Blockade of glucagon signaling prevents or reverses diabetes onset only if residual β-cells persist
Figures
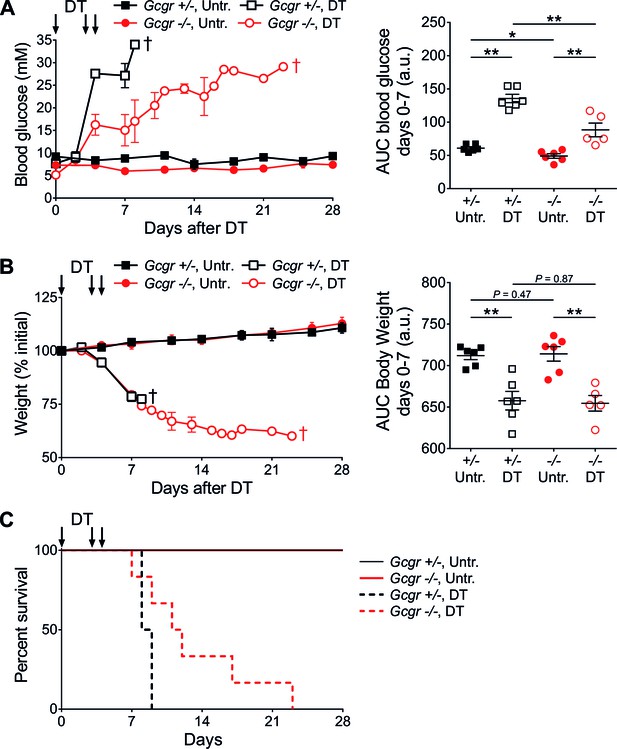
Gcgr-/- mice become diabetic after massive β-cell ablation.
(A) Random-fed glycemia (left) and area under the glycemia curve (AUC) between days 0 and 7 after DT (right) in untreated (Untr.) and DT-treated RIP-DTR;Gcgr+/- and RIP-DTR;Gcgr-/- females. (B) Body weight (left) and AUC body weight (days 0–7 after DT; right). †, all mice of the group were dead at this time point (see Figure 1C). *p<0.05; **p<0.01; Mann-Whitney U test. C: Survival curve of RIP-DTR;Gcgr+/- and RIP-DTR;Gcgr-/- mice after DT treatment (N=5–6). Survival analysis of DT-treated animals (Gcgr+/- versus Gcgr-/-): p=0.044; Log-rank test.
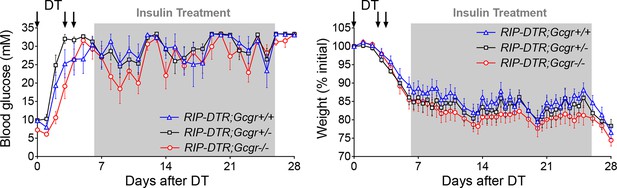
Insulin administration stabilizes body weight and allows survival of DT-treated Gcgr-/-mice.
Glycemia (left) and body weight (right) of RIP-DTR;Gcgr+/+ (blue triangles, N=7), RIP-DTR;Gcgr+/- (black squares, N=9), and RIP-DTR;Gcgr-/- (red circles, N=9) males following DT-mediated β-cell ablation and exogenous insulin treatment. Grey areas indicate the period during which mice were treated with insulin detemir (5 U/kg/day between days 6 and 25).
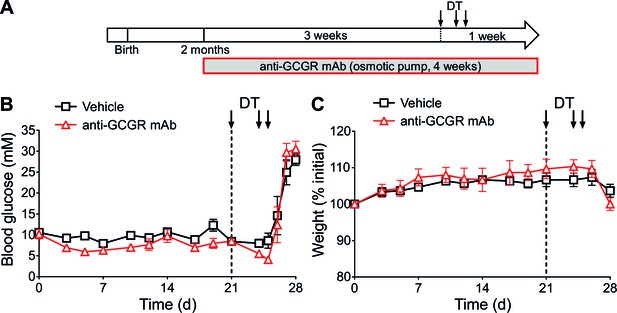
Anti-GCGR mAb-treated mice become diabetic after massive β-cell ablation.
(A) Experimental design. (B-C) Random-fed glycemia (B) and body weight (C) after DT in C57BL/6 males pre-treated with vehicle or mAb (N=3).
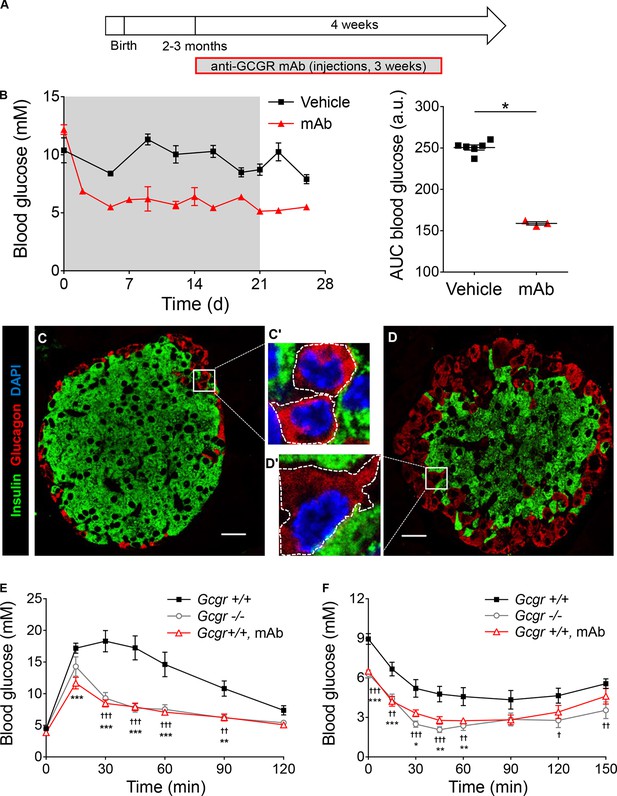
Anti-GCGR mAb administration recapitulates the metabolic and cellular phenotypes of Gcgr-/- mice.
(A) Experimental design. 9 mg/kg anti-GCGR mAb was injected i.p. 3 times per week for 3 weeks in C57BL/6 animals. (B) Left: Random fed glycemia of vehicle- (black squares) or mAb-treated males (red triangles). The grey area indicates the period of antibody treatment. Right: Area under the glycemia curves. *p<0.05; Mann-Whitney U test. C and D. Confocal images of pancreatic islet sections from vehicle- (C) and mAb-treated (D) males. α-cell hyperplasia and hypertrophy (compare C’ and D’, the dashed lines represent the cell perimeters) are observed in islets from mAb-treated mice. Scale bars: 20 μm. E and F. Intraperitoneal glucose tolerance test (E) and insulin tolerance test (F) performed in Gcgr+/+ (black squares, N=9), Gcgr-/- (grey circles, N=10), and mAb pre-treated Gcgr+/+ (red triangles, N=10) males. *p<0.05; **p<0.01; ***p<0.001; Gcgr+/+ versus mAb-treated Gcgr+/+ mice. †, p<0.05; ††, p<0.01; †††, p<0.001; Gcgr+/+ versus Gcgr-/- mice; two-way ANOVA. The difference between Gcgr-/- and mAb-treated Gcgr+/+ mice is not significant.
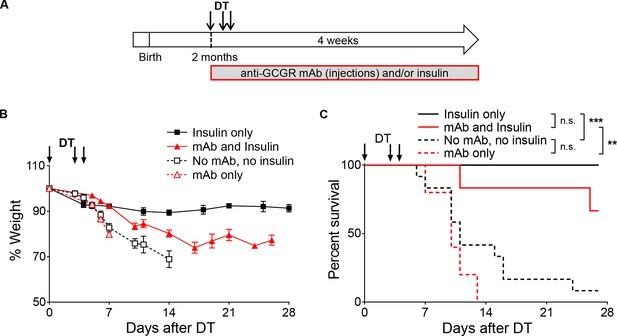
Insulin administration is required to stabilize body weight and allow survival of anti-GCGR-treated mice after DT.
(A-C) Exogenous insulin, but not anti-GCGR mAb treatment, stabilizes body weight and improves survival after extreme β-cell loss. (A) Experimental design. (B) Evolution of body weight following DT administration in RIP-DTR males treated with anti-GCGR mAb and/or exogenous insulin (N=5–12). (C) Survival curves. Survival analyses are indicated next to the legend: n.s., not significant; **p<0.01; ***p<0.001; Log-rank test. Insulin was administered as subcutaneous implants (antibody-untreated mice), or as injections of long-acting insulin (antibody-treated mice) because insulin implants lead to hypoglycemia and death in mice with deficient glucagon signaling.
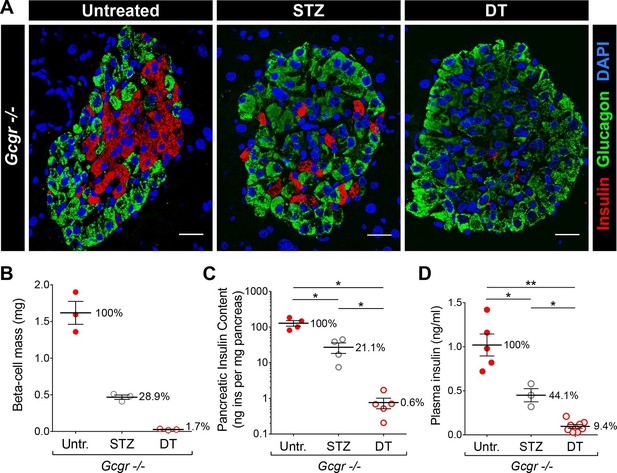
DT administration leads to a more complete β-cell ablation than STZ.
(A) Islet sections stained for insulin (red) and glucagon (green) from untreated, STZ-, or DT-treated RIP-DTR;Gcgr-/- females, 6 days after the last STZ or DT injection. Scale bars: 20 μm. (B-D) β-cell mass (B), pancreatic insulin content (C) and fed plasma insulin levels (D) in untreated (Untr.), STZ-, or DT-treated RIP-DTR;Gcgr-/- males and females, 6 days after the last injection. STZ administration: two injections (200 and 150 mg/kg). *p<0.05; **p<0.01; Mann-Whitney U test.
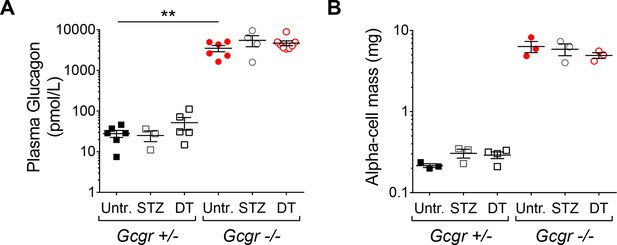
RIP-DTR;Gcgr-/- mice remain hyperglucagonemic and α-cell mass is not affected after STZ- or DT-treatment.
(A-B) fed plasma glucagon levels (A) and α-cell mass (B) in untreated (Untr.), STZ-, or DT-treated RIP-DTR;Gcgr+/- and RIP-DTR;Gcgr-/- males and females, measured 6 days after the last injection. STZ administration: two injections (200 and 150 mg/kg). **p<0.01; Mann-Whitney U test.
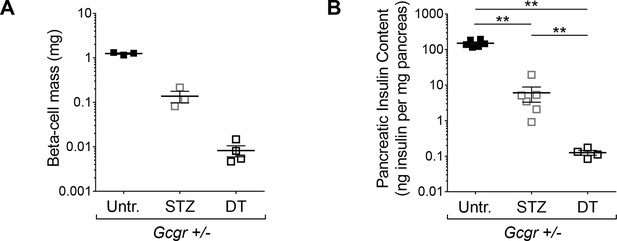
Higher efficiency of β-cell ablation after DT- than after STZ-treatment in mice with normal glucagon signaling.
(A-B) β-cell mass (A) and pancreatic insulin content (B) in untreated (Untr.), STZ-, or DT-treated RIP-DTR;Gcgr+/- females, measured 6 days after the last injection. STZ administration: two injections (200 and 150 mg/kg). **p<0.01; Mann-Whitney U test.
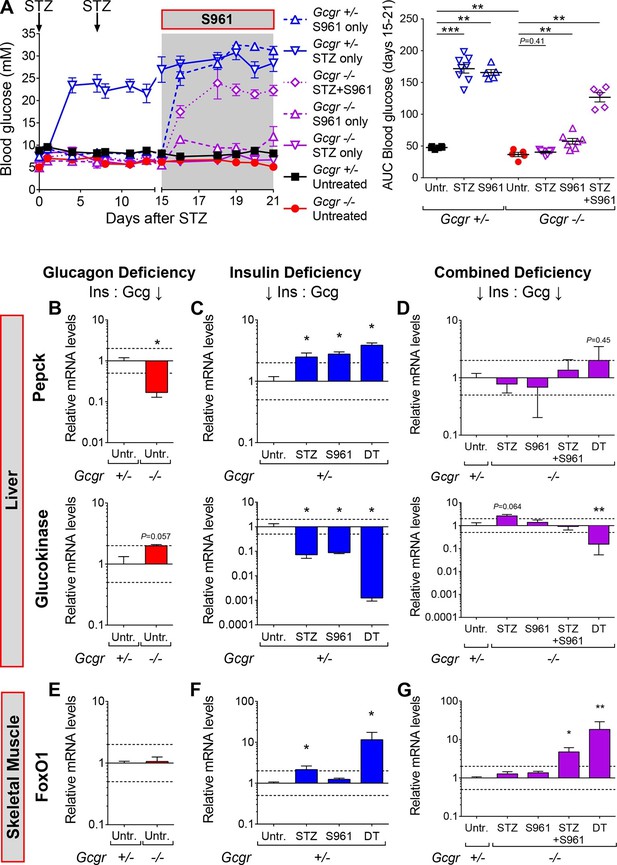
Inhibition of insulin action triggers hyperglycemia in STZ-treated Gcgr-/-mice.
(A) Random-fed glycemia after STZ and/or S961 administration in Gcgr+/- and Gcgr-/- females (left), and area under the glycemia curve (AUC) during S961 treatment (right). (B-D) Hepatic Pepck (top) and Glucokinase (bottom) mRNA levels relative to those of untreated Gcgr+/- (control) mice (N=4–6). (B) Glucagon deficiency: Gcgr-/- background. (C) Insulin deficiency: β-cell ablation or insulin signaling inhibition. (D) Combined deficiency: β-cell ablation and/or insulin signaling inhibition in a Gcgr-/- background. (E-G) FoxO1 mRNA levels in skeletal muscle, relative to those of untreated Gcgr+/- mice (N=4–6). STZ administration: 200 mg/kg at day 0 and 150 mg/kg at day 7. S961 treatment: osmotic pump (days 15 to 21). *p<0.05; **p<0.01; Mann-Whitney U test. Only groups that exhibited a > twofold regulation as compared to controls (dashed lines) were tested.
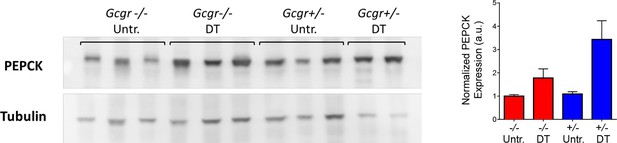
Higher hepatic PEPCK protein expression after DT in both Gcgr+/- and Gcgr-/- mice.
Western blot analysis showing PEPCK and Tubulin expression in the liver of untreated (untr.) and DT-treated RIP-DTR-Gcgr+/- and RIP-DTR-Gcgr-/- females (left). Quantification of PEPCK band intensities relative to Tubulin is shown on the right.
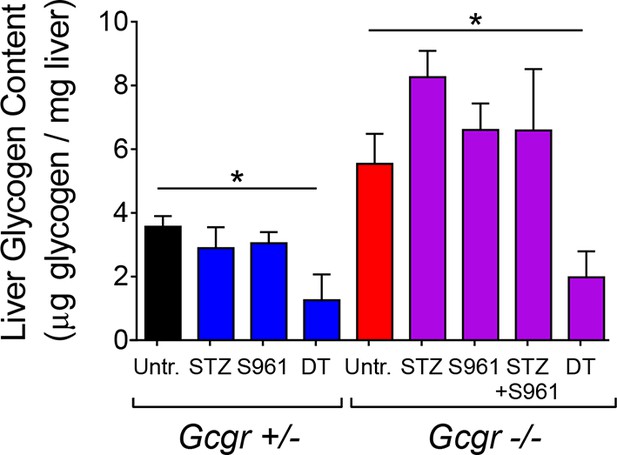
Liver glycogen concentration is reduced after DT-treatment in both RIP-DTR-Gcgr+/- and RIP-DTR-Gcgr-/- mice.
Liver glycogen concentration in different conditions of insulin and/or glucagon deficiency (N=4). *p<0.05; Mann-Whitney U test.
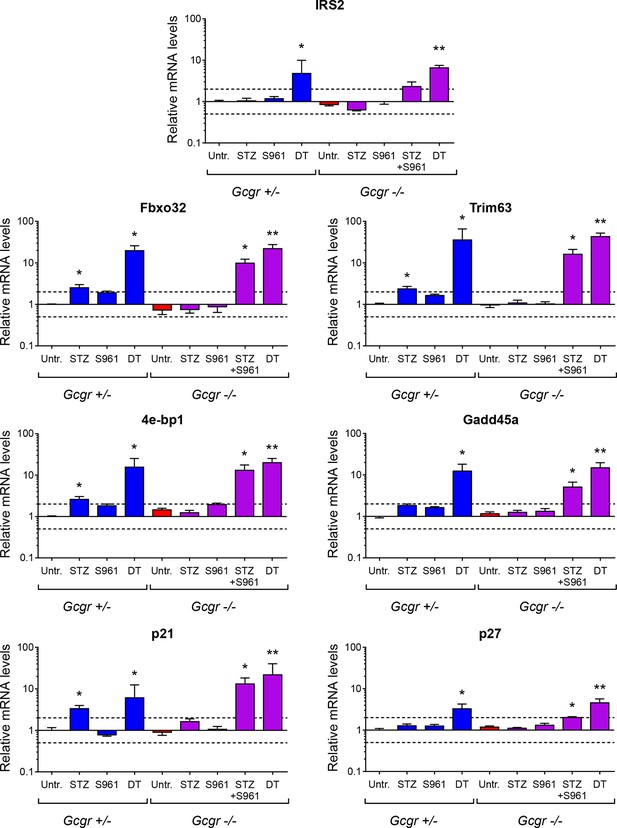
Expression of genes negatively regulated by insulin signaling in skeletal muscle.
mRNA levels of genes inhibited by insulin in skeletal muscle (gastrocnemius), relative to those of untreated Gcgr+/- females (normalized to Actb, Gapdh, and Gusb) (N=4–6). Irs2, Insulin receptor substrate 2; Fbxo32, F-box only protein 32 (Atrogin-1); Trim63, Tripartite motif-containing 63 (MuRF1); 4e-bp1, Eukaryotic translation initiation factor 4E binding protein 1 (Eif4ebp1); Gadd45a, Growth arrest and DNA-damage-inducible 45 alpha; p21, Cyclin-dependent kinase inhibitor 1A (Cdkn1a). p27, Cyclin-dependent kinase inhibitor 1B (Cdkn1b). *p<0.05; **p<0.01; Mann-Whitney U test. Only groups that exhibited a > twofold regulation as compared to controls (dashed lines) were tested.
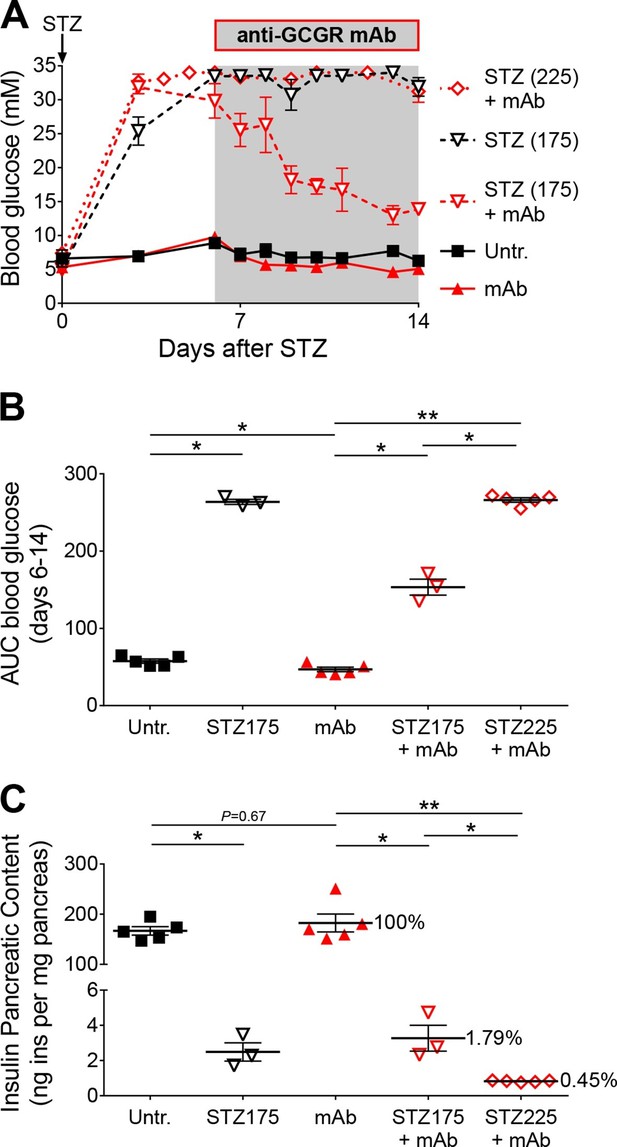
Anti-GCGR mAb treatment does not normalize hyperglycemia after efficient STZ-mediated β-cell ablation.
(A) Random-fed glycemia in C57BL/6 males treated with STZ (single injection at day 0: 175 or 225 mg/kg) and/or anti-GCGR mAb (osmotic pump, days 6 to 14; N=3–6). (B) Area under the glycemia curves during mAb treatment. (C) Pancreatic insulin content. *p<0.05; **p<0.01; Mann Whitney U test.
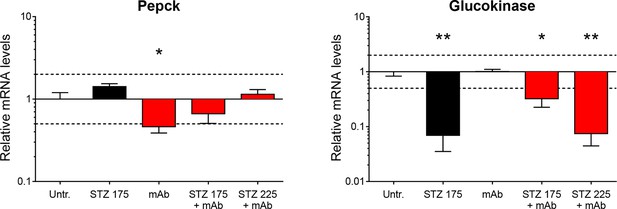
Hepatic Pepck and Glucokinase expression after STZ and/or anti-GCGR mAb treatment.
Liver Pepck (left) and Glucokinase (right) mRNA levels in mice treated with STZ (single injection at day 0: 175 or 225 mg/kg) and/or anti-GCGR mAb (osmotic pump, days 6 to 14) relative to those of untreated Gcgr+/- mice (N=4–7). *p<0.05; **p<0.01; Mann-Whitney U test. Only groups that exhibited a > twofold regulation as compared to controls (dashed lines) were tested.
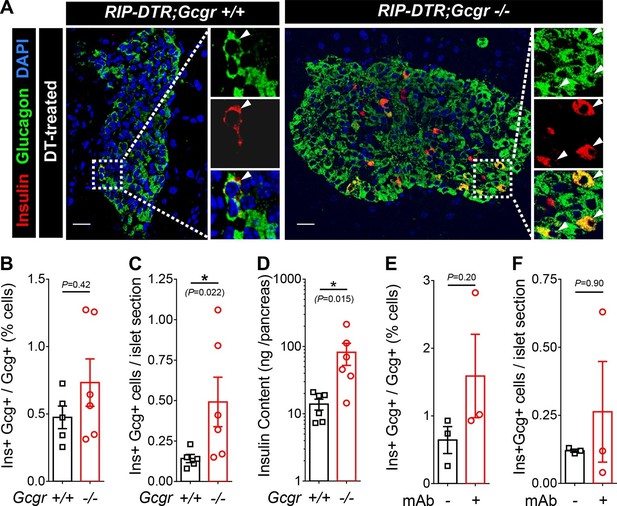
Absence of glucagon signaling does not block the appearance of new glucagon-insulin bihormonal cells after β-cell ablation.
(A) Islet sections exhibiting glucagon-insulin co-expressing cells (arrowheads) from RIP-DTR;Gcgr+/+ and RIP-DTR;Gcgr-/- females (1 m after DT). Scale bars: 20 μm. (B-D) Percentage of glucagon+ cells that co-express insulin (B), bihormonal cells per islet section (C), and pancreatic insulin content (D) in RIP-DTR;Gcgr+/+ and RIP-DTR;Gcgr-/- females (1 m after DT, N=5–6). (E-F) Percentage of glucagon+ cells that co-express insulin (E), and bihormonal cells per islet section (F) in vehicle- or anti-GCGR mAb- treated RIP-DTR males (2 weeks after DT, N=3). *p<0.05; Mann-Whitney U test.
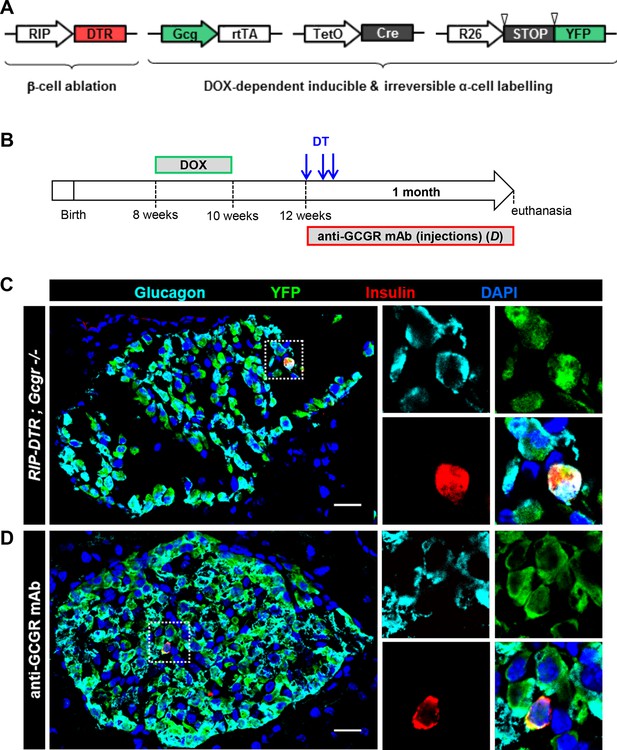
Newly formed bihormonal cells in Gcgr-/- mice are reprogrammed α-cells.
(A) Transgenes required to irreversibly lineage-trace pancreatic α-cells with YFP before β-cell ablation. Inverted triangles represent loxP sites. (B) Experimental design. Upon DOX administration, the transgenic rtTA protein expressed in α-cells binds to the TetO promoter and activates Cre expression, which in turn recombines the STOP sequence in the R26-YFP transgene, leading to irreversible YFP expression. C and D: Example of YFP-traced cells that co-express insulin, as observed after β-cell ablation in a RIP-DTR;Gcg-rtTA;TeTO-Cre;R26-YFP;Gcgr-/- female (C) or in an anti-GCGR mAb-treated RIP-DTR;Gcg-rtTA;TetO-Cre;R26-YFP male (D). Higher magnification of the dotted areas is shown on the right side of panels C and D. YFP was detected using an anti-GFP antibody. Scale bars: 20 μm.
Additional files
-
Supplementary file 1
Primer sequences used for RT-qPCR.
- https://doi.org/10.7554/eLife.13828.019





