The tumor suppressor PTEN and the PDK1 kinase regulate formation of the columnar neural epithelium
Figures
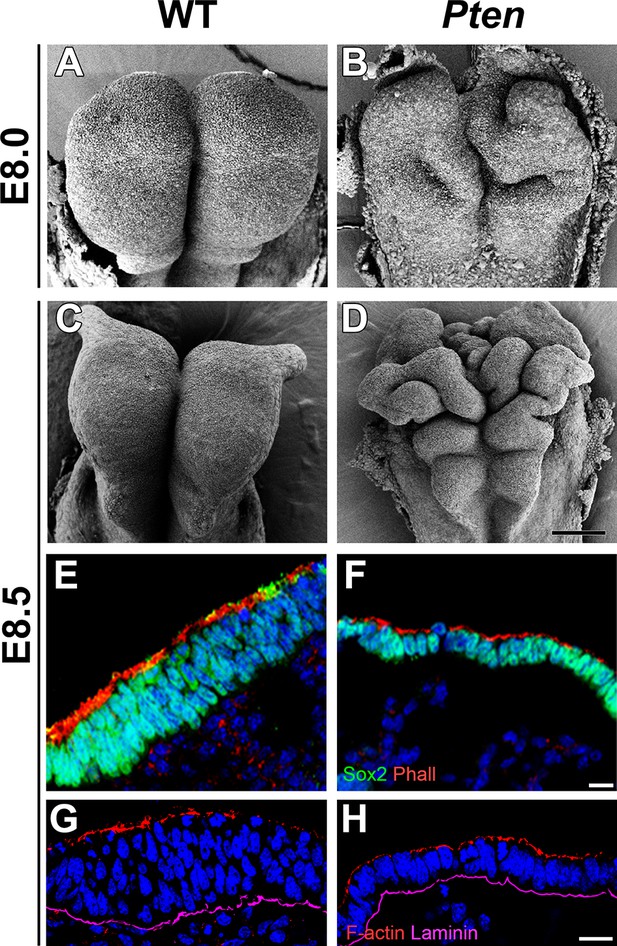
Morphological defects in the Pten mutant cephalic neural plate.
(A, B, C, D) Comparison of neural plate morphology of the dorsal head of wild-type (WT) and Pten △Epi mutant embryos at E8.0 and E8.5 in scanning electron microscope images. Scale bar = 100 μm. (E, F) Transverse sections of E8.5 WT and Pten △Epi embryos show the absence of pseudostratified columnar organization in the Pten mutant cephalic neural plate. Green is SOX2, red is phalloidin (F-actin), blue is DAPI. (G, H) Z-stack projection of three optical sections (total of 3 µm) from transverse sections of the cephalic neural plate of E8.5 WT and Pten △Epi mutant embryos stained for phalloidin (red) and laminin (purple). Scale bar E–H = 10 μm.
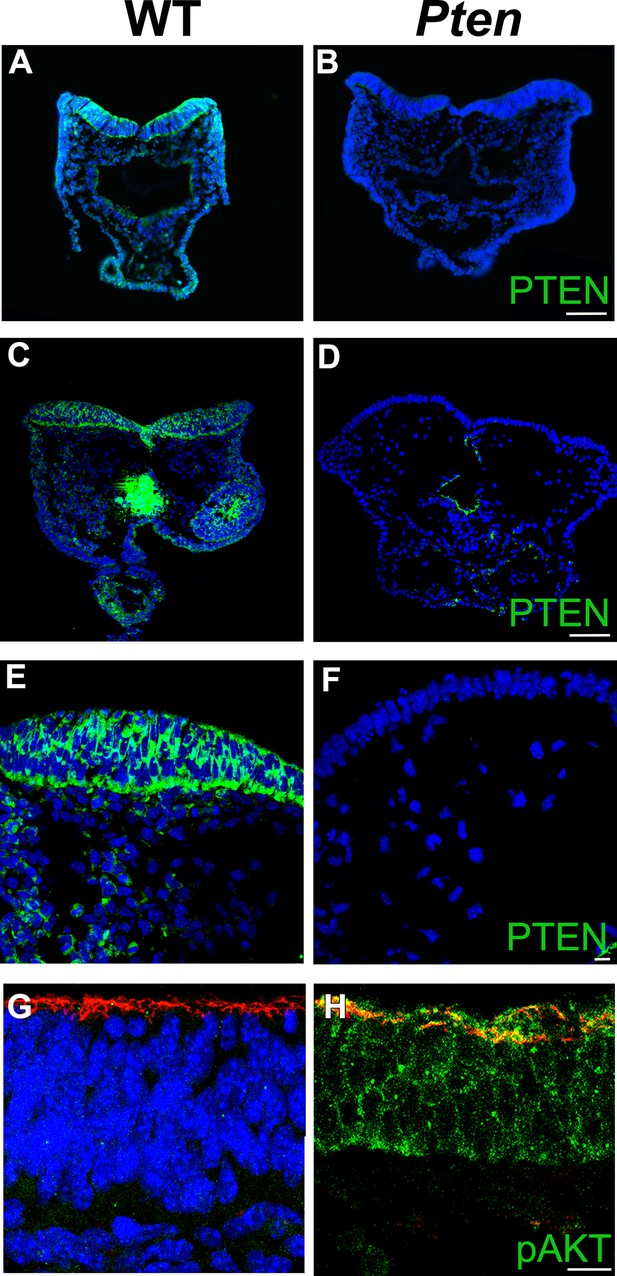
PTEN expression in the cephalic neural plate.
Immunodetection of PTEN in transverse sections of E8.5 WT (A, C, E) and the Pten △Epi embryos (B, D, F) shows the specificity of PTEN staining. (A,B) Antibody from cell signaling; (C–F) antibody from cascade. (G, H) Z-stack projections of 3 optical sections taken every 1 μm, showing expression pAKT S473 expression in transverse sections of the E8.5 WT (G) and Pten △Epi (H) neural plate. Red is phalloidin and blue is DAPI. Scale bar in A–D = 100 μm; in E–H = 10 μm
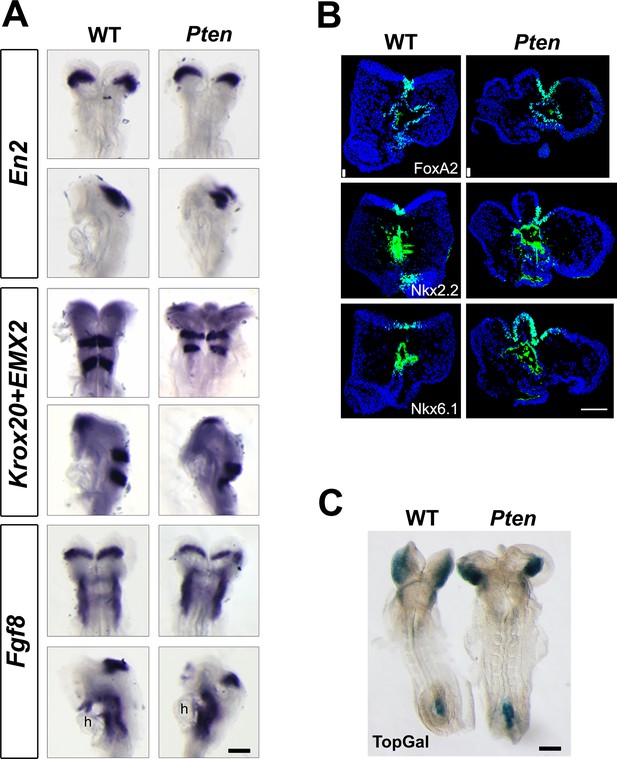
Normal neural patterning in Pten△Epi embryos.
(A) In situ hybridization for Engrailed2, Krox20, Emx2 and Fgf8 in WT and Pten △Epi embryos at E8.5 showed normal anteroposterior regionalization of cephalic neural epithelium. Scale bar = 180 μm. (B) Immunodetection of FoxA2, Nkx2.2 and Nkx6.1 shows normal dorsal-ventral patterning of the E8.5 cephalic neural plate in Pten △Epi embryos. Scale bar = 100 μm. (C) The TOPGAL reporter of canonical Wnt signaling was expressed in the normal domains and at normal levels in E8.5 Pten △Epi embryos. Scale bar = 180 μm.
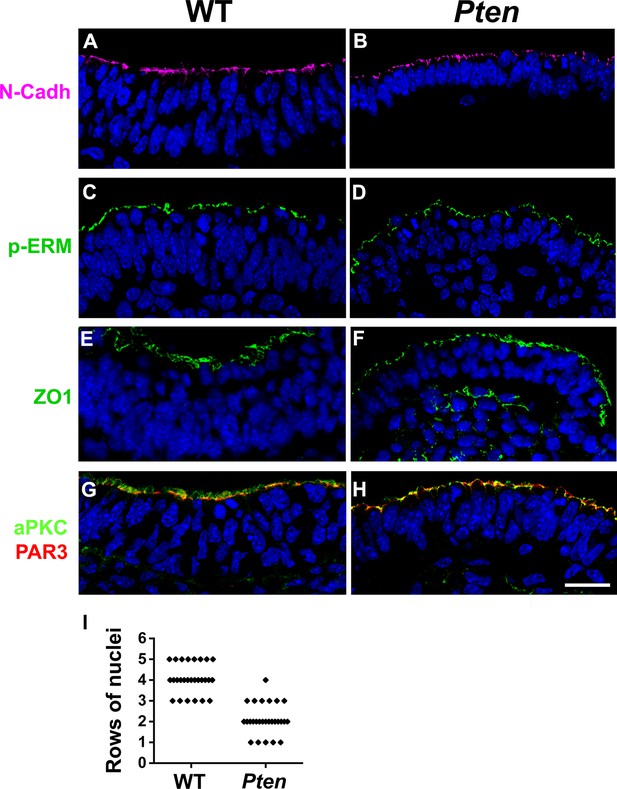
Apical markers in Pten △Epi mutant embryos.
Z-stack projection of three optical sections (total of 3 µm) from transverse sections of the cephalic neural plate of E8.5 WT and Pten △Epi mutant embryos stained for (A, B) N-Cadherin, (C, D) pERM, (E, F) ZO1 and (G, H) aPKC (green) and PAR3 (red). (I) Comparison of the number of rows of nuclei in the pseudostratified cephalic neural plate of E8.5 wild-type and Pten △Epi embryos. n = 27 embryos for each genotype. Blue is DAPI. Scale bar = 20 µm.
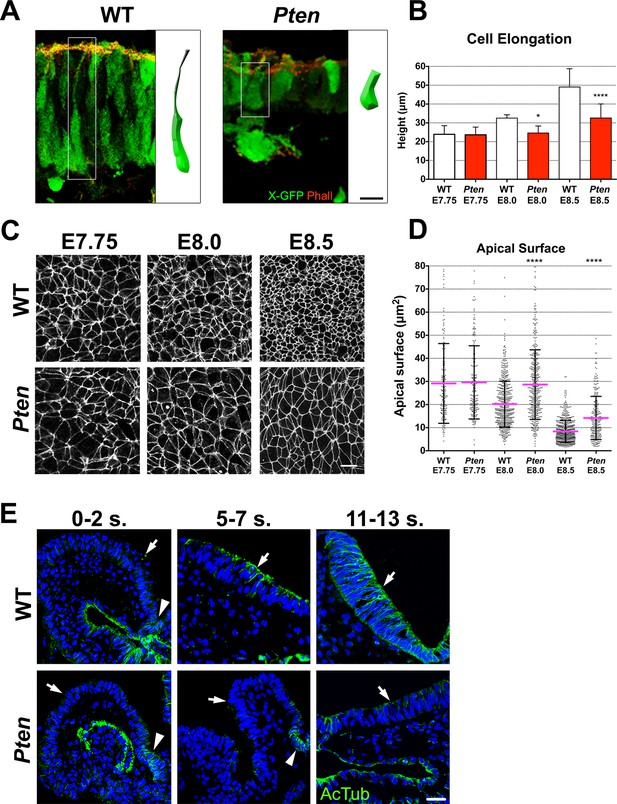
Cellular defects of Pten △Epi mutant neuroepithelial cells.
(A) Comparison of WT and mutant cell shape in the E8.5 cephalic neural plate, using X-linked GFP-expression to mark individual cells. Schematic representations of individual cells for each genotype are shown (white box). Red is phalloidin. Scale bar is 10 μm. (B) Comparison of neural plate height in the cephalic region of WT and mutants. WT E7.75 = 23.9 ± 4.5 μm; Pten △Epi E7.75 = 23.6 ± 4.1 μm: WT and mutant are not different, p = 0.86, by standard t-test. WT E8.0 = 32.5 ± 1.7 μm; Pten △Epi E8.0 = 24.6 ± 3.7 μm: WT is significantly taller than the mutant, *p = 0.0164. WT E8.5 = 49.1 ± 9.6 μm; Pten △Epi E8.5 = 32.6 ± 7.4 μm; WT is significantly taller than the mutant, ****p < 0.0001. For this and similar analyses below, >100 measurements were made from >3 embryos. (C) Comparison of apical cell shape in the cephalic neural epithelium of WT and Pten △Epi embryos viewed en face at E7.75, E8.0 and E8.5. Cell borders are marked by expression of ZO1 (white). Scale bar = 20 μm. (D) Apical surface of cephalic neural epithelial cells, taken from images like those shown in (C). WT E7.75 = 29 ± 17 μm2; Pten △Epi E7.75 = 30 ± 16 μm2: WT and mutant are not different, p = 0.79. WT E8.0 = 20 ± 10 μm2; Pten △Epi E8.0 = 29 ± 15 μm2. The WT surface area is significantly smaller than in the mutant, ****p < 0.0001. WT E8.5 = 8 ± 4 μm2; Pten △Epi E8.5 = 14 ± 9 μm2. The WT surface area is significantly smaller than in the mutant, ****p < 0.0001. (E) Acetylated microtubule arrays in the neural plate in stage-matched WT and mutant embryos. Transverse sections of cephalic regions of WT and Pten △Epi embryos at E8.0 (0– 2 somites), E8.5 (5–7 somites) and E9.0 (11–13 somites). Green is acetylated tubulin; blue is DAPI. Arrows point to the apical surface of neural plate; arrowheads point to the floor plate. The first region of tubulin acetylation in WT is in the floor plate, which is only region of tubulin acetylation in the mutant. Scale bar = 25 μm.
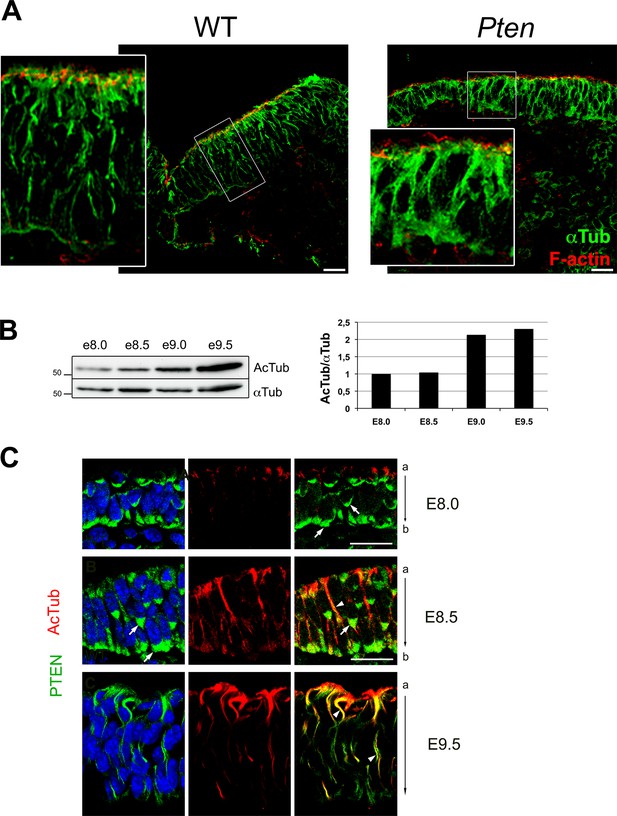
Acetylated microtubules in the wild type cranial neural plate.
(A) α-Tubulin appears to be in apical-basal arrays in E8.5 wild-type cranial neuroepithelial cells but is more diffuse in Pten △Epi embryos. Red is phalloidin. Scale bar = 20 µm. (B) Western blot analysis shows that of acetylated tubulin increases during maturation of the wild-type neural plate. Each lane represents a pool of the cephalic regions of three embryos. (C) Acetylated microtubule arrays increase with time in the cranial neural plate of WT embryos and acetylated tubulin partially colocalizes with PTEN protein beginning at E8.5. Green is PTEN, acetylated tubulin is red; blue is DAPI. Arrows highlight sites of PTEN enrichment and arrowheads show examples of sites of colocalization of PTEN and acetylated tubulin. Scale bar = 25 μm.
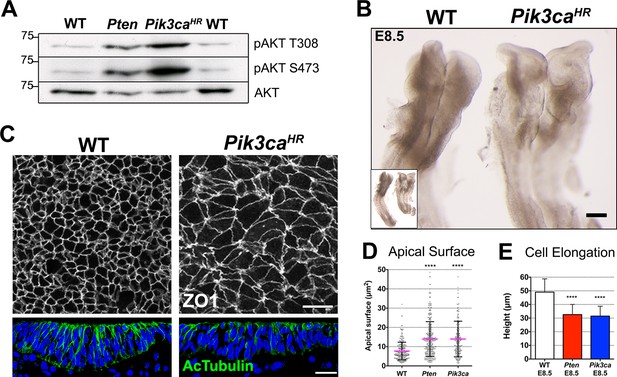
Expression of an activated form of PI3 Kinase mimics the Pten mutant neural plate phenotype.
(A) Loss of Pten (Pten △Epi) or expression of the activating mutation Pik3caH1047R-Epi in the epiblast leads to phosphorylation of AKT in E8.5 embryos. Representative Western blots (n = 3) show the two phosphorylated forms of AKT in WT, Pten △Epi and Pik3caH1047R–Epi embryos. Numbers indicate approximate MW. (B) Pik3caH1047R–Epi embryos phenocopy Pten △Epi embryos. Whole embryos (inset) and expanded view of the cephalic region of E8.5 WT and Pik3caH1047R-Epi embryos; dorsal view. Scale bar = 120 μm. (C) The apical surface of the neural plate, viewed en face; cell borders marked by expression of ZO1 (white) (top row), and acetylated tubulin (green) in transverse sections of the cephalic neural epithelium of E8.5 WT and Pik3caH1047R-Epi embryos. Blue is DAPI. Scale bar = 20 μm. (D) Comparison of apical surface area of cephalic neural epithelial cells at E8.5. WT = 8 ± 4 μm2; Pten △Epi = 14 ± 9 μm2; Pik3caH1047R-Epi = 15 ± 10 μm2. The surface areas of both mutants are significantly larger than wild type, ****p < 0.0001. (E) Cephalic neural plate height at E8.5. WT = 49.1 ± 9.6 μm; Pten △Epi = 32.6 ± 7.4 μm; Pik3caH1047R-Epi = 31.5 ± 7.2 μm. Cells in both mutants are significantly shorter than in wild type, ****p < 0.0001.
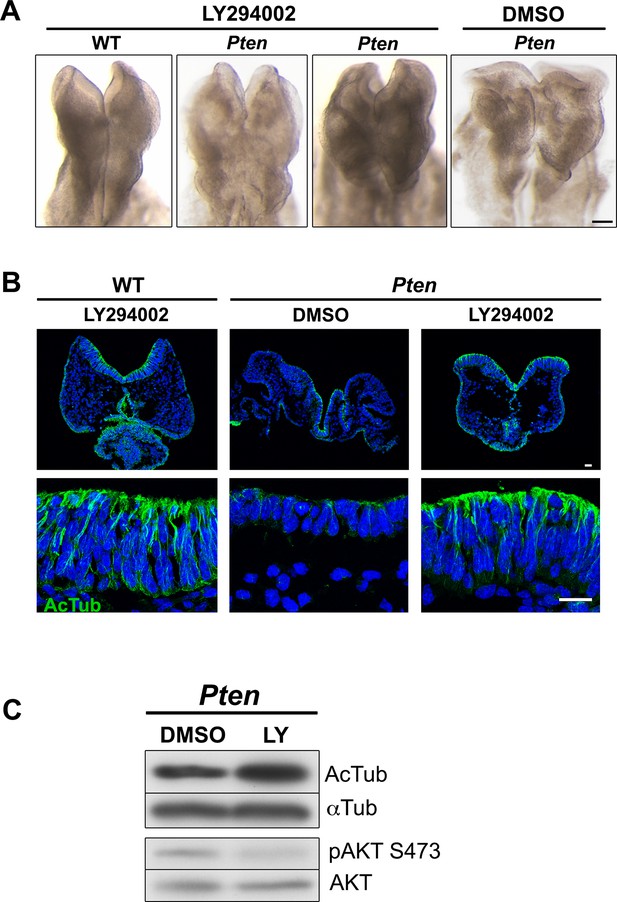
Inhibition of PI3 kinase restores pseudostratification in the Pten △Epi neural plate.
(A) Mothers of Pten △Epi mutant embryos were injected with 25 mg/kg of LY294002 24 hr prior to E8.5 embryo dissection. This treatment leads to elevation of the neural folds and prevents the formation of abnormal folds in the Pten △Epi neural plate phenotype without affecting wild-type development. (B) Immunostaining for acetylated tubulin (green) of transverse sections of the cephalic neuroepithelium of treated E8.5 WT and Pten △Epi embryos. Blue is DAPI. (C) Western blot analysis of untreated and LY294002-treated Pten mutant embryos shows an increase in tubulin acetylation and inhibition of phosphorylation of AKT at Ser473, indicating that the treatment effectively inhibited PtdIns(3,4,5)P3 production. Scale bar in A = 120 μm; in B = 20 μm.
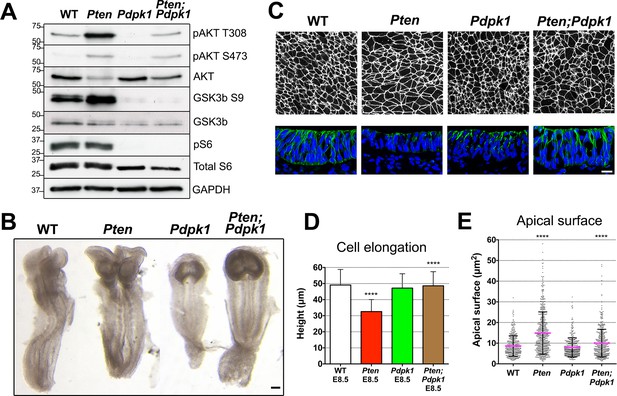
Removal of Pdpk1 rescues the pseudostratified columnar organization of the Pten neural plate.
(A) Phosphorylation of downstream targets of the PI3 kinase pathway in E8.5 wild type, Pten △Epi, Pdpk1 △Epi single mutant and Pten △Epi Pdpk1 △Epi double mutant embryos. Representative western blot shown (n = 3). Numbers indicate approximate MW. (B) Dorsal views of E8.5 wild-type, Pten △Epi, Pdpk1△Epi and Pten △Epi Pdpk1 △Epi embryos. The Pten Pdpk1 double mutants are similar in morphology to Pdpk1 single mutants, but are larger. Scale bar = 100 μm. (C) The apical surface of the neural plate, viewed en face. Cell borders marked by expression of ZO1 (white) (top row) and acetylated tubulin (green) in transverse sections of cephalic neural epithelium in E8.5 wild-type, Pten △Epi, Pdpk1 △Epi and Pten △Epi Pdpk1 △Epi embryos. Blue is DAPI. Scale bar = 20 μm. (D) Cephalic neural plate height at E8.5. WT = 49.1 ± 9.6 μm; Pten △Epi = 32.6 ± 7.4 μm; Pdpk1 △Epi = 47.2 ± 8.9 μm; Pten △Epi Pdpk1 △Epi = 48.6 ± 8.8 μm. Pten △Epi cells are significantly shorter than in wild type, and Pten △Epi Pdpk1 △Epi double mutant cells are significantly taller than in Pten △Epi, ****p < 0.0001. (E) Apical surface area of E8.5 cephalic neuroepithelial cells. Wild type = 9 ± 6 μm2; Pten △Epi = 15 ± 9 μm2. The surface area of Pten △Epi is significantly greater than in wild type, ****p < 0.0001; Pdpk1 △Epi = 8 ± 5 μm2; Pten △Epi Pdpk1 △Epi = 10 ± 7 μm2; the surface area of Pten △Epi Pdpk1 △Epi double mutant cells is significantly less than in Pten △Epi, ****p < 0.0001.
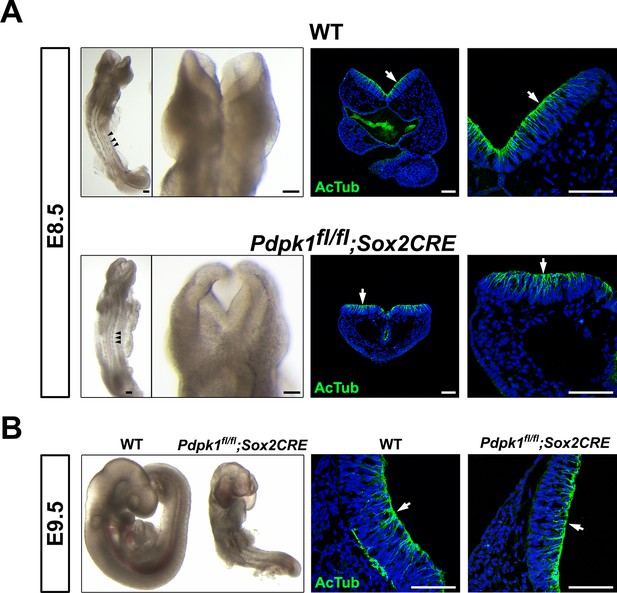
The Pdpk1△Epi phenotype.
(A) Dorsal view and detail of the cephalic region of E8.5 WT and Pdpk1 △Epi embryos. Localization of acetylated tubulin is similar in the neural plate of WT and Pdpk1 mutants, although the shape of the neural plate is abnormal in Pdpk1 mutants. Arrowheads point to somites. (B) At E9.5, Pdpk1 △Epi mutant embryos have a closed neural tube. Transverse sections and staining showed similar expression of acetylated tubulin in wild-type and mutant neural plate. Arrows indicate the apical domain of the neural plate. Scale bar = 80 μm.
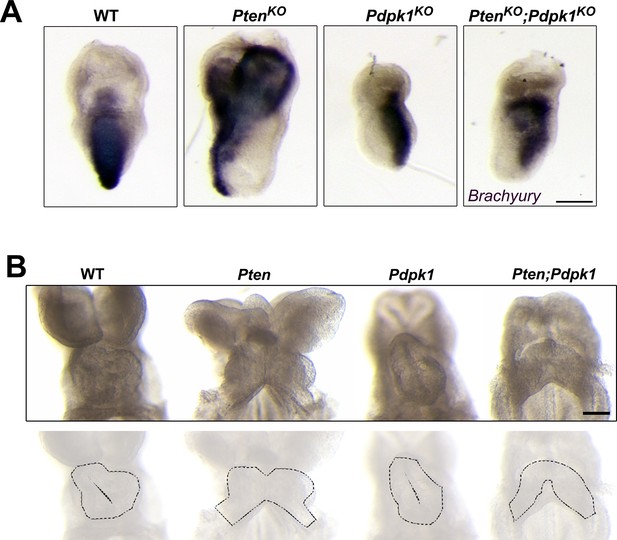
Cell migration phenotypes in Pten Pdpk1 double mutants.
(A) In situ hybridization of the primitive streak marker Brachyury in E7.5 wild-type, Pten-/-, Pdpk1-/- and Pten-/-Pdpk1-/- embryos. Brachyury expression is abnormal in both Pten-/- and Pten-/-Pdpk1-/-, suggesting that removal of Pdpk1 does not rescue the AVE migration and axis specification defects of Pten-/- mutants. (B) Ventral views of E8.5 wild-type, Pten △Epi, Pdpk1 △Epi and Pten △Epi Pdpk1 △Epi embryos, showing that the cardia bifida phenotype caused by loss of Pten is not rescued by removal of Pdpk1. Outlines of the hearts are shown below. Scale bar = 100 μm.
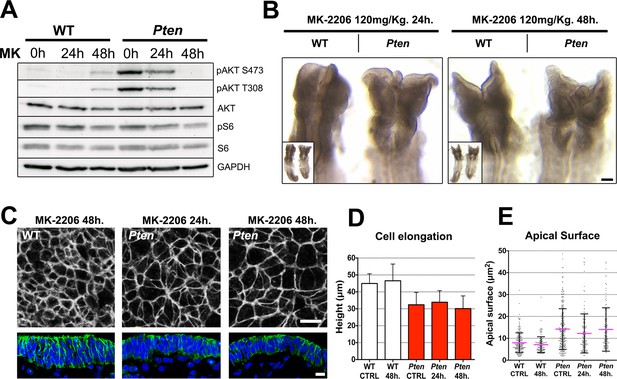
The Pten neural plate phenotype is independent of AKT.
(A) Effect of the AKT inhibitor MK-2206 treatment on targets of the PI3 kinase pathway in E8.5 embryos. Western blot of the two phosphorylated forms of AKT and pS6 S240/4 in WT and Pten △Epi at E8.5 in control embryos (vehicle) and after 24 or 48 hr of MK-2206 treatment in utero prior to embryo dissection. Numbers indicate approximate MW. (B) Dorsal view (inset) and enlarged image of the cephalic region of E8.5 wild-type and Pten △Epi embryos. There is no change in the morphology of the mutant heads after 24 or 48 hr of MK-2206 treatment in utero. Scale bar = 120 μm. (C) The apical surface of the neural plate, viewed en face. Cell borders marked by expression of ZO1 (white) (top row); acetylated tubulin (green) in transverse sections of cephalic neural epithelium in wild type and Pten △Epi at E8.5 after 24 or 48 hr of MK-2206 treatment in utero. Blue is DAPI. Scale bar = 10 μm. (D) Height of the E8.5 cephalic neural plate. Wild type, untreated (control) = 44.9 ± 5.7 μm; WT 48 hr treatment = 46.5 ± 9.9 μm; MK-2206 treatment had no significant effect. Pten △Epi untreated (control) = 32.4 ± 7.3 μm; Pten △Epi 24 hr = 33.9 ± 6.8 μm2; Pten △Epi 48 hr = 30.3 ± 7.5 μm. Treated and untreated mutants were all significantly shorter than wild type, but MK-2206 treatment did not significantly rescue cell elongation in the mutant. (E) Apical surface area of E8.5 cephalic neuroepithelial cells. Control = 8 ± 5 μm2; WT 48 hr = 7 ± 4 μm2; Pten △Epi Control = 14 ± 9 μm2; Pten △Epi 24 hr = 13 ± 9 μm2; Pten △Epi 48 hr = 14 ± 10 μm2. Treated and untreated mutant cells all had significantly larger surface area than wild type, but MK-2206 treatment did not significantly decrease cell surface area in the mutant.
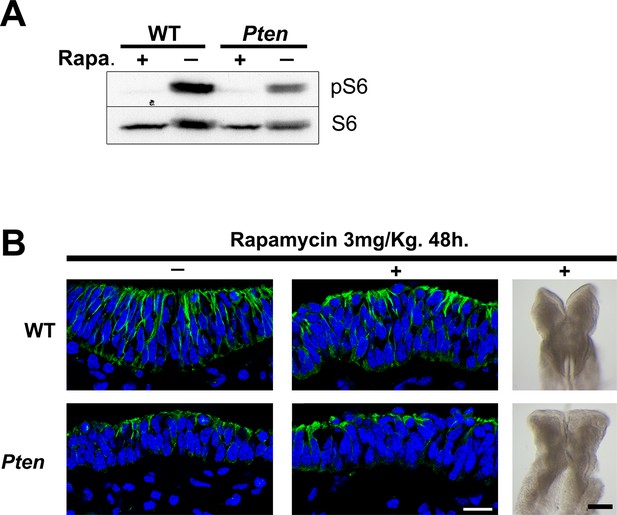
Inhibition of mTORC1 by rapamycin does not rescue the Pten neural plate phenotype.
(A) Western blot of extracts from control embryos and embryos treated in utero with rapamycin for 48 hr shows that the treatment effectively blocked the expression of pS6 in both WT and Pten △Epi embryos. (B) Transverse sections (acetylated tubulin in green) and dorsal view of cephalic region of E8.5 WT and Pten △Epi embryos after a 48 hr treatment with rapamycin. Scale bar = 10 and 200 μm.
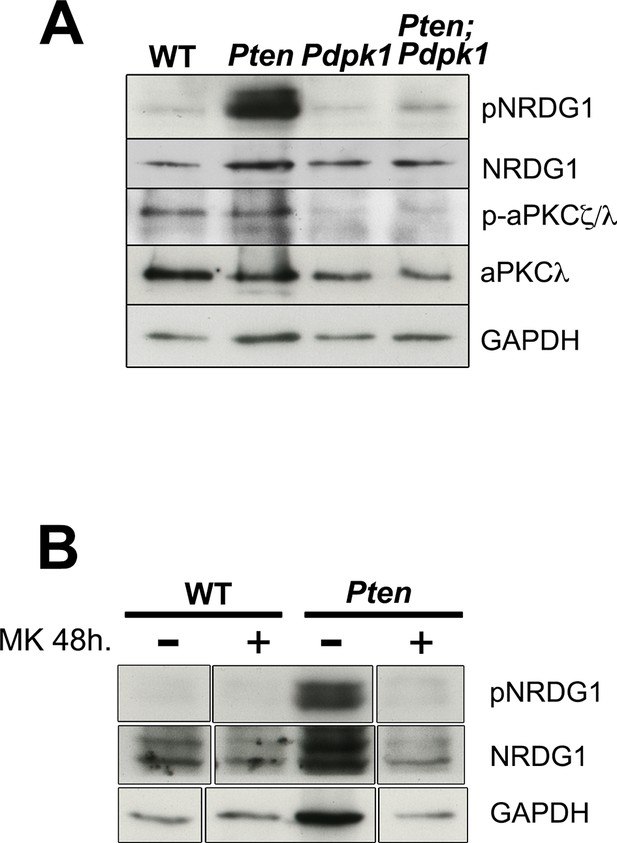
Downstream targets of PDPK1.
(A) Western blots for pNRDG1 and p-aPKCζ/λ in E8.5 WT Pten △Epi, Pdpk1 △Epi and Pten △Epi Pdpk1 △Epi embryos. (B) Western blot analysis of pNRDG1 in WT and Pten △Epi embryos treated with MK2206 for 48 hr.
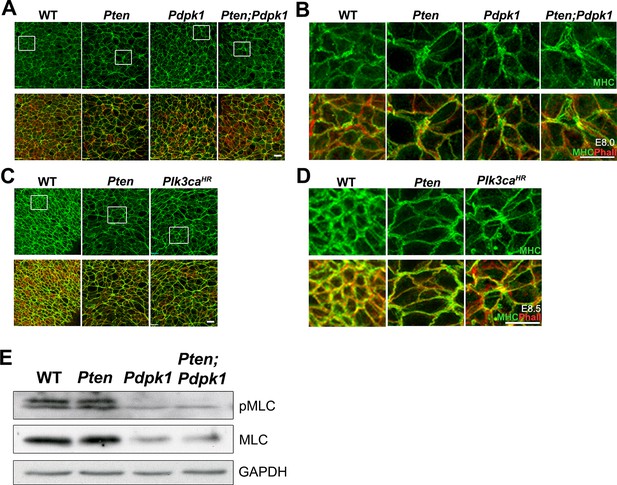
Myosin-II distribution and levels appear normal in the Pten neural plate.
(A–D) En face images of the distribution of MHC-IIB (green). (A–B) E8.0 embryos. Myosin was anisotropically distributed in the neural plate of all genotypes (WT, Pten, Pdpk1 and Pten Pdpk1) and no preferential enrichment at long or short cell edges was noted. (C–D) E8.5 embryos. Apical myosin is enriched in all genotypes at E8.5. This is especially prominent in WT, and presumably reflects the formation of the actomyosin rings that mediate apical constriction in the next phase of neural tube closure. Phalloidin is red. Scale bar = 20 μm. (E) Western blot detection of phospho-myosin light chain (pMLC) and total MLC in E8.5 Pten △Epi, Pdpk1 △Epi and Pten △Epi Pdpk1 △Epi embryos. No striking differences between genotypes were apparent.
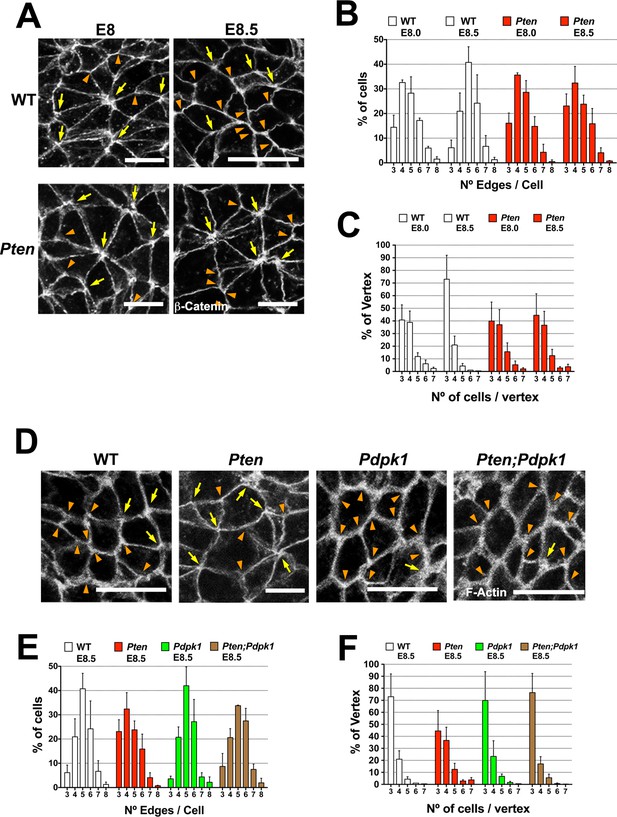
PTEN promotes stable cell packing in the neural plate.
Panels (A) and (D) show high magnification views of the apical surface of the neural plate embryos, with magnification adjusted so that the cells appear to be approximately the same size, in order to highlight the difference in cell packing in the two genotypes. Scale bars in (A) and (D) = 15 μm. Orange arrowheads indicate examples of 3 cells/vertex, and yellow arrows indicate vertices formed by ≥4 cells. Cell borders marked by β-catenin (A) or F-actin (D) expression. (A) At E8.0, rosette-like structures are common in both WT and Pten. Fewer rosette-like arrangements are seen in WT at E8.5, but rosettes persist in the E8.5 Pten neural plate. (B) Quantification of percentage of cells with 3–8 edges. Between E8.0 and E8.5, the percentage of cells with 3–4 edges decreases ∼45%, while the percentage with 5–6 edges increases ∼1.6 fold in WT embryos, but these parameters are unchanged in E8.5 mutants. (C) The percentage of vertices plotted against the number of cells meeting at a vertex. In a honeycomb arrangement, 3 cells meet at a vertex; the number of cases where three cells meet at a vertex increases ∼1.8 fold between E8.0 and E8.5, whereas the Pten neural plate does not changed in this interval. (D) At E8.5, Pdpk1 single and Pten Pdpk1 double mutants show packing similar to that in WT, compared to the more rosette-like packing in Pten. Quantification of % of cells with 3–8 edges (E) and % of vertices formed by 3–7 cells (F) showed similar values in E8.5 WT, Pdpk1 and Pten Pdpk1 embryos. Bars indicate %, lines indicate s.d.
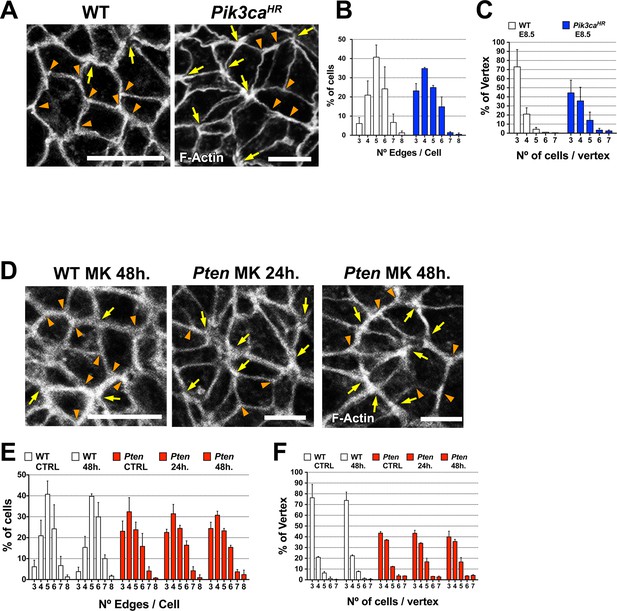
Cell packing in the neural plate with constitutively active PI3 kinase and when AKT is inhibited with MK-2206.
(A) and (D) show high magnification views of the apical surface of the neural plate, with magnification adjusted so that the cells appear to be approximately the same size to highlight the difference in cell packing in the two genotypes. Scale bars in (A) and (D) = 15 μm. Orange arrowheads indicate examples of 3 cells/vertex, and yellow arrows indicate vertices formed by ≥4 cells. Cell borders marked by F-actin expression. (A) At E8.5, more rosette-like structures are seen Pik3caH1047R-Epi than in WT. (B) Quantification of percentage of cells with 3–8 edges (neighbors). As in E8.5 Pten △Epi mutants, the E8.5 Pik3caH1047R-Epi neural plate had ∼two fold more cells with 3 and 4 edges, and ∼55% fewer cells with 5 and 6 edges than WT. (C) There were ∼45% fewer vertices formed by 3 cells (the honeycomb arrangement) and ∼two fold increase in the number vertices formed by ≥4 cells in the E8.5 Pik3caH1047R-Epi neural plate compared to WT. (D–F) MK-2206 treatment does not affect distribution of cells with 3–8 edges or the percentage of vertices in any category. Bars indicate %, lines indicate s.d.
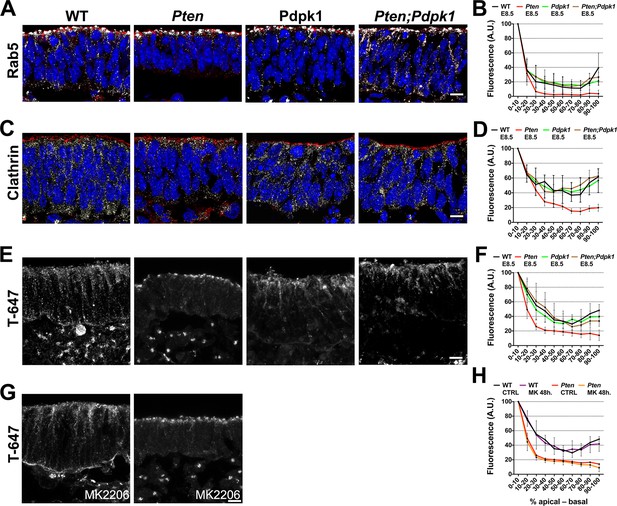
Apical-basal trafficking in PI3 kinase pathway mutants.
(A– D) Distribution of endosome markers along the apical-basal axis in transverse sections of the cephalic neural plate of E8.5 wild-type, Pten △Epi, Pdpk1 △Epi and Pten △Epi Pdpk1 △Epi embryos. (A) Localization of Rab5, an early endosome marker. (B) Distribution of Rab5 along the apical-basal axis, normalized to a maximum value of 100. (C) Localization of clathrin. (D) Distribution of clathrin along the apical-basal axis, normalized to a maximum value of 100. (E) Uptake of Transferrin-Alexa 647 after 8 hr of embryo culture. Transverse sections of cephalic neural plate of E8.5 wild-type, Pten △Epi, Pdpk1 △Epi and Pten △Epi Pdpk1 △Epi embryos. White signal is the native Alexa 647 fluorescence. (F) Distribution of Alexa-647 signal along the apical-basal axis. Transferrin-647 accumulates apically in the Pten △Epi but not in Pten △Epi Pdpk1 △Epi double mutants. (G) Transverse sections of cephalic neural plate of E8.5 wild-type and Pten △Epiembryos treated in utero with MK-2206 for 48 hr and then cultured with 50 μg/ml of Transferrin-647 and MK-2206 for 8 hr. (H) Distribution of Alexa-647 along the apical-basal axis is not affected by MK-2206 treatment. Images are Z-projections of 3 optical sections of 1 μm each. Red is phalloidin. Blue is DAPI. Scale bars = 10 μm.
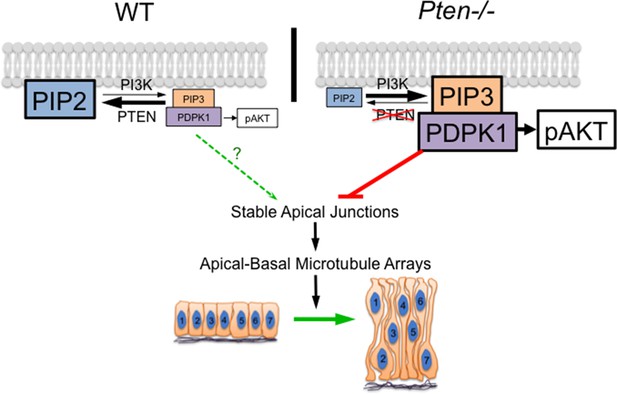
A model for the role of PTEN in the formation of the pseudostratified columnar epithelium.
PDPK1 is anchored to the plasma membrane by PtdIns(3,4,5)P3 (PIP3), which is made by PI3 kinase (PI3K) and degraded by PTEN. In the Pten mutant, increased PIP3 recruits high levels of PDPK1 to the membrane, where it is activated. Activated membrane-associated PDPK1 has two targets: activated PDPK1 generates high levels of pAKT; in a separate pathway, high levels of membrane-associated PDPK1 inhibit the formation of stable apical junctions. Stable apical junctions are required for the formation of stable apical-basal microtubule arrays, which mediate apical-to-basal trafficking in the neural epithelium, allowing elongation and tight packing of cells in the neural epithelium. In WT, PDPK1 is not required for formation of the pseudostratified neural epithelium, although the delay in neural tube closure in Pdpk1 mutants may reflect a subtle role for the protein in epithelial organization.





