Murine Fam65b forms ring-like structures at the base of stereocilia critical for mechanosensory hair cell function
Figures
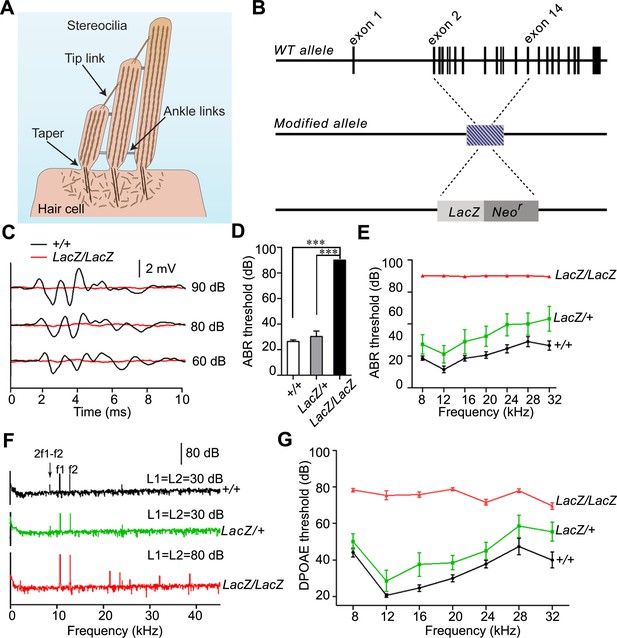
Analysis of hearing function in Fam65b-deficient mice.
(A) Diagram of cochlear hair cells showing the mechanically sensitive hair bundle. Stereocilia are arranged into three rows of decreasing heights. Stereocilia form a taper at their base near the insertion site into the apical hair cell surface. (B) Diagram of the strategy to generate Fam65b knock–out mice. Exons of Fam65b were substituted with a LacZ expression cassette. (C) Representative ABR traces to click stimuli in the indicated control and mutant mice at 4 weeks of age. (D) Statistic results of ABR thresholds to click stimuli at 4 weeks of age. Results are represented as mean ± SE (standard error of the mean). ***p<0.001, by Student’s t-test. (E) ABR thresholds to pure tones at 4 weeks of age. Results are represented as mean ± SE. ***p<0.001 (ANOVA) (F) Representative DPOAE response spectra from control and mutant mice at a single stimulus condition (median primary frequency = 12 kHz). Note the 2f1-f2 peak (black arrow), which is absent in mutant mice. (G) DPOAE thresholds at different frequencies in animals at 4 weeks of age. Results are represent as mean ± SE. ***p<0.001 (ANOVA). More than five animals in each group were tested.
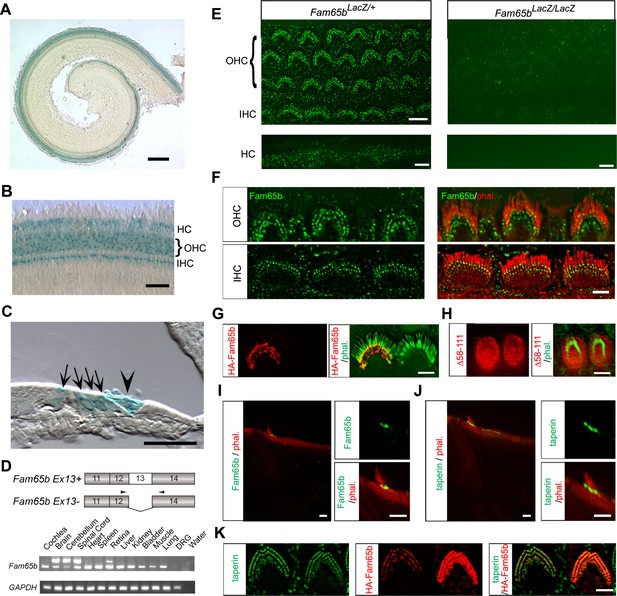
Expression of Fam65b in hair cells.
(A–C) Cochlear whole mounts from P4 Fam65bLacZ/+ mice were stained for LacZ. (A) Whole mount staining reveals expression of Fam65b along the length of the cochlear duct. (B) Higher magnification view of whole mount cochlea. OHCs, IHCs and Hensen’s cells (HCs) express LacZ. (C) Section through a whole mount revealing LacZ expression in OHCs, IHCs, and HCs. Arrows point to OHCs and, IHCs, arrowhead points to HCs. (D) PCR analysis of Fam65b isoform expression in different tissues. Upper panel shows the mouse Fam65b gene structure. Boxes with numbers represent exons. Arrowheads show positions of primers. Lower panel shows expression of Fam65b isoforms in different tissues. GAPDH served as a loading control. Water lane is the negative control. (E) Cochlear whole mounts from Fam65bLacZ/+ and Fam65bLacZ/LacZ mice at P5 were stained for a commercial antibody to Fam65b (Sigma, green) and imaged with fluorescent deconvolution microscopy. The lower panel shows Hensen's cells (HC). Note the absence of a signal in the mutant mice. (F) Co-staining of cochlear whole mounts with phalloidin-rhodamine to reveal stereocilia (red) and antibodies to Fam65b (green). Note the localization of Fam65b at the base of stereocilia. (G) Cochlear explants were prepared at P3 and injectoporated to express HA-Fam65b. Two days later, the cells were stained with HA-antibody. Note the expression of HA-Fam65b at the base of stereocilia. (H) Cochlear explants were prepared at P3 and injectoporated to express HA-Fam65b (∆58–111), corresponding to a mutation that causes deafness in humans (Diaz-Horta et al., 2014). Note the diffuse expression of the mutated protein within the cytoplasm of hair cells with no specific localization at basal regions of hair cells. (I, J) Inner ear sections (P5) were stained with Fam65b (I) and taperin (J) antibodies. Tissues were counterstained with phalloidin. Note expression of Fam65b and taperin at the base of stereocilia. (K) Cochlear explants were prepared at P3 and injectoporated to express HA-Fam65b. Two days later, the cells were stained with antibodies against HA (red) and taperin (green). Note colocalization of HA-Fam65b and taperin in injectoporated hair cells. Note that the cell to the right strongly expressed the transgene, while the cell to the left expressed it weakly. Scale bars: (A) 500 μm; (B, C) 50 μm; (E–K) 4 μm.
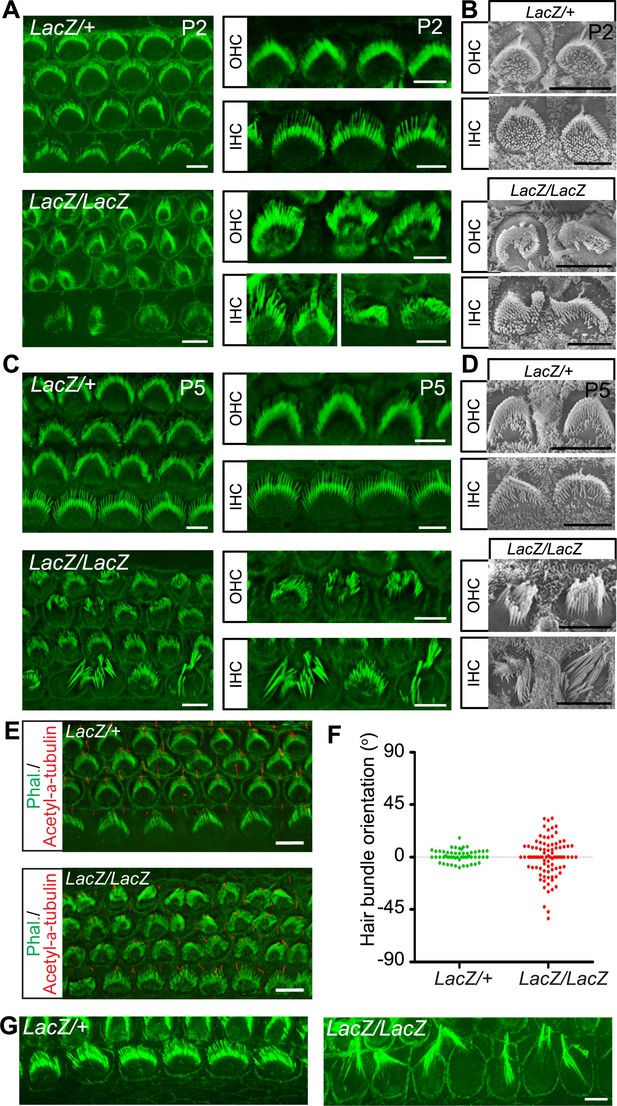
Hair cell morphology in Fam65b-deficient mice.
(A, C) Analysis of the cochlea in P2 (A) and P5 (C) control and mutant animals by whole mount staining with phalloidin (green). Note the morphological changes in hair bundles from mutant mice with defects in bundle polarity, cohesion and length of stereocilia. (B, D) Analysis of hair cells by SEM at P2 (B) and P5 (D). (E) Cochlear whole mounts at P2 were stained with phalloidin and with antibodies to acetylated–α–tubulin to reveal kinocilia. (F) Bundle orientation was determined by drawing a line through the axis of the bundle with 0o indicating the normal medio–lateral axis. Angular deviation from this axis was determined. Each dot represents one hair cell (n=55 for control; n=90 for mutants). (G) Cochlear explants from control and mutant animals were prepared at P2 and cultured for two days in vitro. Samples were stained by phalloidin. Note the hair bundles of most IHCs from mutants lacking Fam65b were disorganized and the stereocilia were extraordinarily long. Scale bars: 6 μm.
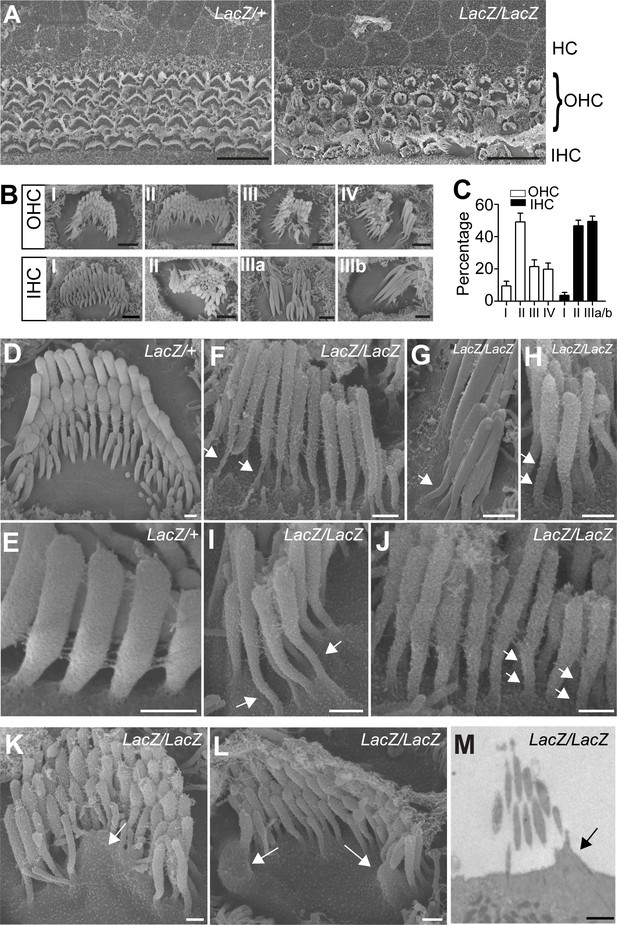
Analysis of hair bundle morphology by SEM.
Whole mounts from the middle part of the cochlea from Fam65bLacZ/+controls and Fam65bLacZ/LacZmutants at P5(A) where analyzed by SEM. (A) Low magnification view showing disorganization of hair bundles in the mutants. (B) Hair bundles were classified both for OHCs and IHCs according to morphological criteria. For OHCs: (I) rounded bundle–shape that deviated from the classical V–shape; (II) elongated but coherent bundles; (III) rounded bundles with signs of degeneration; (IV) fragmented bundles with some thin and elongated stereocilia. For IHCs: (I) coherent bundles with slight abnormalities; (II) coherent bundles with degenerative changes; (IIIa) fragmented bundles with thin and elongated stereocilia; (IIIb) fragmented bundles with few remaining thin and elongated stereocilia. (C) Quantification of the number of hair bundles in different morphological classes (mean ± SEM; n=503 for OHCs; n=191 for IHCs). (D–L) Higher magnification views of hair bundles. Arrows point to abnormally shaped basal domains of stereocilia (F–J) and to protrusions from the apical cell surface (K,L). (M) Transmission electron microscopy image. The arrow points to a protrusion from the apical cell surface. See also Figure 4—figure supplement 1 for further data on the study of hair cell survival. Scale bars: (A) 10 μm; (B) 1 μm; (D–L) 0.3 μm(M) 2 μm.
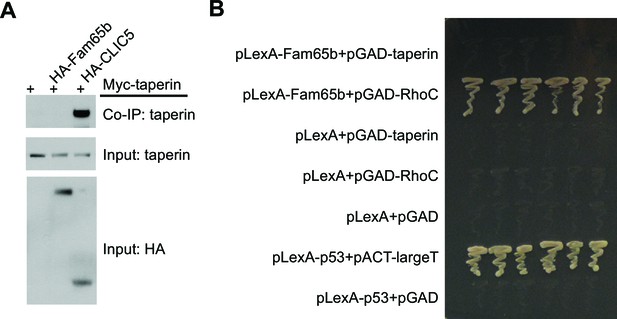
Lack of Fam65b interactions with taperin.
(A) Co–immunoprecipitation experiments. CL4 cells were transfected with the constructs indicated on top of each panel. Immunoprecipitations were carried out with HA antibody followed by western blotting to detect Myc–taperin. The middel and lower panel show input protein, the upper panel shows co–immunoprecipitation (CoIP) results. Taperin interacts with CLIC5 but not with Fam65b. (B) Yeast-two-hybrid analysis. Yeast cells were transformed with the constructs indicated on the left and cultured on an SD-LeuTrp plates to allow for growth of colonies that had taken up the two plasmids. Six independent clones for each condition were picked up from SD-LeuTrp plate and cultured on SD-LeuTrpHisAde selection plates that only allow colonies to grow following interaction between the bait and prey. After two day culture time, the photograph in the panel was taken. pLexA and pGAD are empty bait and prey vectors. pLexA-p53 and pACT-largeT served as positive control. Fam65b directly interacts with RhoC, but not with taperin.
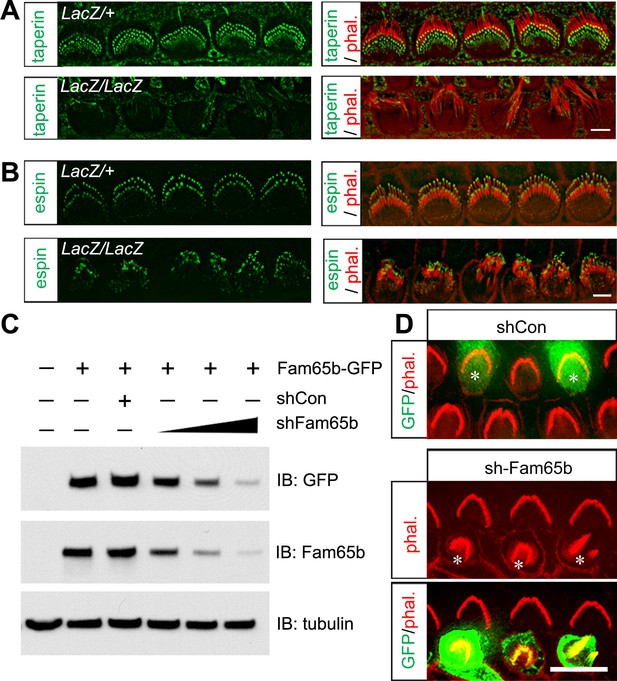
Taperin localization and shRNA perturbation of Fam65b.
(A, B) Taperin (green, A) and espin (green, B) staining in control and mutant IHCs analyzed by fluorescent deconvolution microscopy. Stereocilia were visualized by staining with phalloidin–rhodamine (red). The taperin signal is reduced and diffused in mutant hair cells, while the espin signal is similar. (C) shRNA targeting Fam65b efficiently knocks down Fam65b–GFP expression in transfected HEK293 cells. Different ratios (2:1; 1:1 and 1:2) of Fam65b–GFP and shRNA plasmids were used. Scrambled shRNA was used as knock-down control and tubulin was used as a loading control for western blotting. (D) Cochlear explants were prepared at P2 and injectoporated to express GFP and control shRNA, or GFP and shRNA targeting Fam65b. Three days later, tissues were fixed and stereocilia were visualized by phalloidin staining. Note disorganized hair bundles in shFam65b positive hair cells (asterisks in middle panel) but not in hair cells expressing control shRNA (asterisks upper panel). Scale bars: (A, B) 3 μm; (D) 6 .
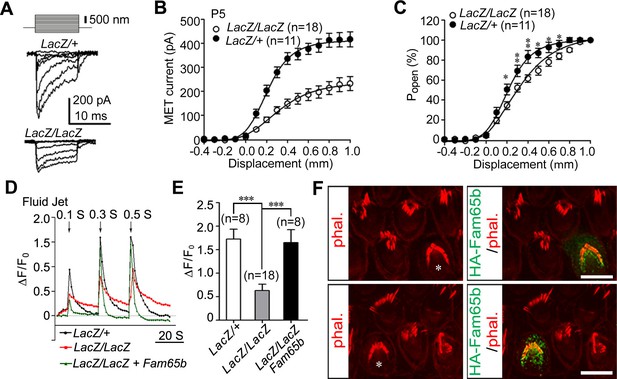
Analysis of mechanotransduction currents in Fam65b-deficient hair cells.
(A) Examples of transduction currents in OHCs from control and mutant mice at P5 in response to a set of 10 msec hair bundle deflections ranging from –400 nm to 1000 nm (100 nm steps). (B) Current displacement plots obtained from similar data as shown in (A). (C) The Po–displacement relationship plot obtained with peak currents following deflection reveals a significant rightward shift and broadening of the curve in mutant hair cells. (D) Representative example demonstrating fluid–jet induced Ca2+ response in G-CaMP3-expressing OHCs from controls, Fam65b mutants, and Fam65b mutants following re-expression of Fam65b. OHCs were transfected at P2 and cultured for 2 days in vitro. Sequential fluid-jet pulse durations were 0.1 s, 0.3 s and 0.5 s. For quantitative analysis (panel E), the amplitude of the 2nd Ca2+ response peak was measured. (E) Quantification of similar Ca2+ responses as shown in (E). The number of analyzed hair cells is indicated in brackets. (F) Fam65b-deficient cochlea explants were prepared at P2 and injectoporated with HA-Fam65b. After in vitro culture for 2 days, samples were fixed and stereociliary morphology was visualized by phalloidin staining. Note the morphological defects could be rescued. Scale bar: 6 μm. All values are mean ± SE ***p<0.001, by Student’s t-test.
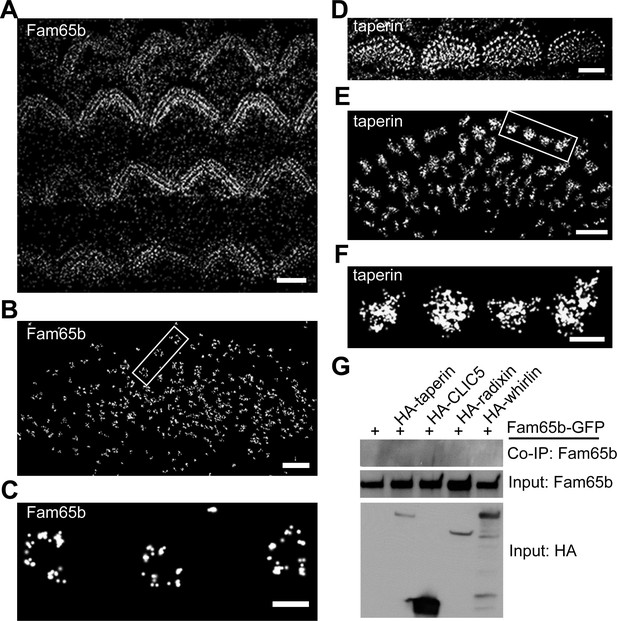
Distinct localization pattern of Fam65b and taperin resolved by STORM.
(A–F) STORM images of Fam65b (A–C) and taperin (D–F) localization in hair cells. A and D show magnification/resolution similar to fluorescent deconvolution microscopy or traditional confocal microscopy. (B, C, E, and F) are higher magnifications only accessible by STORM, demonstrating the distinct localizations of Fam65b and taperin. (C) and (F) are close-up of boxed region in (B) and (E). (G) Lack of interactions between Fam65b and taperin, CLIC5, radixin and whirlin. HEK293 cells were transfected with the constructs indicated on top of each panel. Immunoprecipitations were carried out with HA antibody followed by western blotting to detect Fam65b-GFP. The lowest rows show input protein, the upper row shows co-immunoprecipitation (CoIP) results. See also Figure 7—figure supplement 1 for further data on the analysis of interactions between Fam65b and taperin. Scale bars, (A, D) 3 µm; (B, E) 1 µm; (C, F) 200 nm.
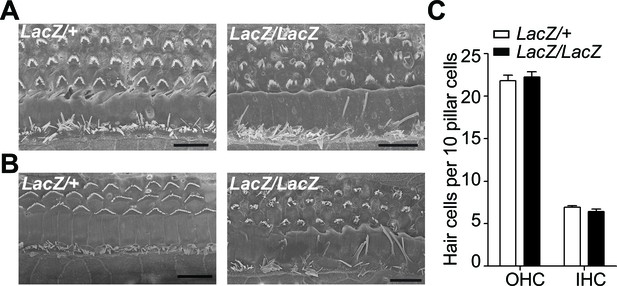
Minimal hair cell loss in Fam65b mutants at P16 and P28.
(A,B) SEM images of the middle part of the cochlea from P16 (A) and P28 (B) Fam65Lacz/+control (left) and Fam65Lacz/Laczmutants (right). Although hair bundles were disorganized at P16 and P28 in Fam65Lacz/Laczmutants, no significant hair cell loss was observed compared to Fam65Lacz/+controls. (C) OHCs and IHCs counts per 10 pillar cells from mutants and control mice at P28 (control: n=127, OHC; n=40 IHCs; n= 58 pillar cells; mutant: n=351, OHCs; n=101 IHCs; pillar cells n=158). Scale bar, 10 µm.
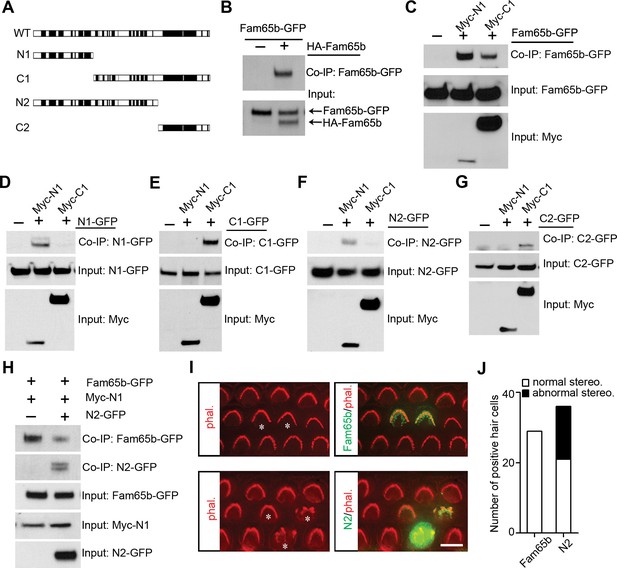
Fam65b forms oligomeric structures.
(A) Diagram of the constructs used for biochemical experiments. Evolutionarily conserved domains are indicated in black. (B–G) HEK293 cells were transfected with the constructs indicated on top of each panel. Immunoprecipitations were carried out with HA (B) or Myc (C–G) antibodies, followed by western blotting to detect co–expressed proteins. The upper rows show CoIP results and the lower rows show input protein. (H) N2-GFP perturbs interactions between Fam65b-GFP and N2-GFP. CoIP blot results above and input protein blot results below. (I) OHCs were injectoporated at P2 to express Fam65b-GFP or N2-GFP, expression of which was evaluated 2 days later by immunohistochemistry (green). Stereociliary morphology of OHCs was visualized by phalloidin staining (red). Note disorganization of stereociliary hair bundles in N2-GFP transfected cells that are marked with an asterisk. Note that one hair cell in the lower panel expressed barely detectable levels of the transgene and showed no structural defects. (J) Number of transfected hair cells and quantification of morphology from (I). Black column represents hair cells with morphological defects. Scale bar: 6 µm.
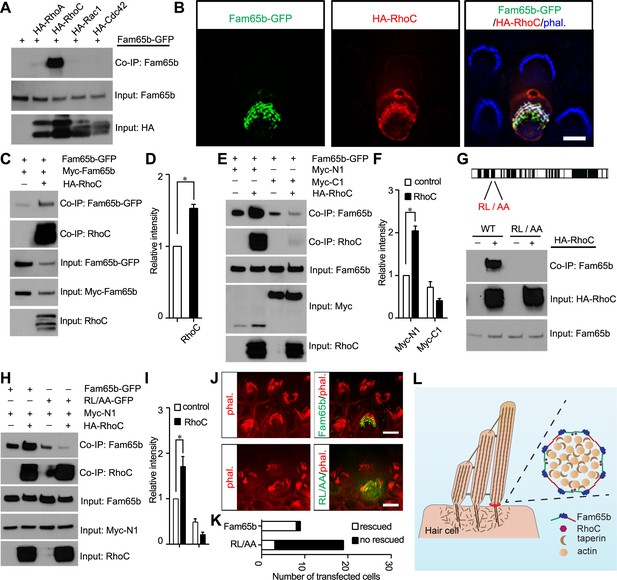
Effects of RhoC on Fam65b oligomerization.
(A) HEK293 cells were transfected with the constructs indicated on top of each panel. Immunoprecipitations were carried out with HA antibody, followed by western blotting to detect co–expressed proteins. The upper row shows CoIP result and the lower rows show input protein. Note strong binding activity was detected between Fam65b and RhoC, weak binding was detected with RhoA, while no binding was detected with Rac1 and Cdc42. (B) OHCs were injectoporated at P2 to express Fam65b–GFP and HA–RhoC. Expression of Fam65b-GFP (green) and HA-RhoC (red) was evaluated 2 days later by immunohistochemistry. Stereocilia were visualized by phalloidin staining (blue). Note colocalization of Fam65b-GFP and HA-RhoC at the base of stereocilia. Scale bar: 4 µm. (C, E, G, H) HEK293 cells were transfected with the constructs indicated on top of each panel. Immunoprecipitations were carried out with Myc antibody (C, E, H) or HA antibody (G), followed by western blotting to detect co-expressed proteins. The upper rows show CoIP results and the lower rows show input protein. Note co-expression of HA-RhoC enhances/stabilizes the oligomerization of Fam65b at N termini (C) but not C termini (E). RhoC did not promote oligomerization of a Fam65b mutant (RL changed to AA; diagram in G) devoid of RhoC binding activity (G, H). (D, F, I) Quantification of CoIP results by scanning of similar gels as shown in (C) (E) and (H). The values are derived by quantifying more than 3 independent experiments. (J) Fam65b-deficient OHCs were injectoporated at P2 to express Fam65b-GFP or mutant Fam65b protein without RhoC binding activity (Fam65bRL/AA-GFP). Expression of Fam65b was evaluated 2 days later by staining for GFP (green). Stereociliary morphology of OHCs was visualized by phalloidin staining (red). Note morphological defects of stereocilia were rescued by Fam65b-GFP but not by Fam65bRL/AA-GFP. Scale bar: 6 μm. (K) Quantification of transfected hair cells from (J). White column represents number of rescued OHCs. (L) Model of Fam65b localization pattern in hair cells. In our model, Fam65b forms a circumferential ring near the basal taper domain of stereocilia, while taperin forms a dense core structure. Fam65b forms oligomers via head-to-head and tail-to-tail interactions and RhoC binds to Fam65b and promotes oligomerization.





