Light-induced depigmentation in planarians models the pathophysiology of acute porphyrias
Figures
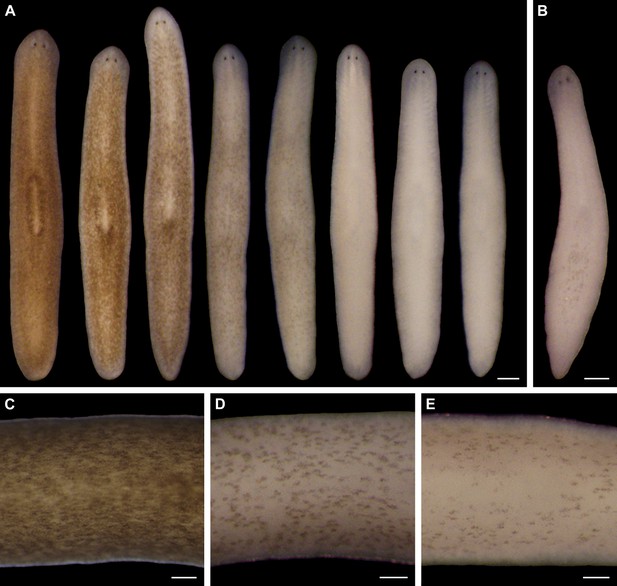
Light-induced depigmentation in S. mediterranea.
(A) Animals exposed to visible light (incident intensity = 5000 lux; see figure supplement 3 for spectrum) exhibit progressive loss of bodily pigmentation. Images show a single live animal photographed (left to right) at time 0 and immediately following each of a series of intermittent light exposure and recovery periods (final timepoint = 10 days; see Materials and methods for details). Continuous exposure results in 100% lethality at this intensity. (B) Ventral surface of a representative depigmented animal. (C–E) Close-ups of control (C) and light-exposed (D,E) animals demonstrating non-uniform pigment loss (photographs in D and E correspond to 4th and 5th images from left in A). Scale bars: A,B = 500 µm; C–E = 200 µm.
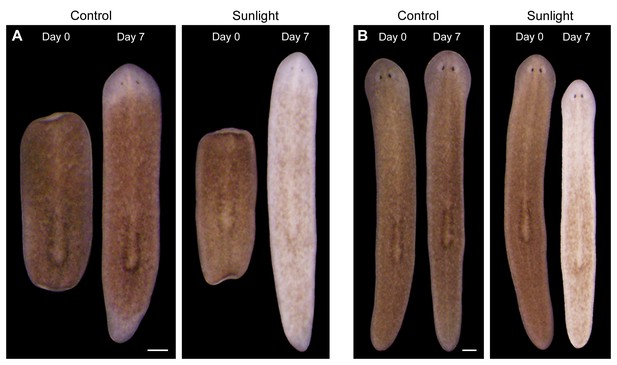
Sunlight-induced depigmentation.
To learn about the scientific method, non-science majors enrolled in a general education course analyzed the effects of environmental variables, including sunlight exposure, on regeneration in S. mediterranea. Control animals, maintained in a dark 20˚C incubator to mimic conditions in their natural environment (Oviedo et al., 2008), are brown. Sunlight-exposed animals, placed on a windowsill receiving indirect sunlight for 4 hr per day and returned to a dark incubator between exposures, regenerated normally but unexpectedly lost almost all bodily pigmentation within 1 week. (A) Representative animals photographed immediately following amputation of cephalic and caudal tissue, and again after 7 days of regeneration. (B) Representative intact animals subjected to the same control and sunlight exposure conditions. Scale bars = 300 µm.
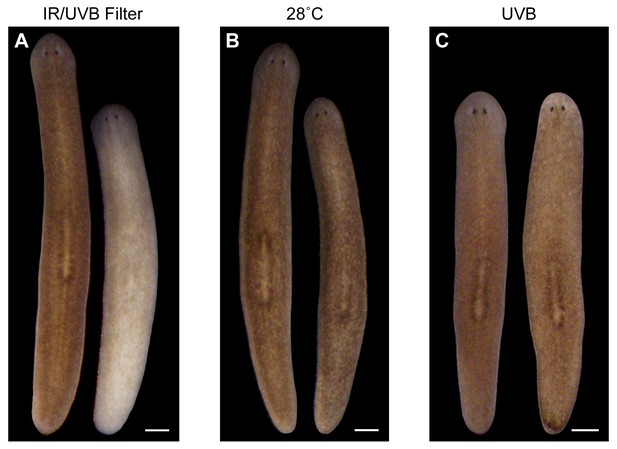
IR and UVB radiation are neither necessary nor sufficient to induce depigmentation.
(A) Animal photographed before (left) and 7 days after (right) a single day of sunlight exposure under IR- and UVB-blocking glass. (B) Animal photographed before (left) and after (right) 7 days in the dark at 28˚C (average high temperature experienced by sunlight-exposed animals). Incubation at 30˚C resulted in 100% lethality within 72 hr, without apparent depigmentation. (C) Animal photographed before (left) and 7 days after (right) exposure to a UVB (302 nm) lamp for 1.5 hr (approximate LT50 exposure time; 8/16 animals died by 7 days post-exposure). All scale bars = 500 µm.
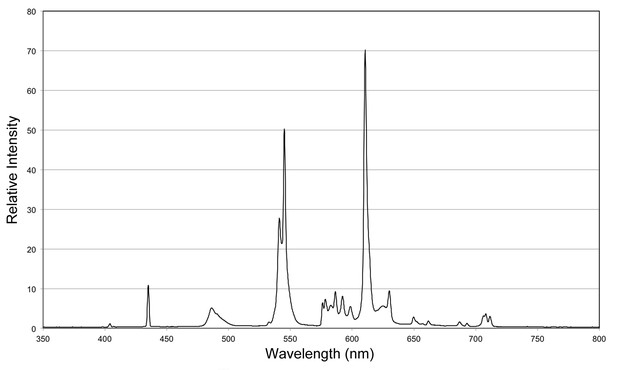
Incident spectrum for visible light exposure.
Relative light intensities (total = 5000 lux) measured under conditions experienced by animals subjected to visible light exposure. Applies to all experiments except those using a red LED (see below).
Feeding, regeneration, and repigmentation in depigmented animals.
(A) Depigmented animal fed with dyed calf liver; 21/26 depigmented animals and 25/26 controls consumed a visible amount of food. (B) Representative control (left) and depigmented (right) animals photographed immediately following amputation of cephalic and caudal tissue (left in each panel), and again after 7 days of regeneration (right in each panel). (C) Light-induced depigmentation is reversible. Images show a single live animal photographed (left to right) immediately after conclusion of light exposure, and again 7 and 21 days later. Animal was fed at 3, 6, and 9 days post-exposure. (D,E) Close-ups of a repigmenting animal photographed 3 (D) and 12 (E) days after conclusion of light exposure. Scale bars: A,B = 300 µm; C = 500 µm; D,E = 200 µm.

Depigmentation is not due to direct photobleaching.
Images show a single live animal photographed (left to right) at time 0, after a 24-hr pulse of light exposure, and every 24 hr thereafter while maintained in a dark incubator. Note that depigmentation continues after light exposure ends. Scale bar = 500 µm.
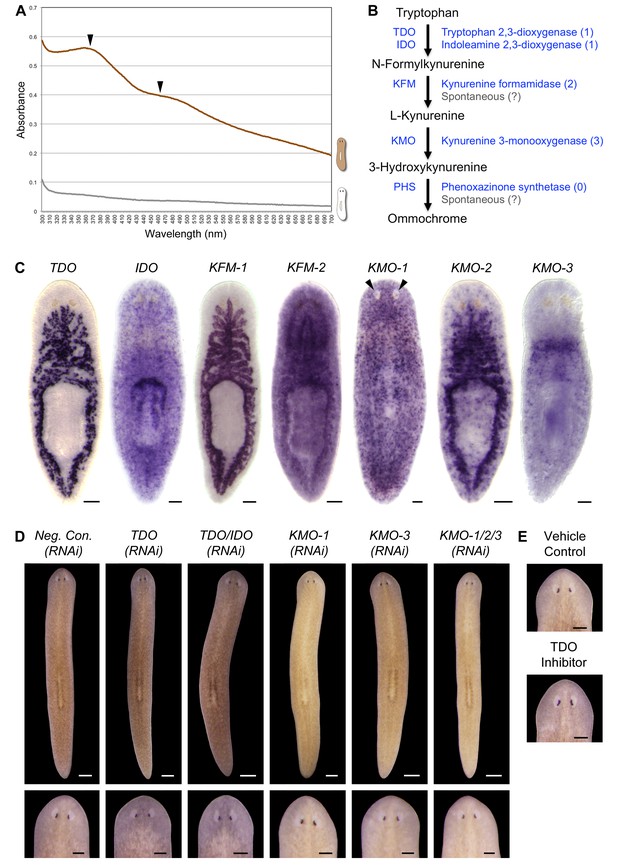
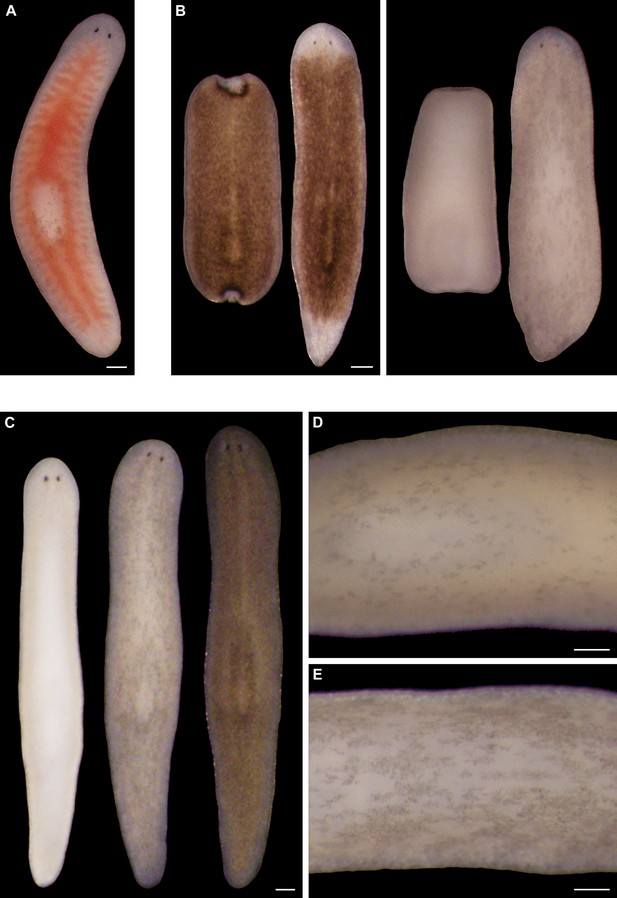
S. mediterranea produces an ommochrome body pigment.
(A) Absorbance spectra of body pigment purified from control animals (brown line) or mock purified from depigmented animals (grey line). Arrowheads denote local maxima at 367 and 463 nm, characteristic of ommochrome pigments. (B) Ommochrome biosynthesis pathway. Numbers in parentheses to the right of each enzyme denote the number of S. mediterranea homologs identified via reciprocal BLAST (Materials and methods; source data 1). Enzyme abbreviations are shown to the left. (C) Whole-mount in situ hybridizations for candidate ommochrome biosynthesis genes. Note absence of KMO-1-expressing cells from unpigmented regions of the photoreceptors (arrowheads) and lower numbers of these cells over the pharynx (center), an area of reduced pigmentation. (D) RNAi phenotypes for candidate ommochrome biosynthesis genes (results not shown for genes that did not generate a visible phenotype). Intact animals (top) were administered a total of 12 RNAi feedings over 3.5 weeks and photographed 3 days after the final feeding. Regenerated animals (bottom) were amputated at this timepoint to remove cephalic and caudal tissue. The resulting trunk fragments were allowed to regenerate for 2 weeks, administered 3 further RNAi feedings, and photographed at 21 days post-amputation. (E) Animals were placed in solutions containing the tryptophan 2,3-dioxygenase inhibitor 680C91 (0.7 µM final concentration) or a vehicle (ethanol) control immediately after cephalic amputation, and photographed after 16 days of regeneration. Scale bars: C = 100 µm; D = 500 µm (top), 200 µm (bottom); E = 200 µm.
-
Figure 2—source data 1
S. mediterranea ommochrome biosynthesis enzymes.
Predicted protein sequences for S. mediterranea genes identified and cloned by reciprocal BLAST and RT-PCR (Materials and methods) were used as queries in BLASTP searches against the non-redundant H. sapiens protein database (NCBI). 1Smed Unigene transcripts are available at the Schmidtea mediterranea Genome Database (Robb et al., 2015).
- https://doi.org/10.7554/eLife.14175.012
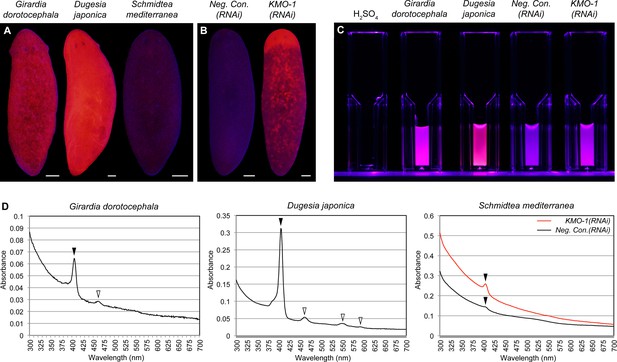
Biochemical evidence of porphyrin biosynthesis in S. mediterranea.
(A) Like G. dorotocephala, D. japonica exhibits bright red fluorescence under black light (400–440 nm excitation). S. mediterranea exhibits negligible fluorescence by comparison. (B) KMO-1(RNAi) animals demonstrate strongly increased fluorescence relative to negative controls. The uniform fluorescence in the anterior (top) corresponds to recently regenerated tissue (animals were photographed 3.5 weeks after cephalic amputation). Fluorescence does not appear to be restricted to pigment cells, particularly within regenerated tissue; this may reflect porphyrin movement across cell membranes (Viljoen et al., 1975). (C) Whole-animal H2SO4 homogenates were photographed in plastic cuvettes over a black (400 nm) LED. (D) Representative absorption spectra of whole-animal homogenates. Black arrowheads denote the Soret peak (405 nm); a visible increase in the height of this peak was evident for 3/3 KMO-1(RNAi) homogenates relative to Neg. Con.(RNAi) homogenates. White arrowheads denote Q bands (459 nm for G. dorotocephala; 459, 549, and 584 nm for D. japonica). All scale bars = 200 µm.
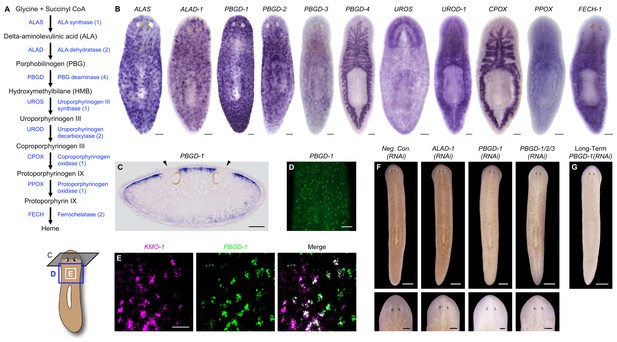
Genetic evidence of porphyrin biosynthesis in S. mediterranea pigment cells.
(A) Heme biosynthesis pathway. Numbers in parentheses to the right of each enzyme denote the number of S. mediterranea homologs identified via reciprocal BLAST (Materials and methods; source data 1). Enzyme abbreviations are shown to the left. (B) Whole-mount in situ hybridizations for porphyrin/heme biosynthesis genes. ALAS, ALAD-1, and PBGD-1, -2, and -3 expression patterns resemble that of KMO-1 (Figure 2C). PBGD-4, UROD-1, CPOX, and FECH-1 exhibit enriched expression in the gut. UROS is highly expressed in the brain. (C) 10 µm transverse section from the anterior of a PBGD-1-labeled animal, stained with nuclear fast red. PBGD-1 expression is subepithelial, higher on the dorsal surface (matching that surface’s higher level of pigmentation), and excluded from the unpigmented regions just above the photoreceptors (arrowheads). (D) PBGD-1 fluorescent in situ hybridization (FISH), showing dendritic morphology of pigment cells. (E) Double FISH showing overlap in KMO-1 and PBGD-1 expression. Over 90% of KMO-1-positive cells were co-labeled with PBGD-1 and vice versa (n = 11 animals analyzed by confocal microscopy). (F) RNAi phenotypes for porphyrin/heme biosynthesis genes (results not shown for genes that did not generate a visible phenotype, or that generated phenotypes unrelated to pigmentation – see figure supplement 1). Intact animals (top) were administered a total of 12 RNAi feedings over 3.5 weeks and photographed 3 days after the final feeding. Regenerated animals (bottom) were amputated at this timepoint to remove cephalic and caudal tissue. The resulting trunk fragments were allowed to regenerate for 2 weeks, administered 3 further RNAi feedings, and photographed at 21 days post-amputation. (G) Long-term RNAi feeding for PBGD-1 leads to complete loss of bodily pigmentation. This animal was from a group fed a total of 50 times over 6 months, with periodic amputation to increase numbers. Scale bars: B–D = 100 µm; E = 50 µm; F = 500 µm (top), 200 µm (bottom); G = 500 µm.
-
Figure 4—source data 1
S. mediterranea porphyrin/heme biosynthesis enzymes.
Predicted protein sequences for S. mediterranea genes identified and cloned by reciprocal BLAST and RT-PCR (Materials and methods) were used as queries in BLASTP searches against the non-redundant H. sapiens protein database (NCBI). 1Smed Unigene transcripts are available at the Schmidtea mediterranea Genome Database (Robb et al., 2015). 2Not cloned (no RT-PCR product).
- https://doi.org/10.7554/eLife.14175.015
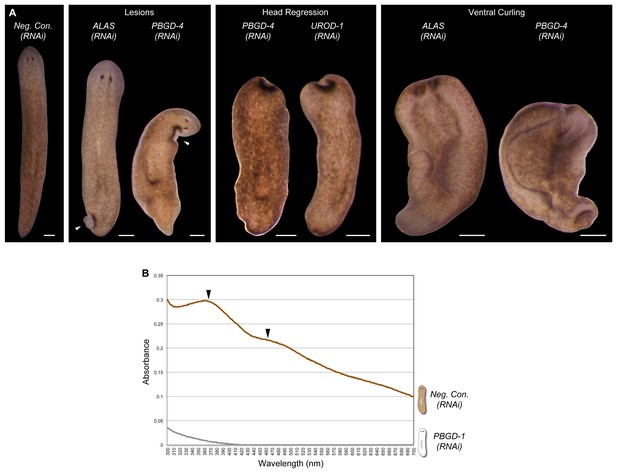
Additional RNAi phenotypes for porphyrin/heme biosynthesis genes.
(A) Animals were administered RNAi feedings every 2–3 days until less than 50% consumed a visible amount of food. This corresponded to a total of 8 (ALAS) or 9 (PBGD-4 and UROD-1, as well as negative control) feedings. Representative animals were photographed as phenotypes arose (0–7 days after final RNAi feeding). Note that ALAS(RNAi) animals are slightly lighter than negative controls. Head regression and ventral curling are phenotypes indicative of stem cell dysfunction. Lesions are consistent with defects in one or more differentiated cell types. 100% of ALAS(RNAi), PBGD-4(RNAi), and UROD-1(RNAi) animals died within 2 weeks of the final RNAi feeding, compared to 0% of negative controls (n > 30 animals per condition). (B) Absorbance spectra of body pigment purified from long-term Neg. Con.(RNAi) and PBGD-1(RNAi) animals (fed more than 50 times over more than 6 months, with periodic amputation to increase numbers). Arrowheads denote local maxima at 367 and 463 nm, characteristic of ommochrome pigments. All scale bars = 300 µm.
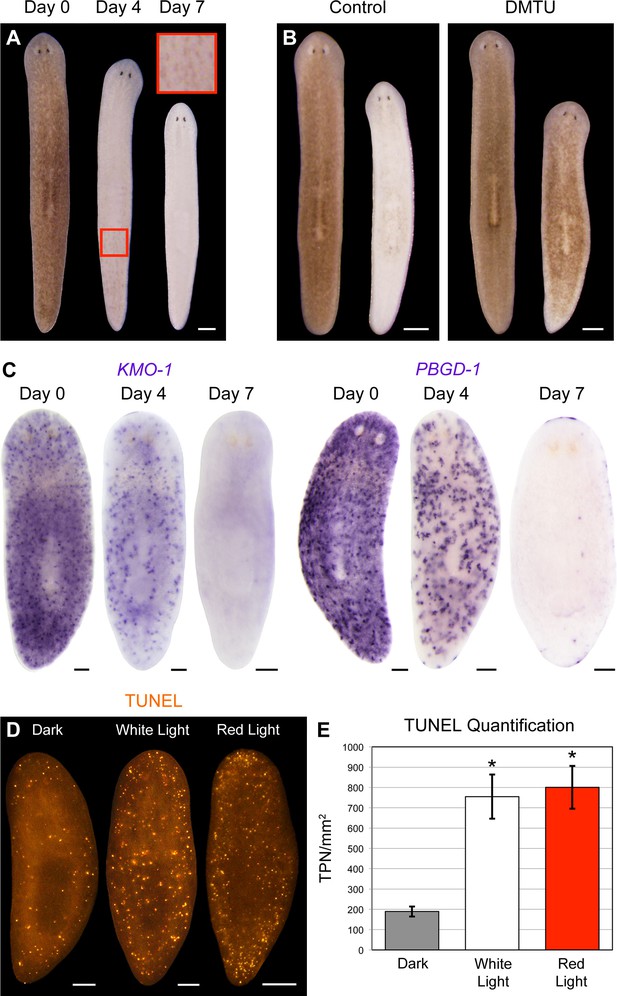
Visible light exposure causes pigment cell loss.
(A) Red light (625 nm LED) is sufficient to induce full bodily depigmentation. Inset shows a magnified view of the dorsal surface, brightness, contrast, and gamma-enhanced to highlight remaining pigment cells. No animals developed lesions or lysed under these conditions (n = 345 analyzed in 23 independent experiments). (B) DMTU inhibits light-induced depigmentation. Representative control and DMTU-treated animals were photographed before and after white light exposure (left and right in each panel, respectively). DMTU treatment (10 mM final concentration) was initiated 5 days prior to the start of light exposure and continued for the duration of the experiment. Total light exposure time = 72 hr (24- and 48-hr exposures were separated by a 24-hr dark recovery). (C) Light-induced depigmentation is due to pigment cell loss. Images show representative red light-exposed animals fixed at the indicated times and labeled with KMO-1 or PBGD-1 riboprobes. (D) Light-induced cell death visualized by whole-mount TUNEL. Light-exposed animals were fixed 12 hr after a 24-hr exposure. (E) Quantitative analysis of TUNEL results. The number of TUNEL-positive nuclei (TPN)/mm2 was averaged over 3 independent experiments (n = total of 38 dark, 31 white and red light-exposed animals). Error bars = +/- s.e.m. *p-value <1 x 10–4 for two-tailed student’s t-test comparing light-exposed animals with controls. Scale bars: A = 300 µm; B = 500 µm; C,D = 100 µm.
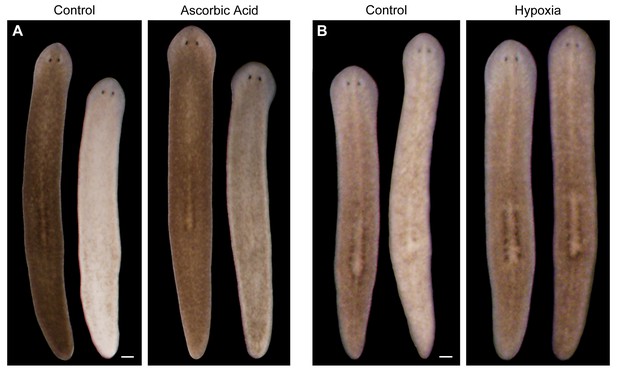
Antioxidants and hypoxia inhibit light-induced depigmentation.
(A) Representative control and ascorbic acid-treated animals photographed before and after white light exposure (left and right in each panel, respectively). Ascorbic acid treatment (10 mM final concentration, pH 7.0) was initiated 5 days prior to the start of light exposure and continued for the duration of the experiment. Total light exposure time = 60 hr (24- and 36-hr exposures were separated by a 24-hr dark recovery). (B) Animals were photographed before and after (left and right images in each panel, respectively) a series of intermittent periods of red light exposure under normoxic or hypoxic conditions. See Materials and methods for details. Scale bars = 200 µm.
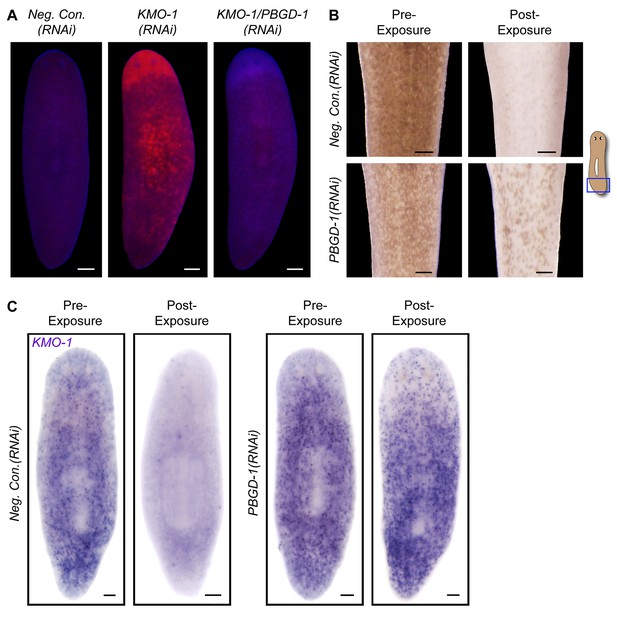
Porphyrins mediate light-induced pigment cell loss.
(A) PBGD-1 knockdown suppresses the porphyrin fluorescence observed in KMO-1(RNAi) animals. The difference in appearance of anterior tissues (top) between negative controls and KMO-1/PBGD-1(RNAi) animals is a consequence of the latters’ failure to repigment newly regenerated tissue (animals were amputated 3.5 weeks prior to photographing). See figure supplement 1 for additional controls. (B) PBGD-1 knockdown suppresses light-induced depigmentation. Animals were photographed before and after 48 hr of red light exposure. Note the greater pigmentation in PBGD-1(RNAi) animals after exposure, despite their lower initial pigmentation. For reasons that are presently unclear, this effect was restricted to the posterior, which typically depigments at a lower rate than anterior tissues. (C) PBGD-1 knockdown suppresses light-induced pigment cell loss. A KMO-1 riboprobe was used to visualize pigment cells in animals fixed before and after 7 days of continuous red light exposure. Scale bars: A,B = 200 µm; C= 100 µm.
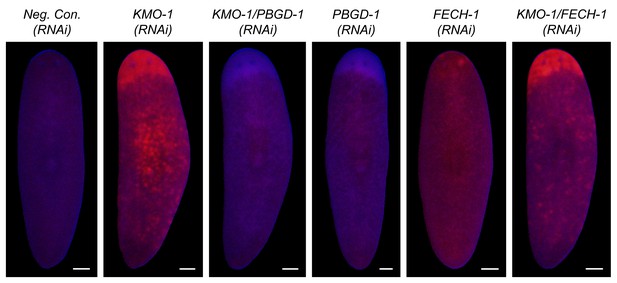
Porphyrin fluorescence controls.
Unlike PBGD-1 knockdown, FECH-1 knockdown did not suppress the porphyrin fluorescence observed in KMO-1(RNAi) animals. The slightly elevated, uniform fluorescence in FECH-1(RNAi) animals relative to negative controls is a reproducible effect that may reflect protoporphyrin IX accumulation in non-pigment cell types. Note that the images of Neg. Con.(RNAi), KMO-1(RNAi), and KMO-1/PBGD-1(RNAi) animals are the same as in Figure 6A (all images are from a single experiment). All scale bars = 200 µm.
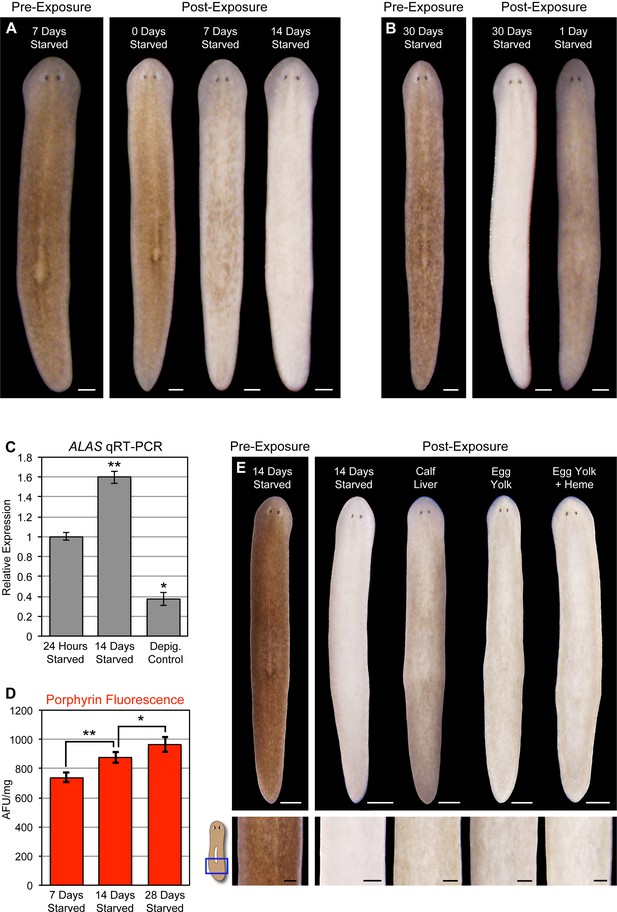
Starvation induces porphyrin biosynthesis and acute photosensitivity.
(A) Animals were fed 4 times in 1 week with dyed calf liver and then starved as indicated prior to 72 hr of red light exposure. Representative animals were photographed pre-exposure and 72 hr after the conclusion of light exposure. (B) Animals given a single feeding after 29 days of starvation and light exposed 24 hr later showed far less depigmentation than 30 day-starved animals. Post-exposure photographs were taken 24 hr after the conclusion of light exposure, as full depigmentation was already apparent in 30 day-starved animals. (C) qRT-PCR analysis of ALAS expression. The fold change relative to 24 hr-starved animals was averaged over 3 biological replicates. Depigmented animals showed reduced expression, as predicted based on the ALAS expression pattern (Figure 4B). Error bars = +/- standard deviation. **p-value <0.001 for two-tailed student’s t-test in comparison with 24 hr starved; *p-value <0.01. (D) Quantitative analysis of porphyrin fluorescence in D. japonica lysates, averaged over 10 biological replicates. AFU/mg = arbitrary fluorescence units/mg wet tissue weight. Error bars = +/- standard deviation. **p-value <1 x 10–7 for two-tailed student’s t-test; *p-value <0.001. (E) Photoprotective effect of different food sources. Well-fed animals were fasted, light exposed, and photographed as in (A), with a subset re-fed as indicated after 13 days. Light exposure was initiated for all groups at 24 hr post-feeding (day 14). Scale bars: A,B = 300 µm; E = 500 µm (top), 200 µm (bottom).
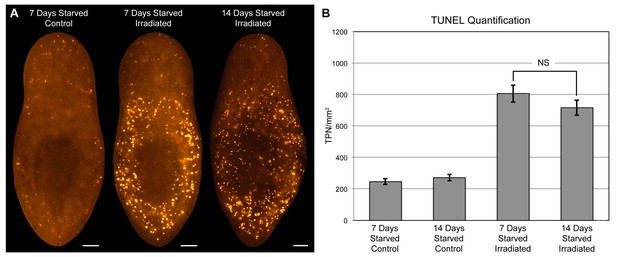
Starvation does not sensitize animals to induction of cell death by gamma radiation.
(A) Cell death was visualized by whole-mount TUNEL in animals starved for 7 or 14 days, and exposed to a 1250 rad dose of gamma radiation as indicated. All animals were fixed 24 hr post-irradiation. (B) Quantitative analysis of TUNEL results. The number of TUNEL-positive nuclei (TPN)/mm2 was averaged over 5 independent experiments (n > 40 animals per condition). Error bars = +/- s.e.m. NS = p-value >0.2 for two-tailed student’s t-test. The difference in animal size between conditions was also statistically insignificant (p-value >0.3). All scale bars = 100 µm.
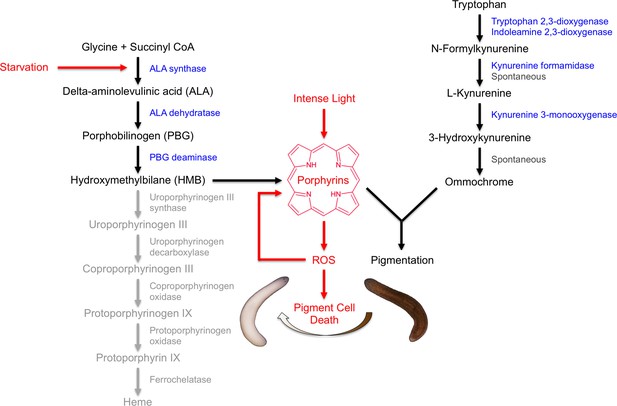
Model of S. mediterranea pigment biosynthesis and photosensitivity.
Body pigment cells produce both porphyrins and ommochrome, using the indicated biosynthetic pathways (black arrows). Together, these molecules confer the normal brown body color, possibly as a result of a physical interaction or joint use as precursors in a downstream biosynthetic step. Porphyrin formation entails PBG deaminase-dependent HMB synthesis; limiting (or absent) expression of downstream enzymes in the heme biosynthesis pathway (grey) leads to non-enzymatic cyclization of HMB to form uroporphyrinogen I, as in the erythroid cells of CEP patients (Discussion). Uroporphyrinogen I is a reduced porphyrin that can undergo spontaneous oxidation to form the potent photosensitizer uroporphyrin I. Intense light exposure causes porphyrin molecules to generate reactive oxygen species (ROS). This has the potential to initiate a positive feedback loop in which ROS drive further uroporphyrinogen I oxidation, leading to oxidative stress and pigment cell death. Starvation accelerates this response, at least partly through induction of ALAS expression and a consequent increase in porphyrin levels.
Videos
Depigmented animals exhibit normal movement.
Control animals maintained under standard laboratory conditions were filmed next to depigmented animals shortly after the conclusion of light exposure.
Depigmented animals exhibit normal touch responsiveness.
Like controls, depigmented animals change direction in response to the touch of a pipet tip.
Additional files
-
Supplementary file 1
Cloning of S. mediterranea ommochrome and porphyrin/heme biosynthesis genes.
See Materials and methods for details. 1Smed Unigene transcripts are available at the Schmidtea mediterranea Genome Database (Robb et al., 2015). 2Smed_ASXL transcripts are available under NCBI BioProject PRJNA215411. 3Not cloned (no RT-PCR product).
- https://doi.org/10.7554/eLife.14175.024





