Complementary shifts in photoreceptor spectral tuning unlock the full adaptive potential of ultraviolet vision in birds
Figures
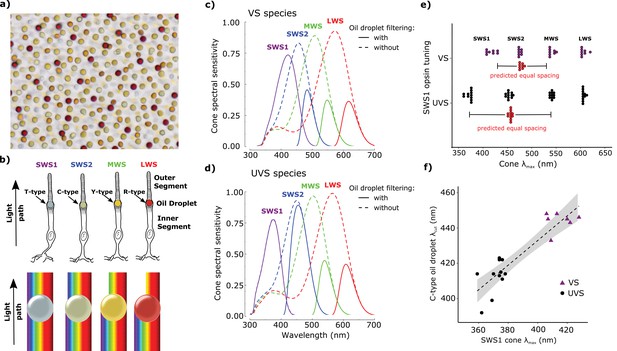
Avian color vision is mediated by four single cone photoreceptors that are tuned by cone oil droplet spectral filters.
(a) A flat-mounted chicken retina under brightfield illumination that shows the distinctive pigmentation of the cone oil droplets. (b) A diagram of the avian single cone photoreceptors showing the relative position of the oil droplet within the cells (top) and a representation of the spectral filtering cutoff effects of the droplet (bottom). The spectral sensitivity of the single cone photoreceptors of the (c) chicken (VS species) and (d) zebra finch (UVS species) with (solid lines) or without (broken lines) the filtering of cone oil droplets. These spectra are scaled to reflect the decrement in absolute sensitivity resulting from oil droplet and ocular media spectral filtering. The SWS1 cone contains a transparent oil droplet, and its short-wavelength sensitivity is limited only by the filtering of the ocular media. (e) The peak sensitivity (λmax) of the four single cone photoreceptors of VS (purple) and UVS (black) species calculated from microspectrophotometric measurements of visual pigment sensitivity and oil droplet filtering in published reports (Supplementary file 2). The red points shown below the SWS2 λmax values are the λmax for each species that would maximize spectral separation from adjacent receptors (λmax of maximum separation = λmax SWS1 + (λmax MWS - λmax SWS1)/2). The actual SWS2 λmax of the UVS species do not differ significantly from these predicted values (paired t-test, t = − 1.27, p = 0.23) and the λmax of the VS species are an average of only 3.6 nm shorter than the predicted values (paired t-test, t = −2.56, p = 0.04). (f) Across bird species there is a significant correlation between the spectral tuning of the SWS1 visual pigment and the blue cone oil droplet filtering cutoff (phylogenetic generalized linear model: t = 5.55, p<0.0001, r2 = 0.63). Each point represents a different species; the dashed line is a linear regression through the points with 95% confidence interval shown in gray.
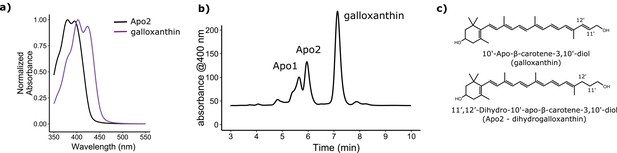
The avian retina contains multiple apocarotenoids that absorb different portions of the light spectrum.
(a) The absorbance spectrum of the major apocarotenoid pigments in the chicken C-type oil droplets. (b) A representative HPLC chromatogram of the apocarotenoids in whole retina extracts of the chicken retina. Apo1 has an absorbance spectrum similar to galloxanthin with more pronounced fine structure, suggesting an ε-ring configuration (Figure 2—figure supplement 2). This pigment is not a major component of the C-type oil droplets (Toomey et al., 2015). (c) The proposed chemical structure of dihydrogalloxanthin and known structure of galloxanthin with the positon of the 11’,12’ double bond indicated.
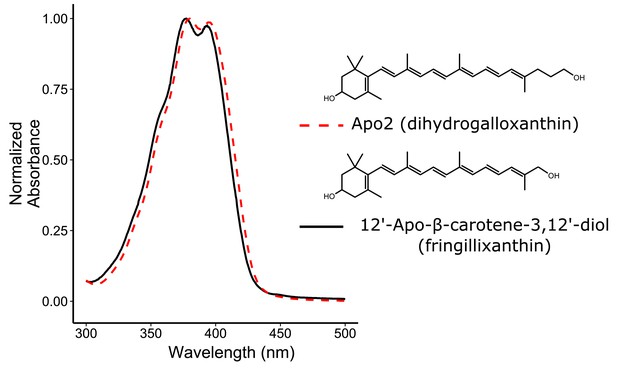
The absorbance spectra of Apo2 and 12'-Apo-β-carotene-3,12'-diol (fringillixanthin) are nearly identical.
A comparison of the UV-Vis spectrum and chemical structure of Apo2 (dihydrogalloxanthin) and 3-OH-apo-12’-carotenol (fringillixanthin).
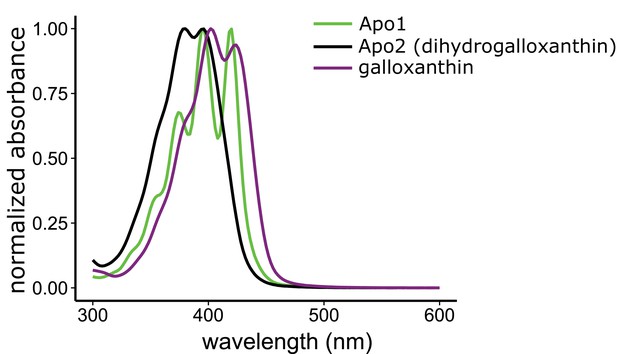
The three major apocarotenoids present in the avian retina have distinct light absorbance spectra.
The UV-Vis absorbance spectra of the three apocarotenoids observed in the chicken retina by HPLC analysis. The structure of apo1 is not known. However, the deeply fingered fine structure of the spectrum is consistent with that of an apocarotenoid containing an ε-bond configuration in the terminal ring.
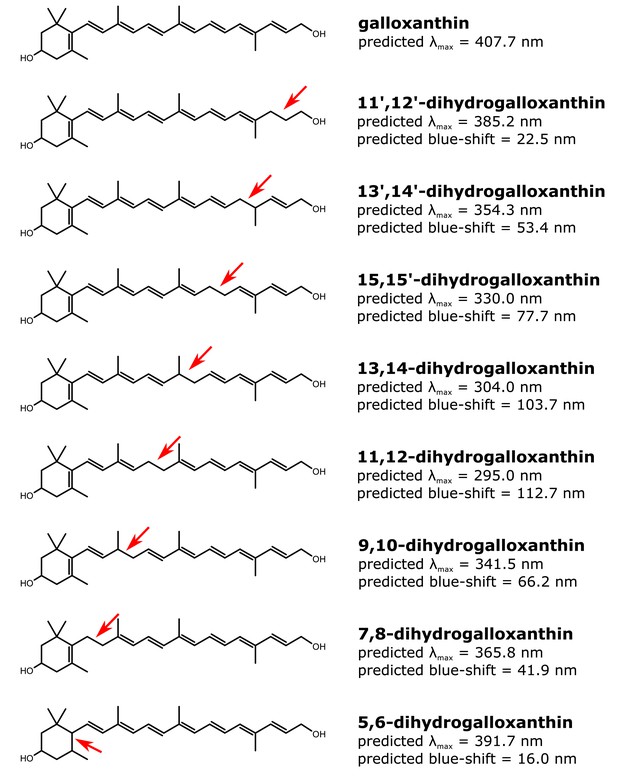
The structure and predicted wavelength of maximum absorbance for galloxanthin and the eight possible monosaturated forms of galloxanthin.
The wavelength of maximum absorbance (λmax) was predicted using the Woodward-Fieser for molecules with conjugated systems of four double bonds or less and the Fieser-Kuhn rule for all others. The blue-shift of the λmax relative to the predicted λmax of galloxanthin is given for each monosaturated structure.
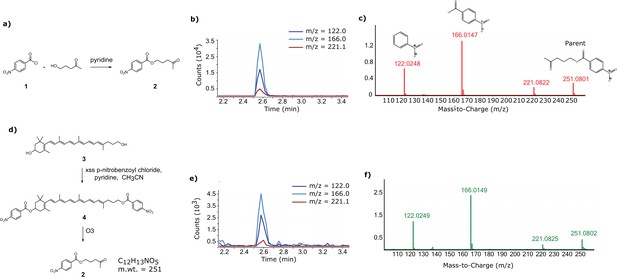
Confirmation of the 11’,12’ saturation of dihydrogalloxanthin via derivatization and ozonolysis.
(a) The reaction scheme for the synthesis of the standard for the expected product of the derivatization and ozonolysis of dihydrogalloxanthin. (b) The LC-MS chromatogram and (c) MS:MS spectra of the standard. (d) The reaction scheme for the derivatization and ozonolysis of dihydrogalloxanthin. (e) The LC-MS chromatogram and (f) MS:MS spectra of the product of Apo2 derivatization and ozonolysis.
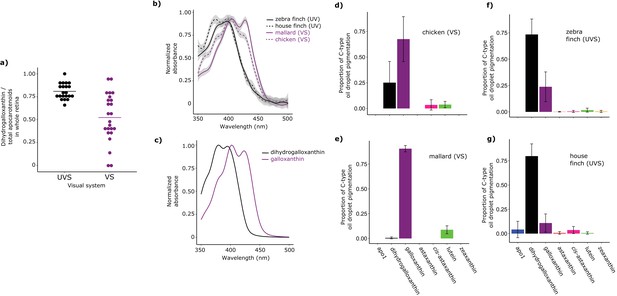
Shifts in the spectral filtering of the SWS2 cone between VS and UVS species are the result of changes in apocarotenoid composition.
(a) The ratio of dihydrogalloxanthin to total apocarotenoid content in the whole retina differs between UV and VS species. Each point represents a different species, and the bar indicates the mean for each visual system. (b) The UV-Vis absorbance spectrum of the expanded C-type oil droplets of selected UVS and VS species, and (c) the spectrum of galloxanthin and dihydrogalloxanthin for comparison. (d–g) The mean ± S.D. proportion of each carotenoid in the additive mixture of pure spectra that best fits the C-type droplet spectra from (d) chicken (n = 5, VS), (e) mallard (n = 3, VS), (f) zebra finch (n = 5,UVS), and (g) house finch (n = 4, UVS). The pure carotenoid spectra, the observed C-type droplet spectra, and the fitted spectra of each droplet used to estimate oil droplet composition are presented in Figure 3—figure supplement 2.
-
Figure 3—source data 1
The measured oil droplet spectra, pure carotenoid spectra, and model fit parameters for each measured C-type droplet.
- https://doi.org/10.7554/eLife.15675.011
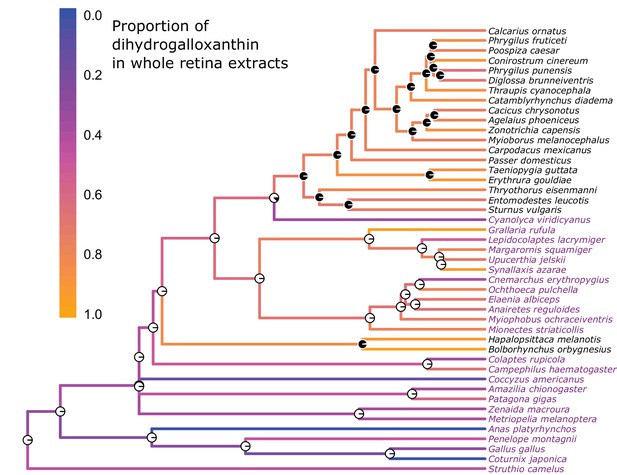
The evolution of the UV sensitive SWS1 opsin is associated with changes in whole retina apocarotenoid composition.
The names of UVS species are written in black and those of VS species in purple. The color of each branch represents the proportion of dihydrogalloxanthin in the total apocarotenoid complement of the whole retina. The proportions of dihydrogalloxanthin for ancestral branches were estimated using maximum likelihood. The pie charts at each node indicate the maximum-likelihood ancestral state for the SWS1 opsin, with black and white indicating the probability of UVS and VS, respectively.
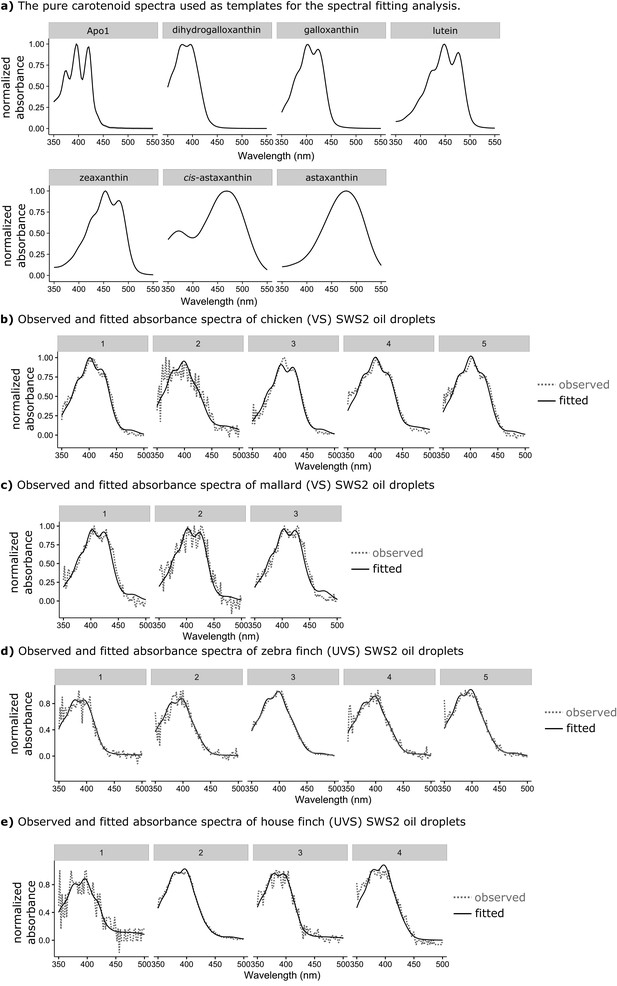
The observed absorbance spectra of the expanded C-type oil droplets and corresponding fitted spectra produced from additive mixtures of pure carotenoid spectra.
(a) The spectra of the seven pure carotenoids used to fit the observed oil droplet spectra. (b–c) Each numbered plot represents the observed spectrum (dotted lines) and fitted spectrum (solid lines) for a different individual C-type oil droplet from (b) chicken (n = 5), (c) mallard duck (n = 3), (d) zebra finch (n = 5), or (e) house finch (n = 4).
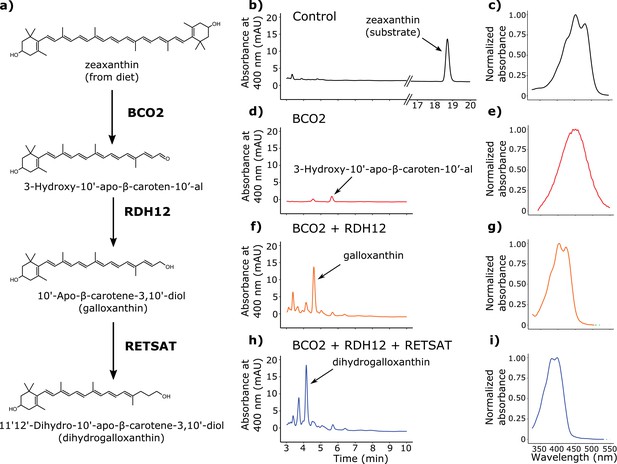
BCO2, RDH12, and RETSAT are sufficient to produce dihydrogalloxanthin from zeaxanthin.
(a) The proposed metabolic pathway for the production of galloxanthin and dihydrogalloxanthin from dietary zeaxanthin. (b–i) Representative chromatograms and UV-Vis absorbance spectra of apocarotenoid products produced by HEK293 cells expressing a control vector, avian BCO2, RDH12, and/or RETSAT enzymes, and supplemented with zeaxanthin.
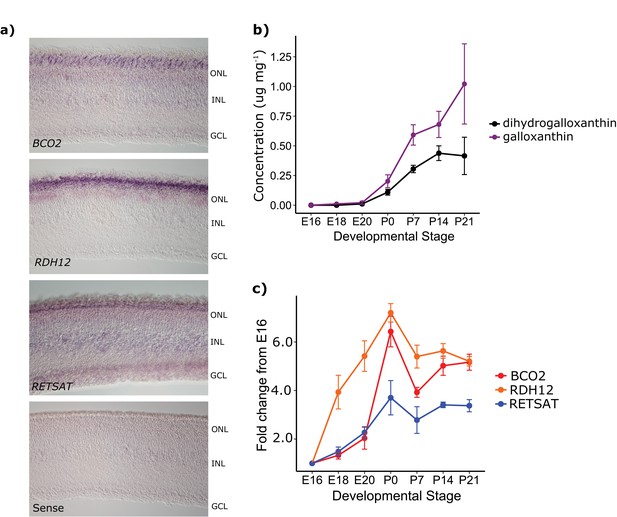
The developmental time course and pattern of expression of BCO2, RDH12, and RETSAT are consistent with their proposed role in apocarotenoid metabolism.
(a) In situ hybridization for transcripts of each of the enzymes in the proposed biosynthetic pathway, performed on retinas from newly hatched chickens indicate enzyme expression in the photoreceptor layer of the retina (ONL). No signal was detected in the negative control (RETSAT sense probe; bottom panel). The outer nuclear (ONL), inner nuclear (INL) and ganglion cell layers (GCL) are indicated in each section. (b) The concentration (μg mg−1 of protein) of dihydrogalloxanthin and galloxanthin in whole retina extracts from chicken (Gallus gallus) increase significantly over the course of development from embryonic day 16 (E16) to 21 days post-hatch (P21). (c) The transcript levels of BCO2, RETSAT, and RDH12 also increase significantly over development. Values are reported as fold-change relative to the earliest time point (E16). Three biological replicates were analyzed at each time point.
-
Figure 5—source data 1
Apocarotenoid concentration and transcript expression levels for each biological and technical replicate.
- https://doi.org/10.7554/eLife.15675.016
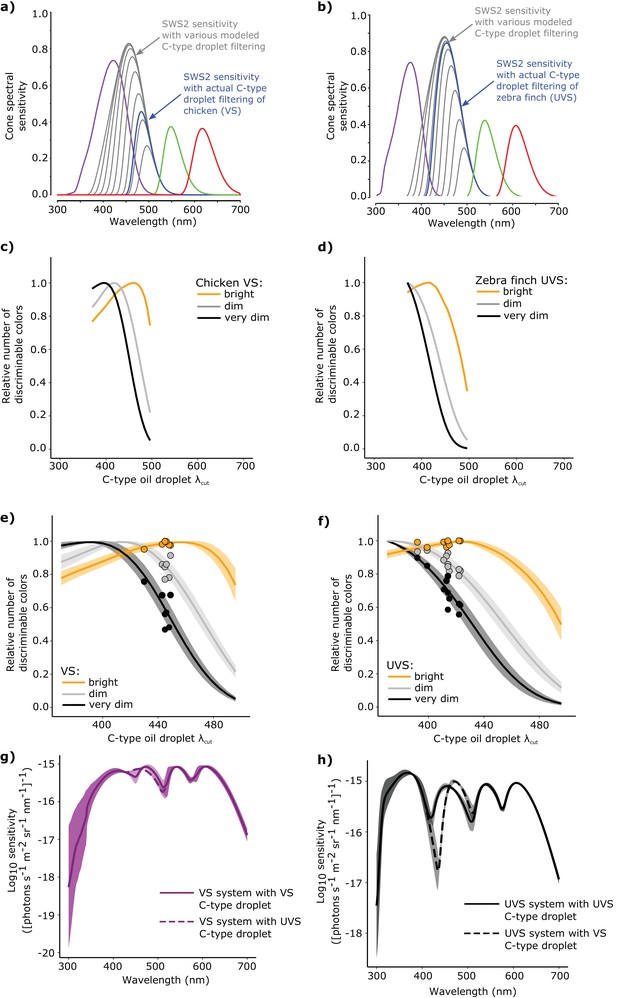
The spectral filtering of the C-type oil droplet is nearly optimal for color discrimination in bright light conditions.
Examples of the spectral sensitivity values of the LWS (red), MWS (green), SWS2 (blue) and SWS1 cones (purple) of (a) chicken (VS) and (b) zebra finch (UVS) used to model color discrimination. To find the optimal C-type oil droplet spectral filtering, we held all other values constant while varying droplet filtering over a wide range, resulting in shifts in the magnitude and wavelength of peak sensitivity of the SWS2 cone (gray lines). We then predicted the total number of colors that each of the hypothetical visual systems (c–d) could discriminate in bright (yellow lines), dim, (gray lines), and very dim light conditions (black lines). We repeated these analyses for a total of (e) 7 VS species and (f) 11 UVS species, and calculated total number of discriminable colors predicted for each C-type oil droplet λcut value under each of the three lighting conditions. The curves represent the mean ± S.D. total discriminable colors as a proportion of the model maximum for each lighting condition: bright (yellow lines), dim, (gray lines), and very dim conditions (black lines). The points above the curves are the observed λcut of the C-type droplets in each VS and UVS species and the predicted number of discriminable colors relative to the modeled optimum. (g) The mean ± S.D. increment spectral sensitivity of the visual systems of the VS species with the typical oil droplet configuration (solid line) or with mismatched C-type oil droplet filtering typical of UVS species (broken line). (h) The mean ± S.D. increment spectral sensitivity of the visual systems of the UVS species with the typical oil droplet configuration (solid line) or with mismatched C-type oil droplet filtering typical of VS species (broken line).
-
Figure 6—source data 1
The number of discriminable colors predicted using the receptor noise-limited model with species-specific ocular media transmittance, spectral sensitivity measures, and varying positions of the C-type oil droplet filtering cutoff.
The increment spectral sensitivity values calculated for the 11 UVS and 7 VS species with matched and mismatched C-type droplet filtering.
- https://doi.org/10.7554/eLife.15675.018
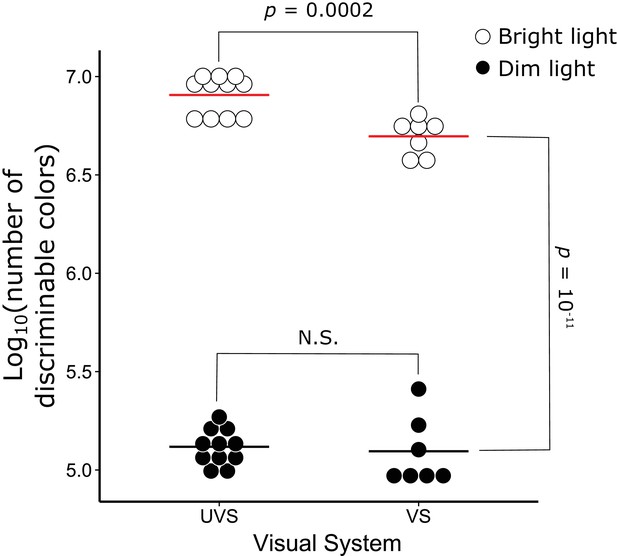
Color discrimination differs significantly between VS and UVS species and lighting conditions.
The number of discriminable colors predicted for the actual visual system parameters of 7 VS and 11 UVS species in bright (open circles) and dim (black points) light conditions. The red and black lines indicate the mean values for each visual system in bright and dim light conditions, respectively. The p-values denote the statistical comparisons described in the text. N.S. indicates that there is no significant difference between the conditions.
Tables
The exact mass measurements of major apocarotenoids in the chicken retina. Galloxanthin lost a water molecule in the ionization process resulting in a difference of 18 units in the measurement of mass.
| Carotenoid | m/z observed | m/z theoretical | ppm error | Ion | m/z of intact molecule |
|---|---|---|---|---|---|
| Apo2 | 397.308 | 397.3101 | -5.29 | M+H | 397.308 |
| galloxanthin | 377.2836 | 377.2844 | -2.12 | M+H-H2O | 395.2836 |
Additional files
-
Supplementary file 1
The species included in our phylogenetic comparison of retina apocarotenoid composition.
The tuning of the SWS1 opsin is inferred from the amino acid at position 90 of the second transmembrane helix (Ödeen and Håstad, 2013; 2009). The amino acid sequence was either derived from previously published studies or was determined by sequencing of genomic DNA in the current study as indicated.
- https://doi.org/10.7554/eLife.15675.020
-
Supplementary file 2
The species and visual system parameters used to model avian color discrimination.
- https://doi.org/10.7554/eLife.15675.021
-
Supplementary file 3
The PCR primers used in studies of enzyme function and expression.
(a) PCR primers used to clone in situ hybridization templates. (b) Primers used for qPCR quantification of apocarotenoid-metabolizing enzyme transcript expression in developing chicken retinas. (c) PCR primers used to clone full-length transcripts of apocarotenoid-metabolizing enzymes for cloning into the pTre expression vector.
- https://doi.org/10.7554/eLife.15675.022





