Interactions between respiratory oscillators in adult rats
Figures
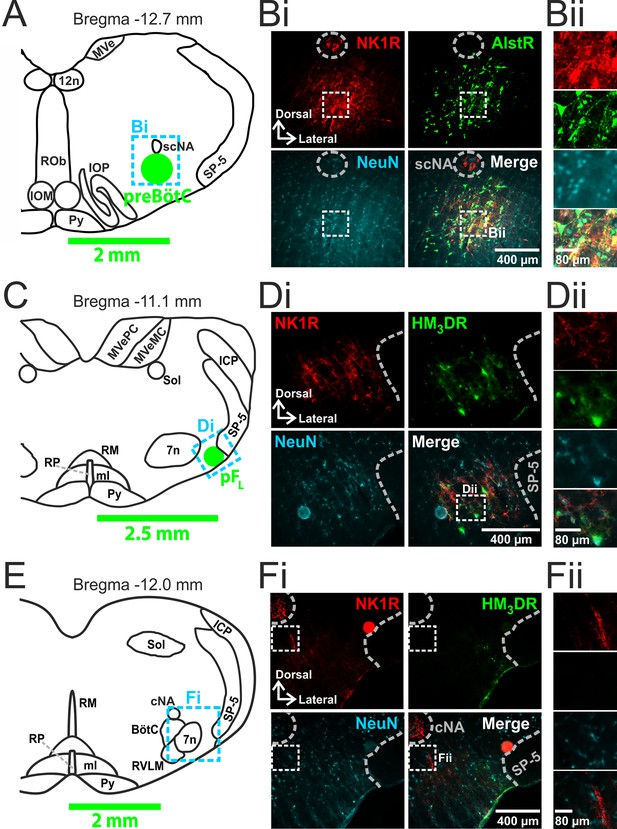
Transfection of neurons in preBötC and pFL.
(A) Localization of preBötC viral injections. Transverse view of medulla at Bregma -12.7 mm, green circle shows location of AlstR-GFP expressing neurons. Dashed blue box indicates location of immunocytochemistry shown in Bi. (B) Histological analysis of preBötC. (Bi) preBötC injection site: neurons identified by NeuN staining (blue) transfected with AlstR expressing GFP (green), co-localized with NK1R (red). (Bii) Expanded from Bi (dashed white boxes). (C) Localization of pFL viral injections. Transverse view of medulla at Bregma -11.1 mm, green circle shows location of HM3DR-mCitrine expressing neurons. Dashed blue box indicates location of immunocytochemistry shown in Di. (D) Histological analysis of pFL. (Di) pFL injection site: neurons identified by NeuN staining (blue) transfected with HM3D receptor (HM3DR) expressing mCitrine (green), co-localized with NK1R (red). (Dii) Expanded micrographs from merged figures in Di (dashed white boxes): NeuN (blue), mCitrine (Green), and NK1R (red). (E) Histological analysis of medulla at the level of the Bötzinger Complex (BötC). Transverse view of medulla at Bregma -12.0 mm. Dashed blue box indicates location of immunocytochemistry shown in Fi. (Fi) No transfected neurons were found in the BötC: neurons identified by NeuN staining (blue) transfected with AlstR or HM3DR co-expressing GFP (green), colocalized with NK1R (red). (Fii) Expanded from Fi (dashed white boxes). cNA – compact nucleus ambiguous, scNA – semi-compact nucleus ambiguous, SP-5 – spinal trigeminal tract, 12n – hypoglossal nucleus, 7n – facial nucleus, Py – pyramidal tract, MeV – medial vestibular nucleus, MeVMC – medial vestibular nucleus: magnocellular part, MeVPC - medial vestibular nucleus: parvocellular part, SOL – nucleus of the solitary tract, ROb – raphe obscurus, RP – raphe pallidus, RM – raphe magnus, ml – medial lemniscus, RVLM – rostral ventrolateral medulla, IOM - inferior olive, medial nucleus, IOP - inferior olive principle nucleus, ICP - inferior cerebellar peduncle (restiform body).
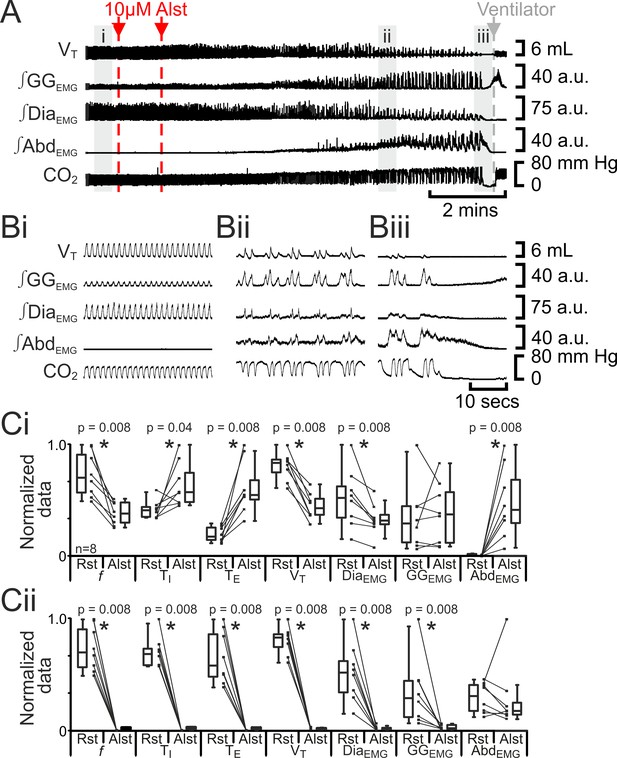
Hyperpolarizing preBötC neurons reduced ventilation and induced active expiration, but eventually resulted in apnea.
(A) Effect of Alst application to left preBötC (unilateral, first red arrow and dashed line) then right preBötC (bilateral, second red arrow and dashed line); gray arrow and dashed line mark onset of mechanical ventilation. (B) Expanded traces from A, indicated by shaded epochs (i-iii): (Bi) Activity at rest. (Bii) and (Biii) Activity following bilateral Alst injection. (C) Comparison between ventilation in rats at rest (Rst) and following Alst: (Ci) before Alst had taken full effect (≈Bii). (Cii) After Alst had taken full effect (≈Biii). Lines connect data from individual experiments, box and whisker plots show combined data. Data are normalized to highest parameter, i.e., f, TI, TE, VT, ∫GGEMG, ∫DiaEMG, or ∫AbdEMG, value regardless of whether it belonged to control or Alst group. f – frequency, TI – inspiratory period TE, – expiratory period, VT – tidal volume, ∫GGEMG – integrated genioglossus electromyogram, ∫DiaEMG – integrated diaphragm electromyogram, ∫AbdEMG – integrated abdominal electromyogram.
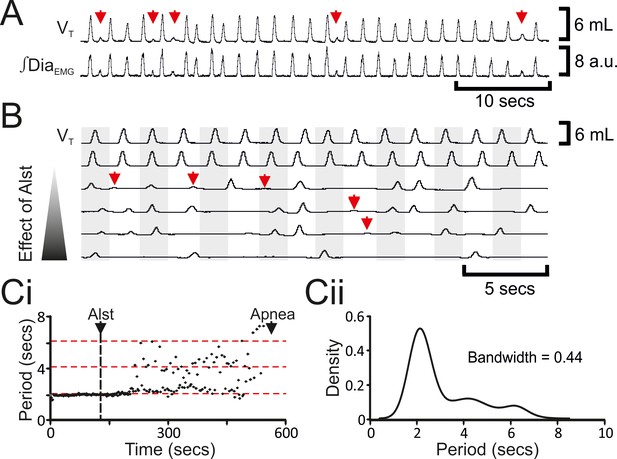
Hyperpolarizing preBötC neurons leads to quantal slowing of breathing and burstlet-like activity in ∫DiaEMG.
(A) Burstlet-like activity in airflow and ∫DiaEMG traces (red arrows). (B) Traces at different time points (top to bottom ranging from -5 to +10 min) after Alst infusion showing burstlet-like activity. (C) Quantal slowing of breathing. (Ci) Raster plot of respiratory period before and after Alst. (Cii) Kernel density estimations determined the optimal bandwidth, i.e., bin size, of 0.44 s, revealing a multimodal distribution with respiratory periods at quantal intervals of ~2.1 s.
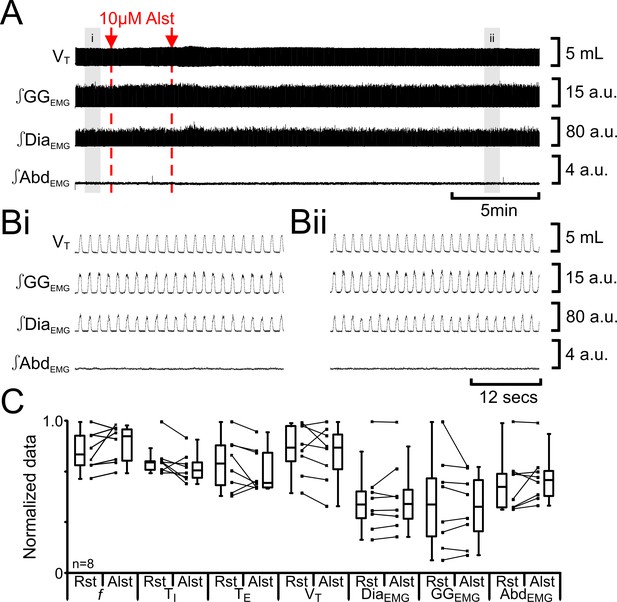
Alst in absence of AlstRs does not affect breathing.
(A) Effect of Alst application to left preBötC (unilateral, first red arrow and dashed line) then right preBötC (bilateral, second red arrow and dashed line). (B) Expanded traces from A, indicated by shaded epochs (i–ii): (Bi) Activity at rest. (Bii) Activity following bilateral Alst injection. (C) Comparison between ventilation in rats at rest (Rst) and following Alst. Lines connect data from individual experiments, box and whisker plots show combined data. Data are normalized to highest parameter, i.e., f, TI, TE, VT, ∫GGEMG, ∫DiaEMG, or ∫AbdEMG, value regardless of whether it belonged to control or Alst group. Abbreviations defined in Figure 2.
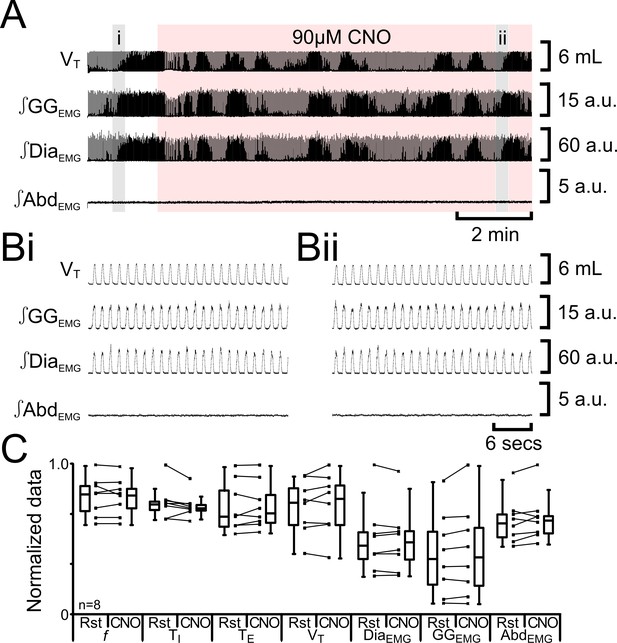
CNO in absence of HM3DRs does not affect respiration.
(A) Effect of CNO applied to ventral surface (pink shaded area). (B) Expanded traces from A, indicated by shaded epochs (i-–ii): (Bi) Activity at rest. (Bii) Activity in presence of CNO. (C) Comparison between ventilation in rats at rest and in presence of CNO. Lines connect data from individual experiments, box and whisker plots show combined data. Data are normalized to highest value for that parameter, i.e., f, TI, TE, VT, ∫GGEMG, ∫DiaEMG, or ∫AbdEMG regardless of whether it belonged to control or CNO group. Abbreviations defined in Figure 2.
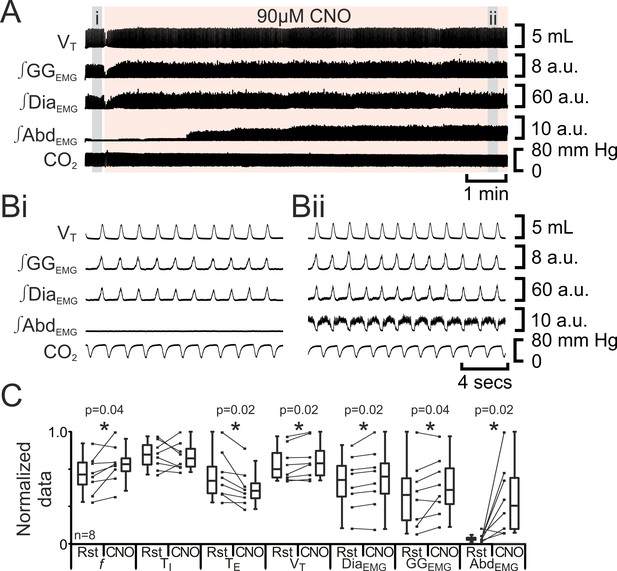
Depolarizing pFL neurons elicits active expiration.
(A) Effect of CNO applied to ventral surface (pink shaded area). (B) Expanded traces from A, indicated by grey shaded epochs (i-ii): (Bi) Activity at rest. (Bii) Activity in presence of CNO. (C) Comparison between ventilation in rats at rest and in presence of CNO. Lines connect data from individual experiments, box and whisker plots show combined data. Data are normalized to highest value for that parameter, i.e., f, TI, TE, VT, ∫GGEMG, ∫DiaEMG, or ∫AbdEMG regardless of whether it belonged to control or CNO group. Abbreviations defined in Figure 2.
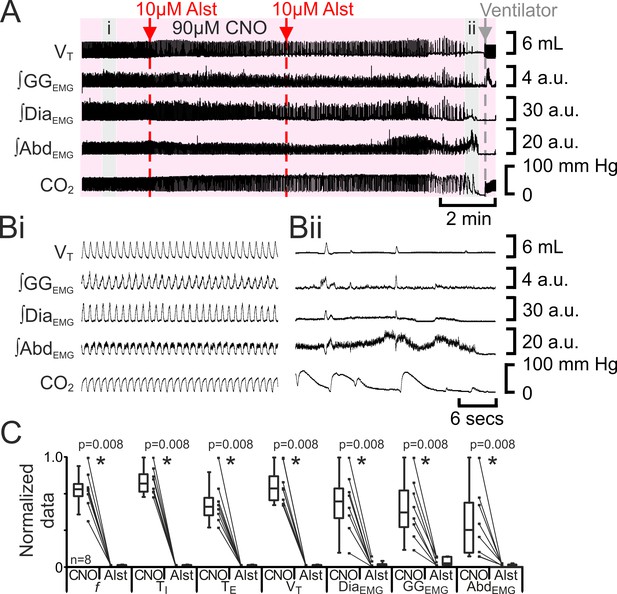
Hyperpolarizing preBötC neurons leads to apnea, and loss of active expiration even with activation of pFL.
(A) Integrated traces from a single experiment showing effect of Alst injection to left preBötC (unilateral, first red arrow and dashed line) then right preBötC (bilateral, second red arrow and dashed line), in presence of CNO (pink shaded area); gray arrow and dashed line mark onset of mechanical ventilation. (B) Expanded traces from A indicated by shaded epochs: (Bi) In presence of CNO only. (Bii) Following Alst in presence of CNO. (C) Comparison between ventilation in rats in presence of CNO and following Alst in presence of CNO. Lines connect data from individual experiments, box and whisker plots show combined data. Data are normalized to highest value for that parameter, i.e., ∫GGEMG, ∫DiaEMG, or ∫AbdEMG regardless of whether it belonged to CNO or CNO with Alst group. Abbreviations defined in Figure 2.
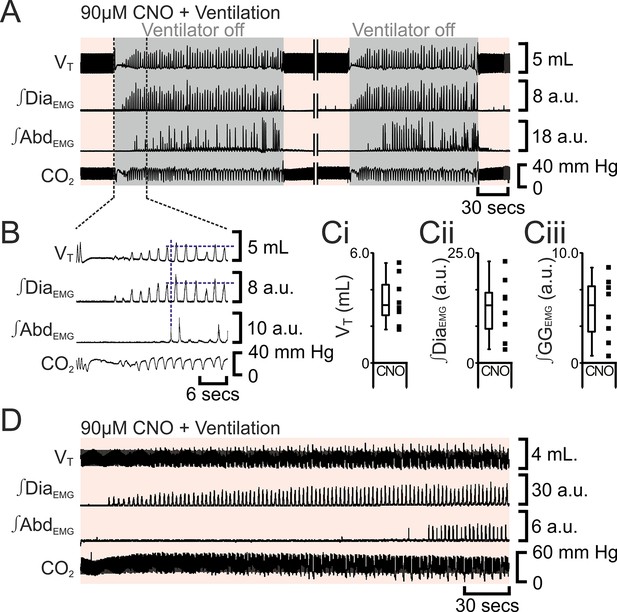
Following apnea, in presence of CNO, active expiration only returns after inspiratory activity returns.
(A) Integrated traces from one experiment showing effect of removing rat from ventilator (dark grey shaded area), in presence of CNO (pink shaded area), following induction of apnea by microinjection of Alst into the preBötC of preBötC-AlstR transfected rats. Intervening period between the two traces, whilst ventilation was ongoing and continuous, has been removed (double break), so traces can be expanded. (B) Expanded traces from A (indicated by black dashed lines), showing how measurements were taken for inspiratory parameters when active expiration returned. (C) Inspiratory parameters when active expiration returned. Dots represent individual experiments, box and whisker plots show combined data. (Ci) Tidal volume (VT) (Cii) ∫DiaEMG, (Ciii) ∫GGEMG. (D) Integrated traces from one experiment showing the return of inspiratory and expiratory activity during mechanical ventilation, in presence of CNO (pink shaded area), following induction of apnea by microinjection of Alst into the preBötC of preBötC-AlstR transfected rats. Abbreviations defined in Figure 2.
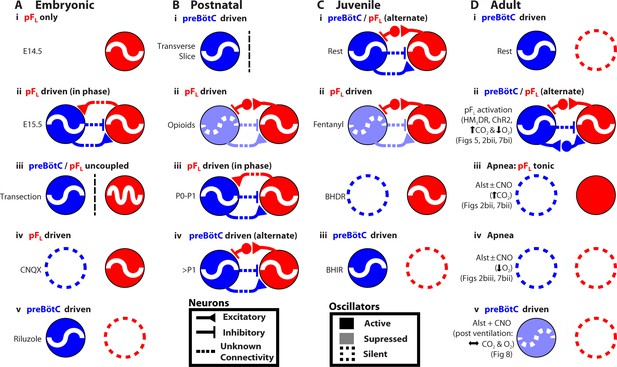
Developmental and state-dependent changes in coupling between pFL and preBötC.
Functional connections of undetermined connectivity are indicated as broken lines. As pFL neurons are excitatory (Onimaru et al., 2008; Thoby-Brisson et al., 2009) and lack inhibitory markers (Ellenberger, 1999; Tanaka et al., 2003), inhibitory connections from pFL to preBötC are indirect (see Figure 7 in Huckstepp et al., 2015). (A) Embryonic stage (all data in vitro). (Ai) pFL (red circle) oscillates at embryonic day 14.5 (E14.5). (Aii) preBötC (blue circle) oscillates at embryonic day 15.5 (E15.5) and couples to the pFL, where it excites and inhibits different groups of pFL neurons. (Aiii) preBötC and pFL can oscillate independently of each other following a transverse section caudal to facial nucleus. (Aiv) pFL can oscillate in the absence of preBötC following bath application of a glutamatergic antagonist (CNQX). (Av) preBötC can oscillate in the absence of the pFL following bath application of a sodium channel blocker (riluzole) (Thoby-Brisson et al., 2009). (B) Postnatal stage. (Bi) In late fetal (Thoby-Brisson et al., 2005; Bouvier et al., 2008) and postnatal rats (Smith et al., 1991), the preBötC can oscillate in the absence of the pFL in transverse slices, and (Bii) the pFL can oscillate independently following suppression of preBötC rhythm by bath application of opioid agonists (Takeda et al., 2001; Janczewski et al., 2002). Biii) Immediately following birth, respiratory rhythm is driven by pFL (Onimaru and Homma, 2003; Oku et al., 2007). (Biv) Shortly after birth (>1 day), the breathing CPG becomes driven by the preBötC (Oku et al., 2007). (C) Juvenile stage. (Ci) Expiration and inspiration alternate and are reciprocally coupled. (Cii) PreBötC and pFL are differentially affected by fentanyl, which shifts breathing to an expiratory-dominant pattern. (Cii + iii) preBötC and pFL can be independently suppressed by activation of Breuer-Hering deflation reflex (BHDR; Cii) or inflation reflex (BHIR; Ciii) (Janczewski and Feldman, 2006). (D) Adult Stage: (Di) breathing is inspiratory driven by preBötC while pFL activity is normally suppressed at rest (also see Pagliardini et al., 2011 and Huckstepp et al., 2015); (Dii) activation of HM4DR transfected pFL neurons by CNO (see Figure 6) or optogenetic activation (Pagliardini et al., 2011), or suppression of AlstR transfected preBötC neurons with Alst (see Figure 2, 7) can induce active expiration; (Dii) as preBötC neurons project to the pFV but do not appear to project to the pFL (Tan et al., 2010), excitatory drive from the preBötC to the pFL is most likely through an intermediate excitatory relay, such as the pFV. (Diii) Depression of inspiration by Alst, in presence or absence of CNO, leads to tonic expiratory activity during hypercapnia (see Figures 2, 7) or (Div) apnea during hypoxia (see Figures 2, 7). (Dv) As breathing returns, abdominal activity remains absent until inspiratory activity is near normal levels (see Figure 8), implicating an indirect involvement of preBötC excitatory neurons in expiration either through its excitatory projections throughout breathing CPG (Tan et al., 2010), including pFV that contributes to expiratory activity (Huckstepp et al., 2015), or through mechanosensory feedback that can provides expiratory drive (Remmers, 1973; Davies and Roumy, 1986; Janczewski and Feldman, 2006).





