Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/α-albumin
Figures
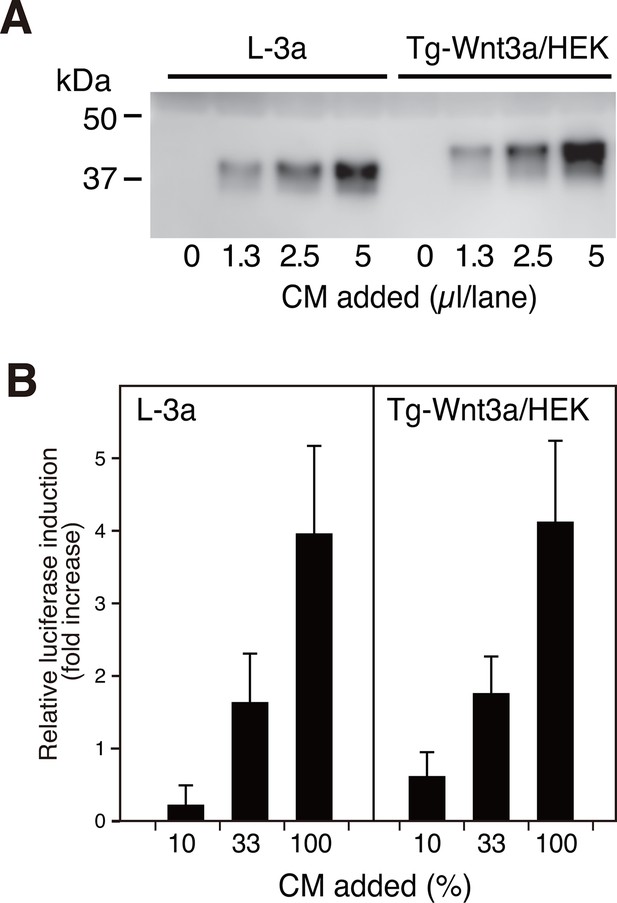
N-terminally tagged Wnt3a secretion from HEK cells.
(A) Indicated amounts of CM from the confluent L cells stably expressing untagged Wnt3a (L-3a) or HEK293S GnT1- cells stably expressing TARGET-tagged Wnt3a (Tg-Wnt3a/HEK) were subjected to a Western blotting using anti-mouse Wnt3a antibody. Note that Tg-Wnt3a migrate slower than the untagged Wnt3a due to the presence of extra 35-residue (~4 kDa) tag sequence. (B) The stable TCF reporter cells were incubated with the indicated concentration of CM for 6 hr. Luciferase activities in the cell lysates were determined and expressed as the relative increase from the control value obtained in the mock-treated cells. Data are mean ± SD of three independent experiments, in which quadruplicate determinations were made. See also Figure 1—source data 1.
-
Figure 1—source data 1
The Excel spreadsheet source file for Figure 1B.
- https://doi.org/10.7554/eLife.11621.004
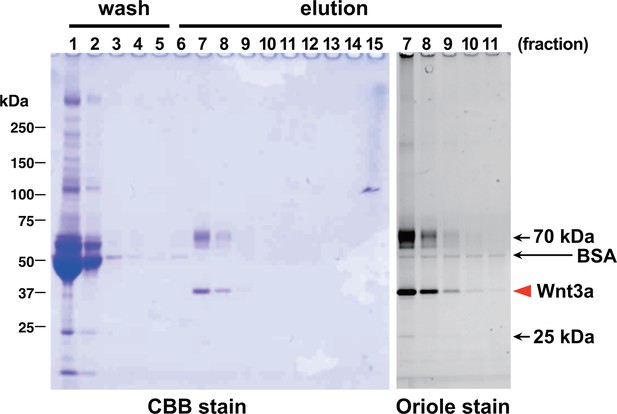
Affinity purification of tagged Wnt3a from the culture supernatants.
A small column of Sepharose coupled with anti-TARGET tag antibody P20.1 (~3 ml) that had been incubated with ~220 ml of CM from the confluent culture was washed with TBS, followed by elution with TBS containing 0.2 mg/ml competing C8 peptide. Ten µl sample from each fraction (fraction size = 3 ml) was subjected to 5–20% SDS-PAGE under nonreducing condition and stained with Coomassie Blue. A portion of the same gel (fractions 7–11) was stained with Oriole fluorescent stain to visualize minor contaminating bands.
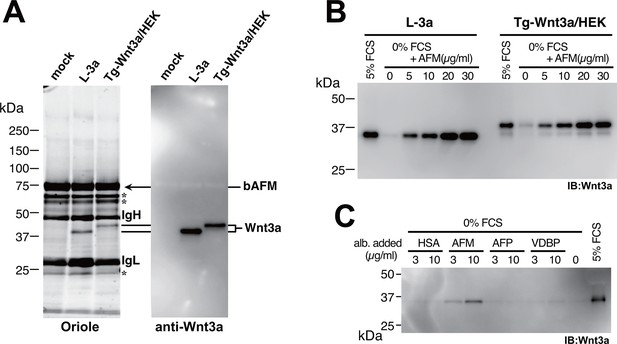
Serum afamin binds to Wnt3a secreted from cells.
(A) Serum-containing CM from the mock, L-3a, or Tg-Wnt3a/HEK cells were incubated with Sepharose beads immobilized with an anti-bovine AFM monoclonal antibody (clone B91) and the immunoprecipitated materials were separated by SDS-PAGE under reducing condition, followed by Oriole Fluorescent protein stain (left) and anti-Wnt3a immunoblotting (right). Positions for bovine AFM and tagged/untagged Wnt3a are shown in the right. Asterisks denote nonspecific serum-derived proteins. (B) L-3a or Tg-Wnt3a/HEK cells were cultured in DMEM containing various concentrations of serum or purified recombinant human AFM for 5 days, and the resultant CMs (3 µl) were subjected to the anti-Wnt3a immunoblotting. (C) Effect of various albumin family proteins to support Wnt3a secretion from L-3a cells is evaluated as in (B). HSA, human serum albumin; AFM, human afamin; AFP, human α-fetoprotein; VDBP, mouse vitamin D binding protein.
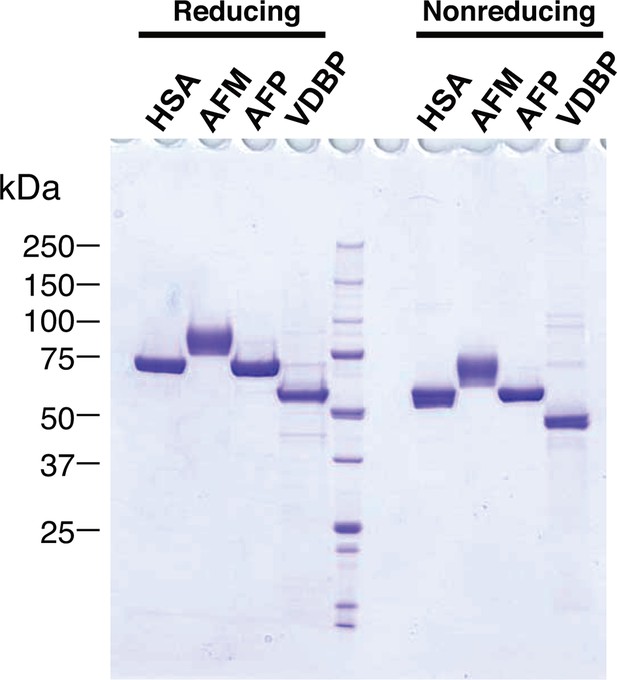
Purified recombinant albumin family proteins.
Purities of the recombinant albumin family proteins used in the experiment shown in the Figure 3C were checked by running on 5–20% SDS-PAGE under reducing (left) and nonreducing (right) conditions, followed by Coomassie Blue staining.
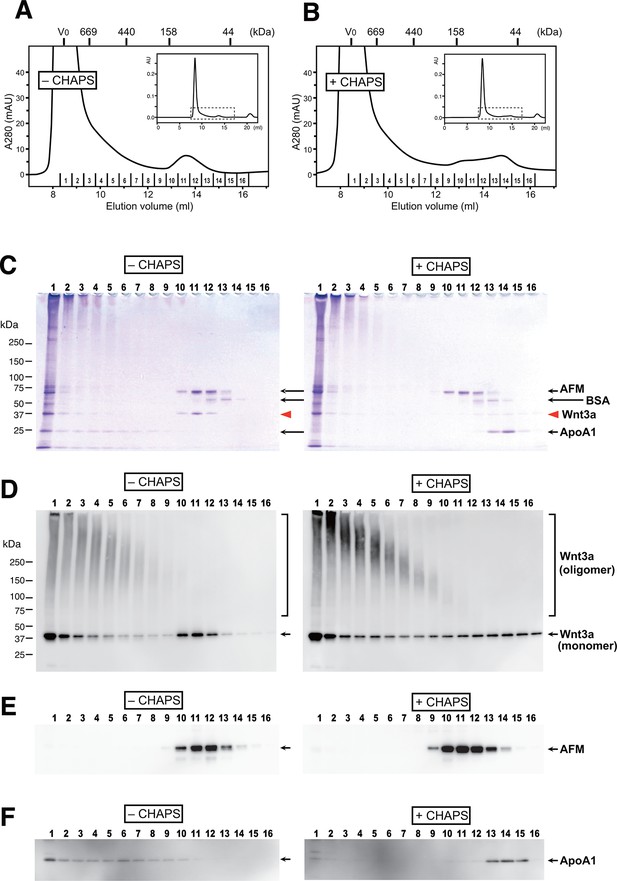
AFM and Wnt3a form stable 1:1 complex in the absence of detergents.
(A,B) SEC profiles of affinity-purified Wnt3a preparation in the absence (A, –CHAPS) or presence (B, +CHAPS) of 1% CHAPS. Graphs are expanded view of the areas indicated by dotted line box in the whole chromatograms (insets). Elusion positions for molecular mass standards including thyroglobulin (669 kDa), ferritin (440 kDa), aldorase (158 kDa) and ovalbumin (44 kDa) are indicated at the top. Sixteen fractions collected from each chromatography are subjected to nonreducing SDS-PAGE on 5–20% gradient gels, followed by Coomassie Blue staining (C) or immunoblotting with anti-Wnt3a (D), anti-bovine AFM (E), or anti-bovine ApoA1 (F). In (C) ~ (F), analysis of the samples from (A) and (B) are shown in the left (–CHAPS) or right (+CHAPS) panels, respectively.
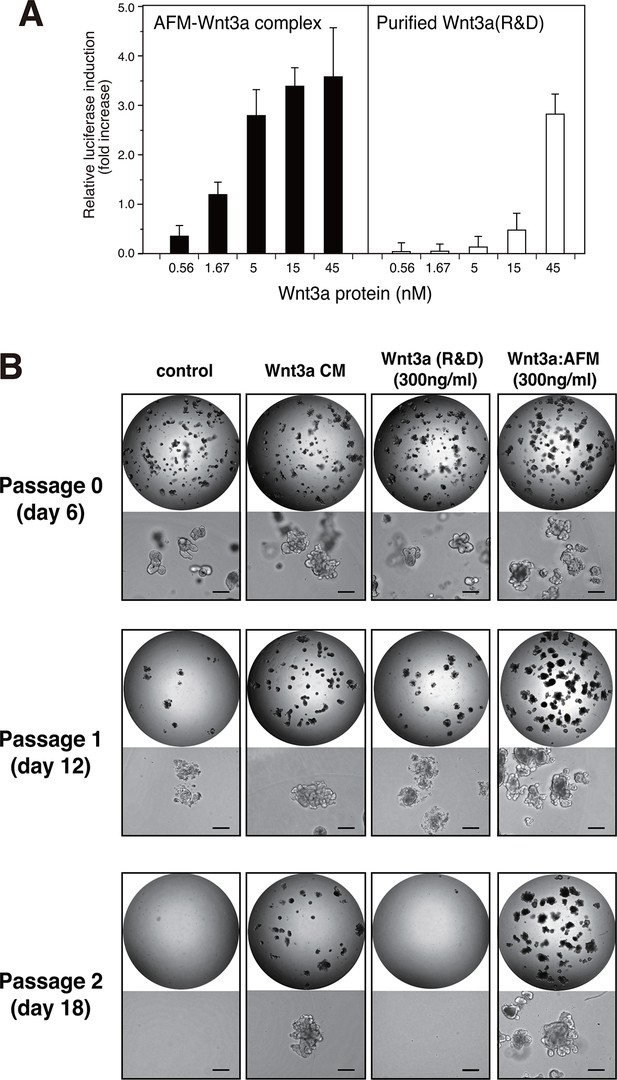
Wnt3a in complex with AFM is biologically active.
(A) Comparison between the purified Wnt3a from a commercial source and the AFM-Wnt3a complex. Increasing concentrations of Wnt3a proteins are added to TCF reporter cells and the Wnt signaling activities are evaluated as in Figure 1B. The data are mean ± SD of three independent experiments, in which quadruplicate determinations were made. See Figure 5—source data 1. (B) AFM-Wnt3a complex supports self-renewal of human intestinal stem cells. The ability of single-cell dissociated human intestinal organoids to expand was evaluated by culturing in the absence (control) or presence of various forms of Wnt3a preparations. Photographs are taken every 7 days at a low (x2, entire view of one 48-well, upper panels) and the high (x 10, lower panels) magnifications. Bar: 200 µm.
-
Figure 5—source data 1
The Excel spreadsheet source file for Figure 5A.
- https://doi.org/10.7554/eLife.11621.010
-
Figure 5—source data 2
The Excel spreadsheet source file for Figure 5—figure supplement 1.
- https://doi.org/10.7554/eLife.11621.011
-
Figure 5—source data 3
The Excel spreadsheet source file for Figure 5—figure supplement 2.
- https://doi.org/10.7554/eLife.11621.012
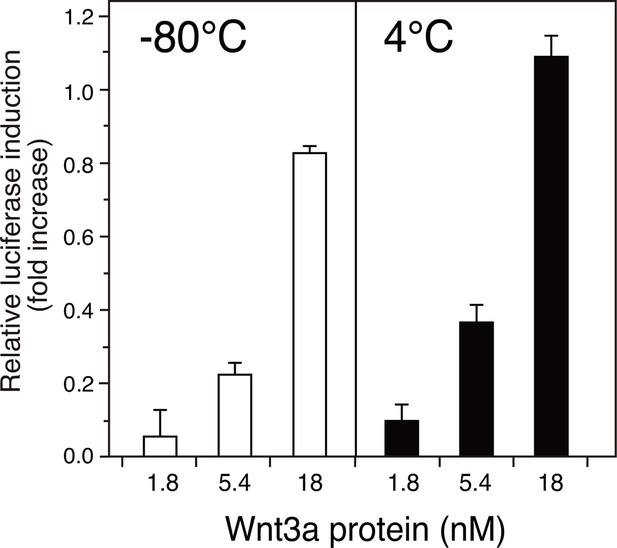
Stability of the AFM-Wnt3a complex.
The AFM-Wnt3a complex preparation was flash-frozen and stored at -80°C (left panel, white bars) or stored in a refrigerator at 4°C for 1 month (right panel, black bars) after the purification, and subjected to the reporter assay using HEK293T cells transiently transfected with reporter plasmids. Briefly, HEK293T cells seeded in 24-well plates were transfected with 50 ng of TOPFlash plasmid (Upstate) together with 5 ng of the Renilla luciferase construct, pRL-TK (Promega). Eighteen hours after the transfection, culture media were gently replaced with the test reagents described above diluted in a fresh DMEM containing 10% FCS. The test reagents were added and incubated with the reporter-transfected cells for an additional 18 hours prior to the harvest and cell lysis. Reporter activities were measured using a dual luciferase reporter assay system (Promega). The data are mean ± SD (n = 4). See Figure 5—source data 2.
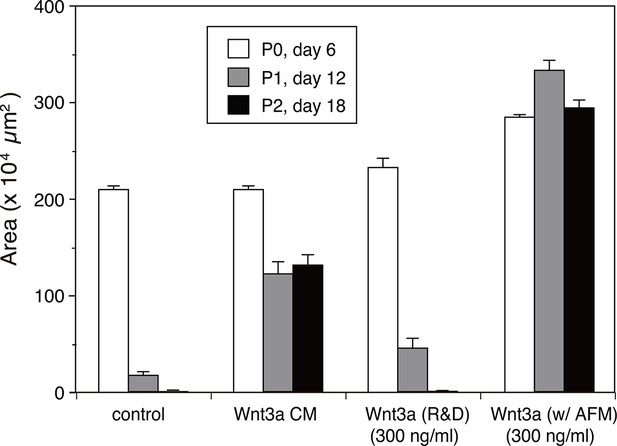
Quantitative analysis of the human intestinal organoid growth and expansion.
The organoid growth was evaluated by measuring the total areas of viable organoids per well from the photographs taken at each experimental point shown in Figure 5B. The data are mean ± S.E.M. from triplicates. See Figure 5—source data 3.
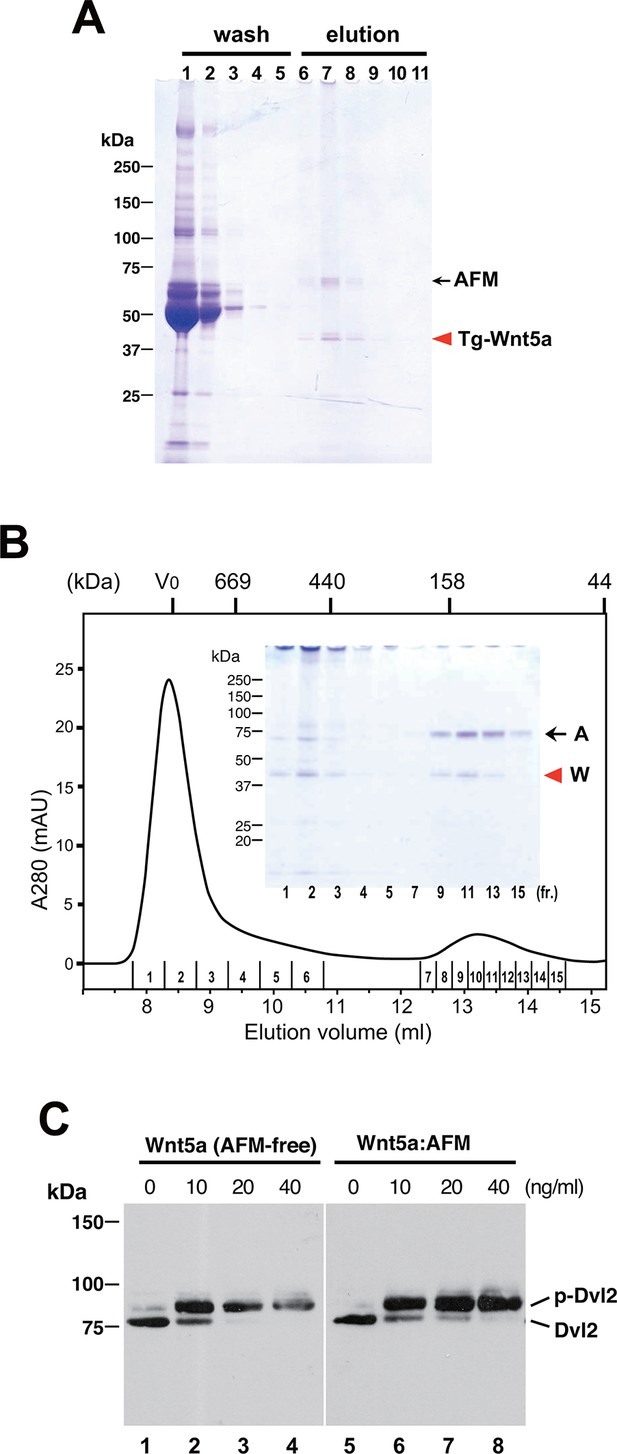
Serum AFM binds and solubilize Wnt5a.
(A) Purification of Tg-Wnt5a from the CM of stable HEK cell line was conducted as in the case for Tg-Wn3a, and the resulting column fractions were analyzed by SDS-PAGE and Coomassie Blue staining. Positions for the 70-kDa bovine AFM and the 40-kDa mouse Wnt5a are shown at the right. (B) SEC profile of the tag-purified Wnt5a. Note that the ~150-kDa peak (fractions 9–13) contains roughly equimolar amounts of AFM (A) and Wnt5a (W) proteins as judged by the non-reducing SDS-PAGE (inset). The 40-kDa Wnt5a was present in both HMW and monodispersed fractions. See also Figure 6—figure supplement 1. (C) Biological activity of AFM-Wnt5a complex. NIH3T3 cells were incubated with either AFM-free, untagged Wnt5a (lanes 1–4) or the purified Tg-Wnt5a:AFM complex (lanes 5–8) at increasing concentrations. The level of cellular Dvl2 phosphrylation was assessed by the mobility shift of Dvl2 band in the anti-Dvl2 immunoblots using the whole cell lysates.
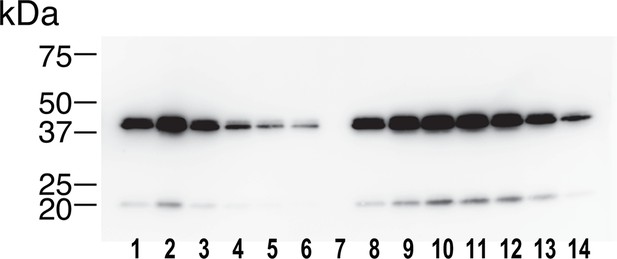
Identification of the 40-kDa band as Wnt5a.
The SEC fractions 1–14 in Figure 6B were subjected to an immunoblotting with anti-mouse Wnt5a monoclonal antibody. The minor band migrating at ~20 kDa likely represents a truncated fragment of Wnt5a.
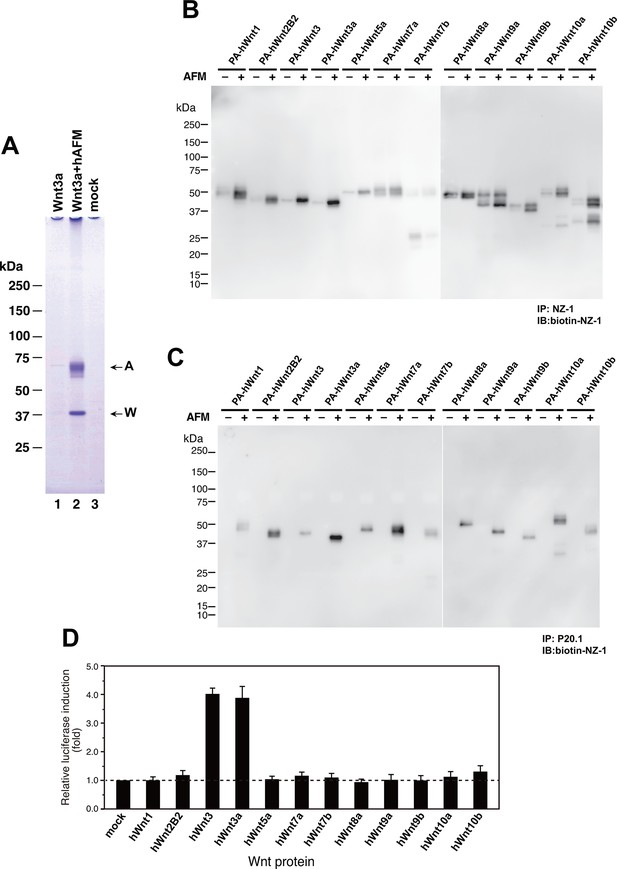
Increased production/secretion of various Wnt proteins by the co-expression with AFM.
(A) Expi293F cells were transiently transfected with PA-tagged mouse Wnt3a (lane 1), PA-tagged mouse Wnt3a + Tg-tagged human AFM (lane 2), or mock (lane 3) plasmids under the serum-free condition and the resultant culture supernatants were subjected to the immunoprecipitation with anti-PA tag antibody NZ-1 and analyzed on a nonreducing 5–20% SDS-PAGE gel followed by Coomassie Blue staining. A, Tg-hAFM; W, PA-mWnt3a. (B–D) AFM co-expression facilitates various Wnt secretion. N-terminally PA-tagged human Wnts are transiently co-transfected with Tg-tagged human AFM into Expi293F cells, and the culture media are immunoprecipitated with either anti-PA NZ-1 (B) or anti-Tg P20.1 (C), followed by immunoblotting with biotinylated NZ-1 to visualize PA-tagged Wnts. In (D), the same set of culture media are subjected to the TCF reporter assay as in Figure 1B, to see if they can induce Wnt/β-catenin signaling. The data are mean ± SE (n = 4) from a representative experiment. See Figure 7—source data 1.
-
Figure 7—source data 1
The Excel spreadsheet source file for Figure 7D.
- https://doi.org/10.7554/eLife.11621.018
-
Figure 7—source data 2
The Excel spreadsheet source file for Figure 7—figure supplement 2.
- https://doi.org/10.7554/eLife.11621.019
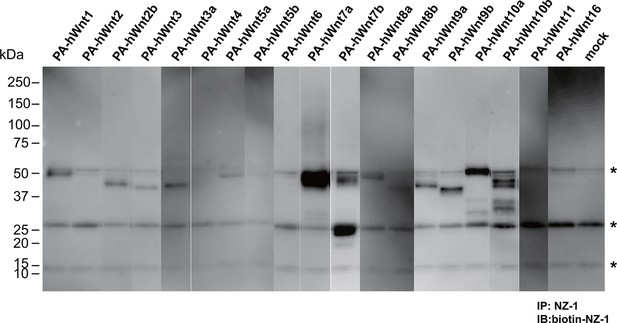
Expression/secretion profile of all human Wnt constructs in the presence of serum.
N-terminally PA-tagged human Wnt constructs (constructs #4 - #22 shown in Supplementary file 1) are singly transfected into HEK293T cells in the presence of 10% bovine serum and the resultant culture supernatants were subjected to the immunoprecipitation with NZ-1 followed by immunoblotting with biotinylated NZ-1 as in Figure 7B. Asterisks denote nonspecific bands present in all samples including that obtained with mock-transfected culture supernatant.
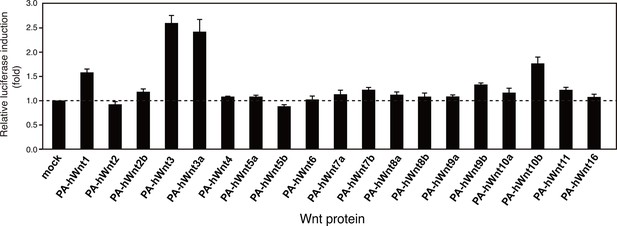
Signaling activity of 19 PA-tagged Wnts in an autocrine type assay.
HEK293T cells seeded in 96-well plates were simultaneously transfected with 10 ng of TOPFlash plasmid, 0.5 ng of the Renilla luciferase construct, and 100 ng of N-terminally PA-tagged human Wnt constructs (constructs #4 - #22 shown in Supplementary file 1) using Lipofectamine2000 (ThermoFisher Scientific, Inc.). Eighteen hours after the transfection, culture media were gently removed and the cells were washed once with PBS, followed by cell lysis and the determination of the luciferase activity using a dual luciferase reporter assay system (Promega). The data are mean ± SD (n = 4). See Figure 7—source data 2.
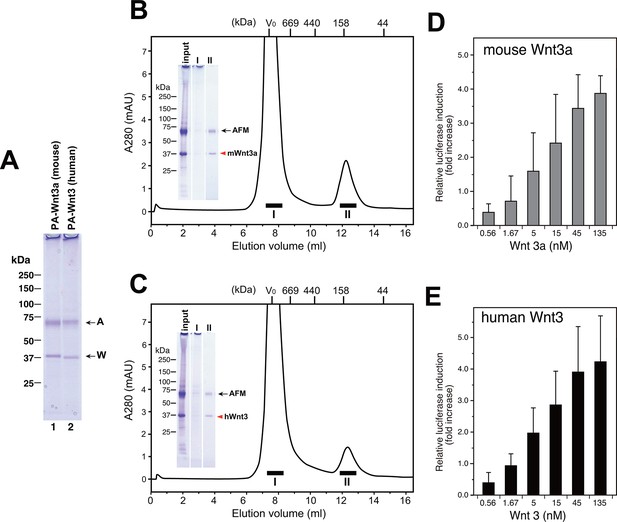
Large scale production of Wnt3a and Wnt3 by using AFM co-expression method.
(A) A 300 ml culture of Expi293F cells were used to co-express Tg-tagged human AFM and PA-tagged mouse Wnt3a or human Wnt3. The AFM-Wnt complexes were purified from the co-transfected culture media using NZ-1-immunoaffinity resin and checked for its purity on a nonreducing 5–20% SDS-PAGE gel followed by Coomassie Blue staining. (B,C) SEC profile of the NZ-1-purified AFM-Wnt3a (B) or AFM-Wnt3 (C) complex. The eluted material from the NZ-1 immunoaffinity chromatography (input, same as the sample shown in (A)) was subjected to the SEC on Superdex 200, showing two peaks. The void peak (fraction I) comprised only of HMW aggregates that barely enter the top of separation gel, while the ~140-kDa peak (fraction II) contained AFM and Wnt3a/Wnt3 at similar amounts. (D,E) Signaling activity of AFM-Wnt complex prepared by AFM co-expression method. The NZ-1-purified AFM-Wnt3a (D) or AFM-Wnt3 (E) complex was subjected to the TCF reporter assay as in Figure 5A. The data are mean ± SD of three independent experiments, in which quadruplicate determinations were made. See Figure 8—source data 1.
-
Figure 8—source data 1
The Excel spreadsheet source file for Figure 8D and E.
- https://doi.org/10.7554/eLife.11621.023
Additional files
-
Supplementary file 1
Construct design of tagged Wnt proteins.
Amino acid sequence of the N-terminal regions for the all tagged Wnt protein constructs used in this study starting at the initiation Met are shown.
- https://doi.org/10.7554/eLife.11621.024





