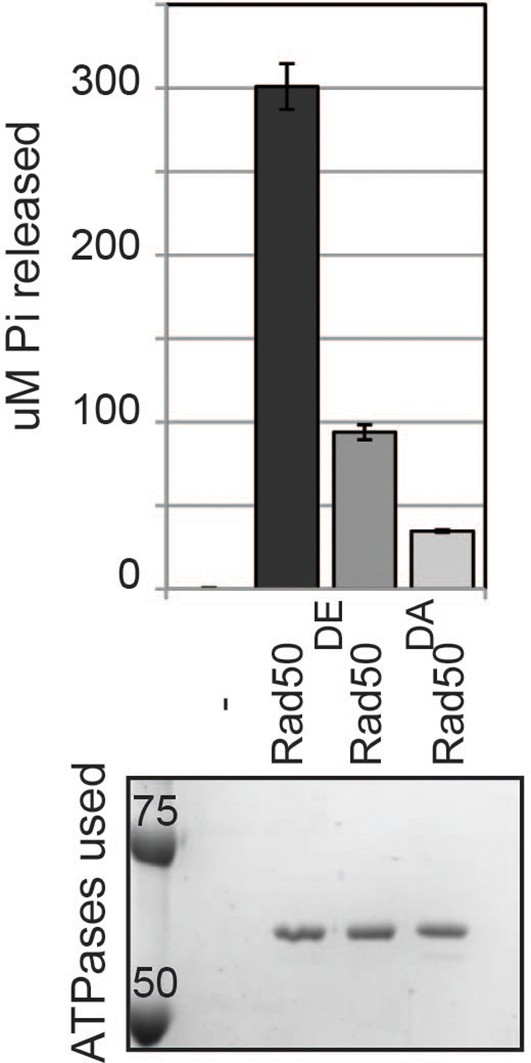
ATPase activity of wild type and D-loop mutant Rad50 homodimer.
ATPase assays were carried out with purified Rad50 homodimer from T4 bacteriophage in ATPase buffer 1 for 2 hours at 30°C.
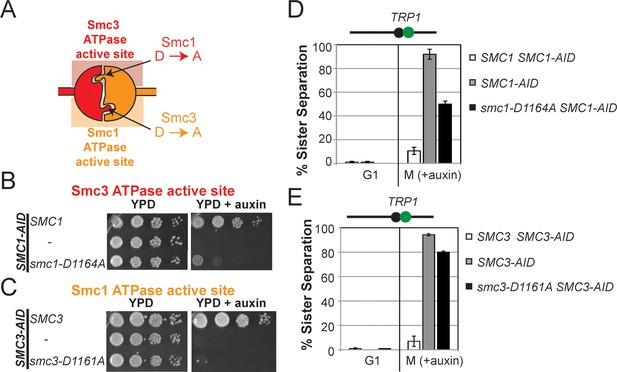
D-loop-A (DA) mutations perturb cohesin function more severely than D-loop D to E (DE) mutations
(A) Cartoon representation of the D-to-A substitution mutants in the Smc3 ATPase active site (smc1-D1164A) and Smc1 ATPase active site (smc3-D1161A). (B,C) Assessing whether the DA D-loop mutants can support viability. (B) Smc3 ATPase active site D-loop-A mutant, smc1-D1164A, failed to promote viability. SMC1-AID smc1-D1164A (VG3766-3C) cells were grown and dilution plated as described in Figure 4B. SMC1-AID SMC1(VG3764-3A), SMC1-AID (VG3311-5D) cells were re-plated here for comparison. The smc1-D1164A SMC1-AID was moved from a different region of this same plate for clarity of presentation. (C) Smc1 ATPase active site D-loop-A mutant, smc3-D1161A, failed to promote viability. SMC3-AID smc3-D1161A (VG3772-13A) cells were grown and dilution plated. SMC3-AID SMC3(VG3726-6A) and SMC3-AID (VG3711-5D) cells were re-plated here for comparison. The smc3-D1161A SMC3-AID was moved from a different region of this same plate for clarity of presentation. (D,E) Cohesion loss at the CEN-proximal locus (TRP1) in M phase cells depleted for AID tagged proteins from G1 through M phase as described in Figure 4D. The percentage of cells with two GFP spots (sister separation) was plotted. (D) Cohesion loss in smc1-D1164A cells at the CEN-proximal TRP1 locus. Haploid strains SMC1 SMC1-AID (VG3794-2E), SMC1-AID (VG3711-5D) and smc1-D1164A SMC1-AID (VG3796-1F) assayed for cohesion. (E) Cohesion loss in smc3-D1161A cells at the CEN-proximal TRP1 locus. Haploid strains SMC3 SMC3-AID (VG3797-1A), SMC3-AID (VG3651-3D) smc3-D1161A SMC3-AID (VG3798-2B) assayed for cohesion. Note: smc1-D1164A SMC1-AID and smc3-D1161A SMC3-AID cells were analyzed in the same experiments as Figure 4D,E, respectively. smc-DA data was omitted from Figure 4 but presented here with controls from those experiments for clarity of presentation. DNA content analysis of cells in Figure 6D,E is shown in Figure 6—figure supplement 3. Please see Figure supplements 1–6 for further characterization of smc1-D1164A and smc3-D1161A alleles.
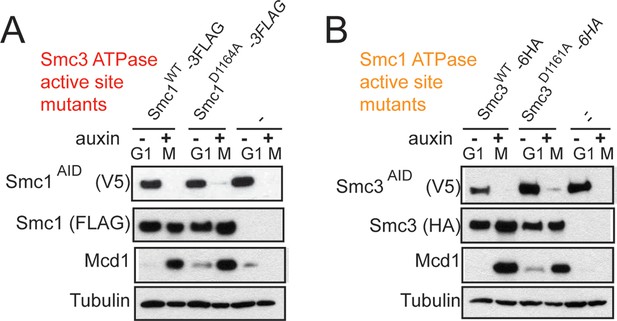
Assessing SMC-AID depletion and Mcd1 levels after depletion in DA strains.
(A) Western blotting of extracts from cells expressing Smc1AID-3V5 in G1 (-auxin) and M (+auxin), as in Figure 4—figure supplement 2. Cells expressing Smc1AID-3V5 Smc1D1164A-3FLAG (VG3566-3C) had Mcd1 in M phase after being treated with auxin to deplete Smc1AID-3V5, indicating that cohesin complex assembly is not perturbed. (B) Western blotting of extracts from cells expressing Smc3AID-3V5 in G1 (-auxin) and M (+auxin). Cells expressing Smc3AID-3V5 Smc3D1161A-6HA (VG3731-5B) that were treated with auxin to deplete Smc3AID-3V5 had Mcd1 in M phase, indicating that cohesin complex assembly is not perturbed.
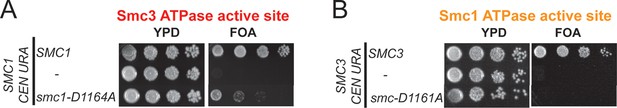
Characterization of smc1-D1164A and smc3-D1161A mutants.
(A) Plasmid shuffle assay showing that smc1-D1164A is severely compromised for viability as the sole SMC1 source. Haploid SMC1 shuffle strain (VG3568-8A) alone or with an integrated copy of either SMC1 or smc1-D1164A were grown and plated on YPD or FOA plates to assess viability in the absence of the covering plasmid (SMC1 URA3 CEN) as described in Figure 4—figure supplement 1. (B) Plasmid shuffle assays showing that smc3-D1161A is inviable as sole source of Smc3. Haploid SMC3 shuffle strain (VG3464-16C) alone or with an integrated copy of either SMC3 or smc3-D1161A were grown and plated on YPD or FOA plates to assess viability in the absence of the covering plasmid (SMC3 URA3 CEN) as described in Figure 4—figure supplement 3.
DNA content analysis of cells in Figure 6D(A) and Figure 6E(B).
https://doi.org/10.7554/eLife.11315.029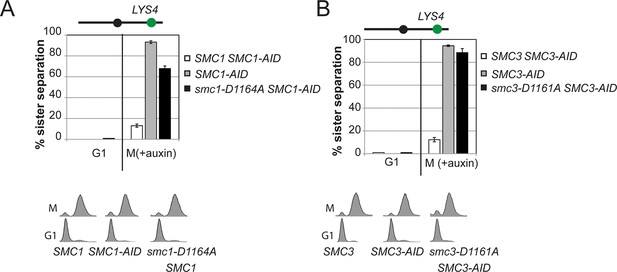
Cohesion of smc1-D1164A and smc3-D1161A at the CEN-distal locus.
(A) smc1-D1164A cells arrested in M phase + auxin have a large cohesion defect at the CEN-distal LYS4 locus. Haploid strains from Figure 6B were depleted from Smc1AID-3V5 with auxin, released from G1 and arrested in M phase + auxin as described in Figure 4D. The percentage of cells with 2-GFP signals (sister separation) is plotted. (B) smc3-D1161A cells arrested in M phase + auxin have no cohesion at the CEN-distal LYS4 locus. Haploid strains from Figure 6C were assayed for cohesion as described. Note: smc1-D1164A SMC1-AID and smc3-D1161A SMC3-AID cells were analyzed in the same experiments as Figure 4—figure supplement 5. smc-DA data was omitted earlier but presented here with controls from those experiments for clarity of presentation.
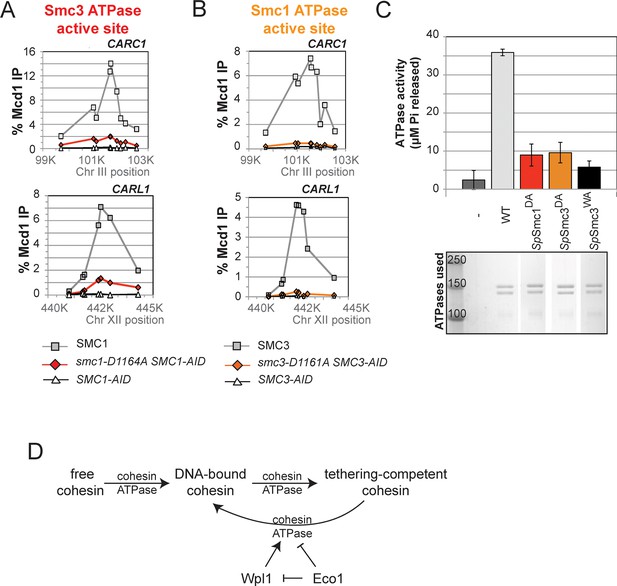
DA mutations in Smc1 and Smc3 ATPase active sites abolish chromosome binding and ATPase activity.
(A) ChIP of Mcd1 in M phase smc1-D1164A cells grown in auxin-containing media. Haploid M phase cells from Figure 6B were fixed and processed for ChIP using Mcd1 antibodies.% Mcd1 binding plotted as described in Figure 3—figure supplement 4. Mcd1 ChIP at centromere-proximal CARC1 (top panel) and centromere-distal CARL1 (bottom panel). (B) ChIP of Mcd1 in M phase smc3-D1161A cells grown in auxin-containing media. Haploid M phase cells from Figure 6C were fixed and processed for ChIP. Mcd1 ChIP at centromere-proximal CARC1 (top panel) and centromere-distal CARL1 (bottom panel). (C) ATPase activity of purified cohesin complexes from S. pombe bearing D-loop-A mutations in Smc3 and Smc1 ATPase sctive sites. Psm1 D1167A (SpSmc1DA) and Psm3 D1132A (SpSmc1DA), analogous to smc1-D1164A and smc3-D1161A, respectively, were purified from overexpression strains listed in Supplementary file 1. Same amount of cohesin was used in the ATPase experiments (lower panel). ATPase assays were carried out in ATPase buffer 1 for 2 hours at 30°C. ATPase activity of wild type (WT) and Walker A-mutant (SpSmc3WA) cohesin was represented here for comparison. Bands were spliced from the same gel for representation purposes. (D) Model for how the two ATPase active sites regulate cohesin function. Free cohesin is converted to a stable DNA-bound form by the action of the loader complex (not shown) and ATP binding/hydrolysis by ATPase active sites. A particular unknown nucleotide state at the Smc3 ATPase active site induces the tethering (cohesive) form. Upon finding the correct sister to be paired with, the Eco1-mediated acetylation of Smc3 leads to the stabilization of this cohesive state. Absent this stabilization, the Smc3 ATPase active site destabilizes the tethering form or induces cohesin dissociation from chromosomes. Wpl1 could either promote this Smc3 ATPase active site function or destabilize the cohesin bound to DNA in the non-tethering form. Figure supplement 1 shows cohesin binding to DNA in smc1-D1164E and smc3-D1161E strains.
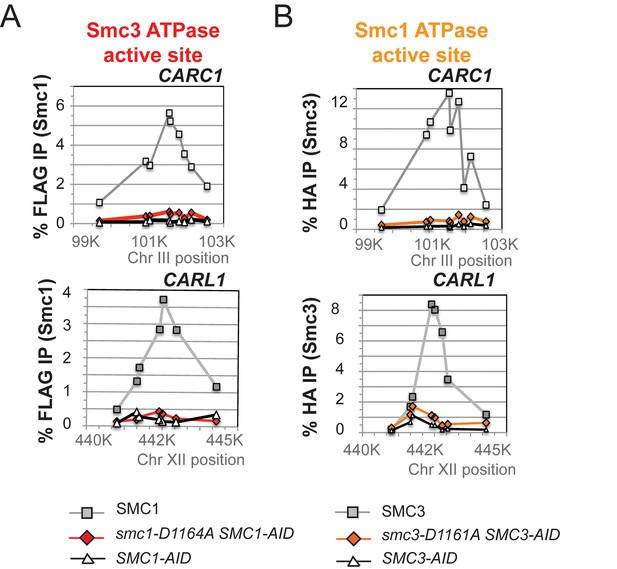
Chromosomal binding of smc1-D1164A and smc3-D1161A mutants assayed with antibodies against tagged Smc proteins.
(A) ChIP of Smc13FLAG in M phase smc1-D1164A cells at centromere-proximal CARC1 and centromere-distal CARL1. Haploid M phase cells from Figure 6B were fixed and processed for ChIP using anti-FLAG antibodies. (B) ChIP of Smc36HA in M phase smc3-D1161A cells at centromere-proximal CARC1 and centromere-distal CARL1. Haploid M phase cells from Figure 6C were fixed and processed for ChIP using anti-HA antibodies.
Additional files
-
Supplementary file 1
Strain table.
- https://doi.org/10.7554/eLife.11315.033





