ESCRT-III drives the final stages of CUPS maturation for unconventional protein secretion
Figures
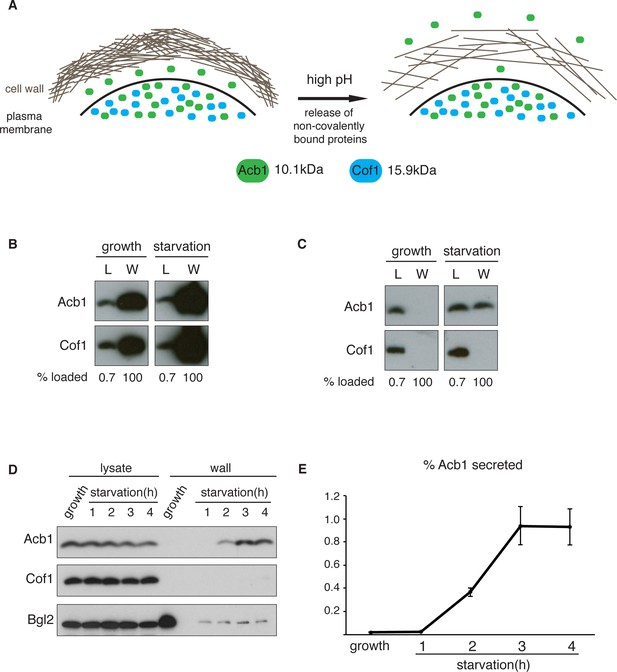
A quantitative assay for Acb1 secretion.
(A) The cell wall is a highly-charged, porous meshwork of glucans, chitin, and mannoproteins. Incubation in high pH buffers loosens the cell wall, thus allowing some non-covalently bound proteins to be released. (B) Standard cell wall extraction procedures employed thus far cause cell lysis. Wild type cells were grown to mid-logarithmic phase, washed twice, and cultured in 2% potassium acetate for 2.5 hr (starvation). Cell wall proteins were extracted from equal numbers of growing and starved cells in 100 mM Tris-HCl pH 9.4, 10 mM DTT with mixing at 350 rpm for 30 min at 37°C, followed by precipitation with TCA. Lysates (L) and cell wall-extracted proteins (W) were analyzed by western blot. (C) Mild cell wall extraction conditions do not cause lysis and reveal starvation-specific release of Acb1. Wild type cells were grown to mid-logarithmic phase, washed twice, and incubated in 2% potassium acetate for 2.5 hr (starvation). Cell wall proteins were extracted from equal numbers of growing and starved cells in 100 mM Tris-HCl pH 9.4, 2% sorbitol for 10 min on ice followed by precipitation with TCA. Lysates (L) and cell wall-extracted proteins (W) were analyzed by western blot. (D) Time course of Acb1 secretion during starvation. Wild type cells were grown to mid-logarithmic phase, washed twice, and cultured in 2% potassium acetate for the indicated times. Cell wall proteins were extracted in the mild conditions described in (C) and analyzed by western blot. (E) The ratio of wall/lysate Acb1 was determined and the average amount of secreted Acb1 at 3 hr was calculated to be 0.94% (SEM = 0.16%) and at 4 hr 0.93% (SEM = 0.16%) of total cellular Acb1 (n = 3).
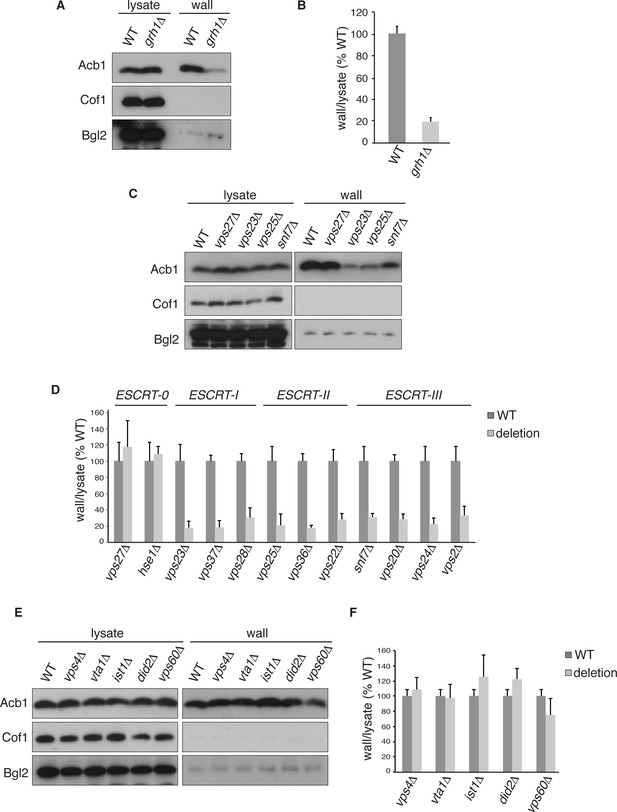
Acb1 secretion requires Grh1 and a subset of ESCRT proteins.
Wild type and deletion strains were grown to mid-logarithmic phase, washed twice, and incubated in 2% potassium acetate for 2.5 hr. Cell wall proteins were extracted from equal numbers cells in 100 mM Tris-HCl pH 9.4, 2% sorbitol for 10 min on ice followed by precipitation with TCA. Lysates and cell wall-extracted proteins were analyzed by western blot. The ratio of wall/lysate Acb1 during starvation was determined and compared to that of wild type in each experiment. Statistical analyses were performed for each gene deletion and are represented as% of wild type (paired student’s t-test). (A) Grh1 is required for Acb1 secretion. Representative cell wall extractions monitored by western blot of wild type and grh1Δ cells. (B) grh1Δ cells secreted on average 80.3% less Acb1 than wild-type cells (p = 0.0045, SEM = 3.8%, n= 4). (C) Acb1 secretion requires ESCRT complexes I, II and III but not ESCRT-0. Representative cell wall extractions monitored by western blot of wild type and one deletion strain from each ESCRT complex. (D) Quantification of all ESCRT deletion strains tested (n = 4 or more). ESCRT-0; vps27Δ (+17%, SEM = 3.2%, p > 0.2), hse1Δ (+8%, SEM = 0.9%, p > 0.2). ESCRT-I; vps23Δ (-81.8%, SEM = 7.9%, p = 0.0046), vps37Δ (-81.5%, SEM = 8.4%, p = 0.001), vps28Δ (-69.4%, SEM = 11.8%, p = 0.026). ESCRT-II; vps25Δ (-78.8%, SEM = 14.1%, p = 0.021), vps36Δ (-81.7%, SEM = 2.9%, p = 0.0055), vps22Δ (-71.6%, SEM = 7.7%, p = 0.032). ESCRT-III; snf7Δ (-68.5%, SEM = 4.4%, p = 0.055), vps20Δ (-71.5%, SEM = 6.5%, p = 0.022), vps24Δ (-77.4%, SEM = 7.5%, p = 0.049), vps2Δ (-66.7%, SEM = 11.3%, p = 0.0006). (E) Vps4 and accessory proteins are not required for Acb1 secretion. Representative cell wall extractions monitored by western blot of wild type and indicated deletion strains. (F) Quantification of indicated deletion strains (n = 4 or more). No differences were determined to be statistically significant.
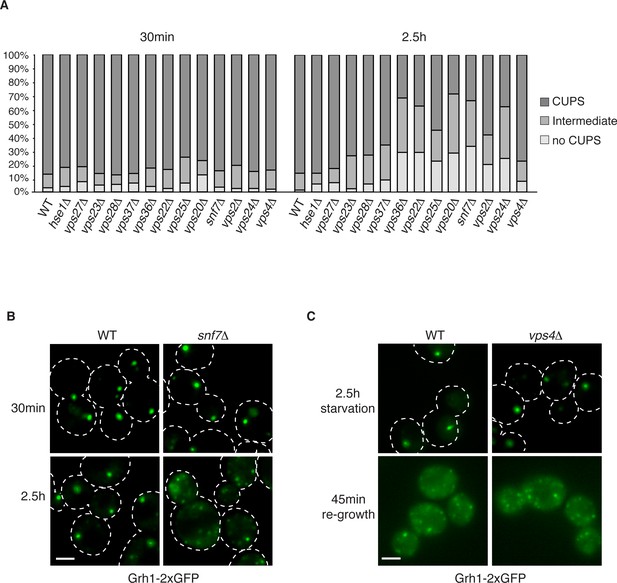
The involvement of ESCRTs in CUPS formation, stability and disassembly.
(A) Wild type and the indicated deletion strains expressing Grh1-2xGFP were grown to mid-logarithmic phase, washed twice, and incubated in 2% potassium acetate for 30 min and 2.5 hr to assess CUPS biogenesis and stability, respectively. Cells were grouped in 3 classes: CUPS (1–3 large punctae per cell), intermediate (large and small punctae), no CUPS (multiple small punctae). Between 50–200 cells were counted for each strain/condition from 3 independent experiments. (B) Effect of loss of Snf7 of ESCRT-III at 30 min and 2.5 hr starvation. (C) Wild type and vps4Δ cells were starved for 2.5 hr as in (A) to allow CUPS formation. Cells were washed once and cultured in rich media for 45 min. Scale bars = 2 μm.
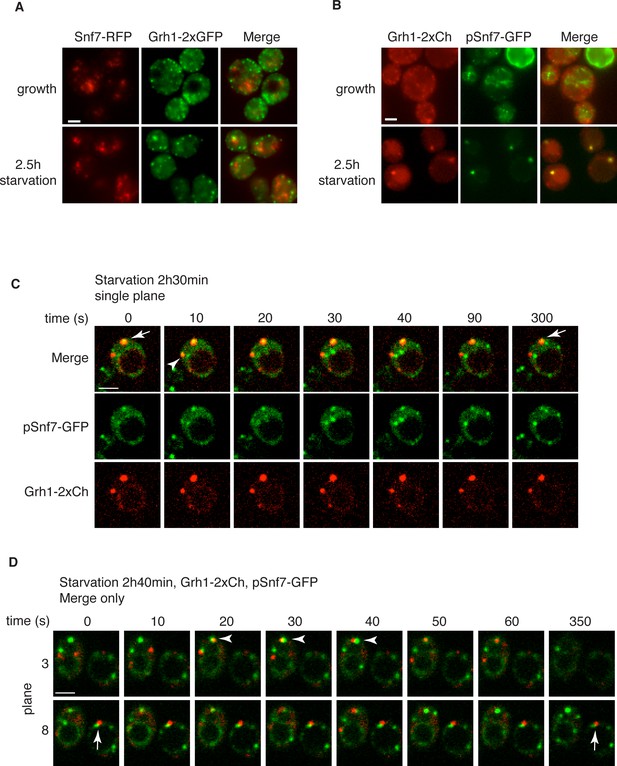
Snf7 localizes transiently to CUPS.
(A) Genomically integrated Snf7-RFP and Grh1-2xGFP were visualized by fluorescence microscopy during growth in mid-logarithmic phase and after incubation in 2% potassium acetate for 2.5 hr. (B) Snf7-GFP was expressed exogenously in cells expressing genomically integrated Grh1-2xmCherry (Grh1-2xCh) and visualized by fluorescence microscopy during growth in mid-logarithmic phase and after incubation in 2% potassium acetate for 2.5 hr. (C–D) The same cells from (B) were visualized over time throughout starvation by confocal spinning disk microscopy. Cells with Grh1-2xmCherry on CUPS and Snf7-GFP expression were quantitated for frequency and duration of co-localization and/or overlap of the two structures. Rapid (less than 30 s) and stable (more than 30 s) co-localization examples are indicated by arrowheads and arrows, respectively. All scale bars = 2 μm.
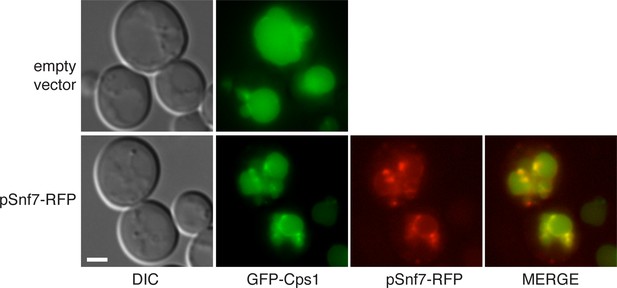
GFP-Cps1 transport is unaffected in pSnf7-RFP expressing cells.
Wild type cells co-expressing GFP-Cps1 (pRS415) with either empty pRS416 vector or pSnf7-RFP were grown mid-logarithmic phase and visualized by fluorescence microscopy. Scale bar = 2 μm.
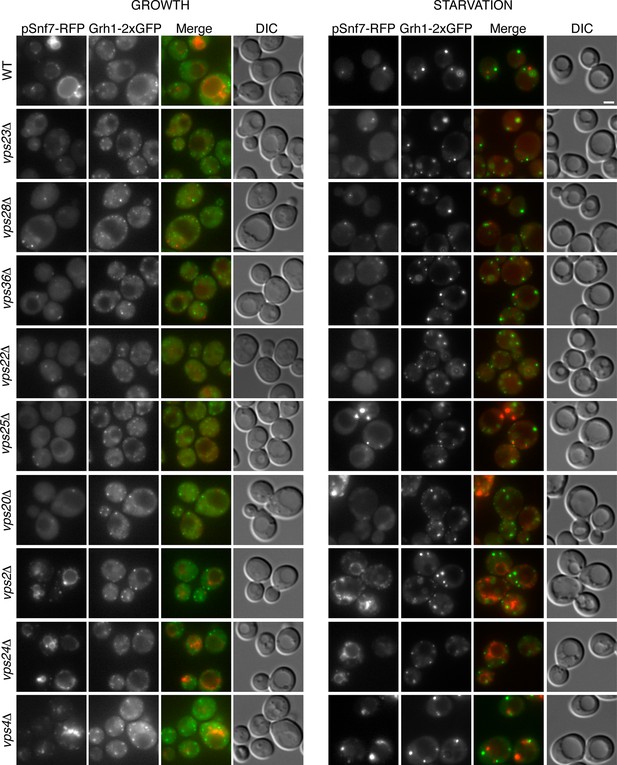
Recruitment of Snf7 to membranes for Acb1 secretion and MVB pathway share a similar mechanism.
Snf7-RFP was expressed exogenously from its own promoter in wild type and the indicated ESCRT deletion strains expressing Grh1-2xGFP. Cells were visualized by fluorescence microscopy during growth in mid-logarithmic phase (growth) and after incubation in 2% potassium acetate (starvation) for 2–2.5 hr. Scale bar = 2 μm.
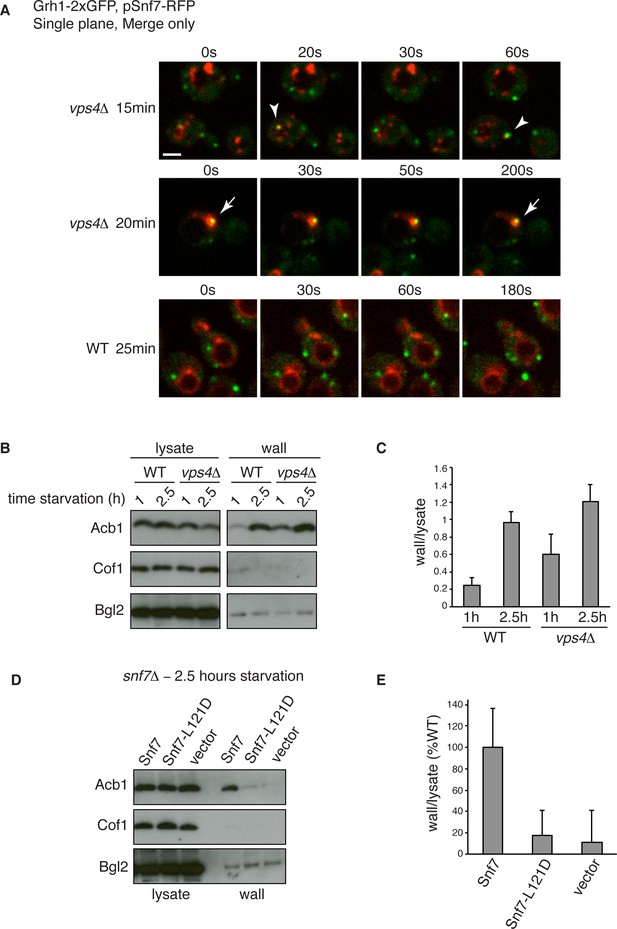
Snf7 recruitment to CUPS and Acb1 secretion are accelerated in vps4Δ cells.
(A) Snf7-RFP was expressed exogenously from its own promoter in wild type and vps4Δ cells expressing Grh1-2xGFP. Cells were starved and immediately visualized by time-lapse confocal microscopy (Scale bar = 2 μm). (B–C) Wild type and vps4Δ were grown to mid-logarithmic phase, washed twice, and incubated in 2% potassium acetate for 1 hr and 2.5 hr. Cell wall proteins were extracted as before and analyzed by western blotting. Acb1 levels were quantified and the ratio of wall/lysate Acb1 was determined. n=3 (D–E) Snf7 polymerization is required for Acb1 secretion. snf7Δ cells expressing wild type Snf7, Snf7-L121D or empty vector were grown to mid-logarithmic phase, washed twice, and incubated in 2% potassium acetate for 2.5 hr. Cell wall proteins were extracted as before and analyzed by western blotting. The ratio of wall/lysate Acb1 was determined (n=3).
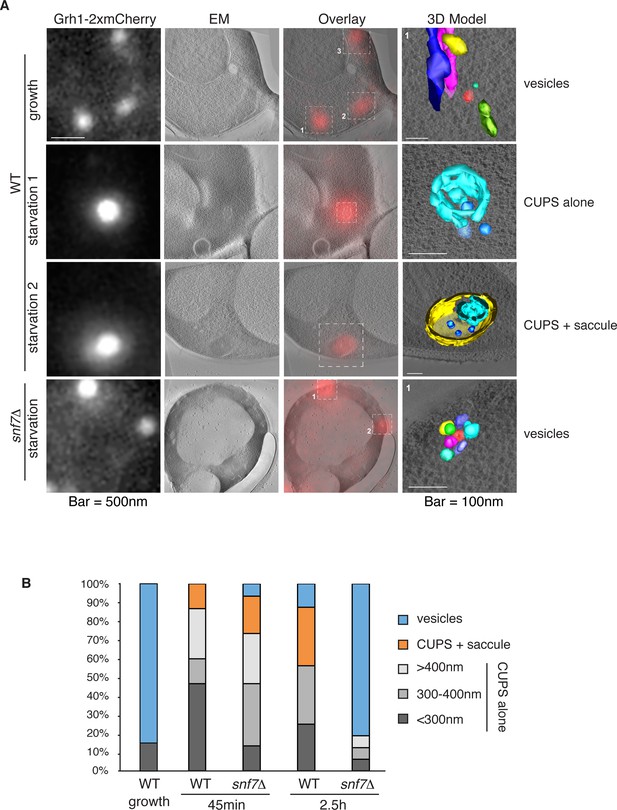
Ultrastructure of CUPS and the involvement of Snf7 in their stability.
CUPS are revealed as a spheroidal collection of highly curved membranes of average 200 nm diameter (WT starvation 1, 3D Model). (A) Grh1-2xmCherry expressing cells were grown to mid-logarithmic phase, washed twice, and incubated in 2% potassium acetate for 2.5–3 hr. Growing or starved cells were subjected to cryofixation and correlative light and electron microscopy (CLEM) (see Materials and methods). High magnification tomograms were acquired and 3D models were reconstructed of the membranes positive for mCherry signal. In the case of wild type cells, 13 tomograms were acquired in growth and 16 during starvation. For snf7Δ cells, 14 tomograms were acquired in starvation (the organization of Grh1-2xmCherry positive membranes during growth was indistinguishable from wild type). In 50% of CUPS structures identified in wild type cells a saccule surrounding CUPS was observed (WT starvation). (B) Classification of Grh1-positive membranes and measurements of CUPS structures at 45 min and 2.5 hr starvation. At 45 min starvation 12 tomograms each from wild type and snf7D cells were analyzed. 'Vesicles' refers to vesicles and small cisternae. The diameter of the CUPS structures was measured along the longest axis. In the case of 'CUPS + saccule' the CUPS structures were small, with an average diameter of 200 nm.
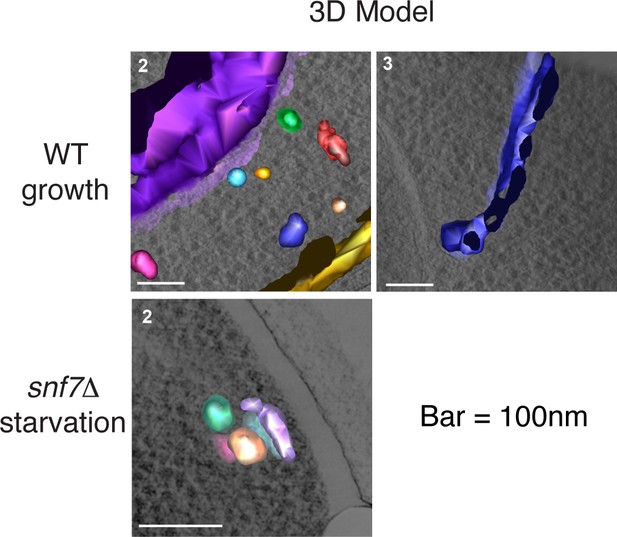
Remaining 3D models of Grh1-positive membranes from Figure 7A.
https://doi.org/10.7554/eLife.16299.011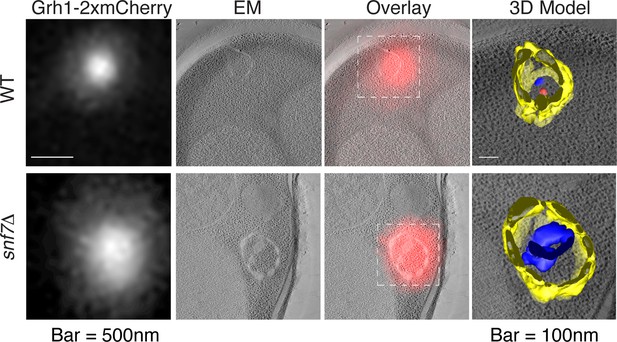
Examples of CLEM analysis from wild type and snf7Δ cells at 45 min of starvation.
Grh1-positive membranes from 12 tomograms each from wild type and snf7Δ cells of were analyzed.
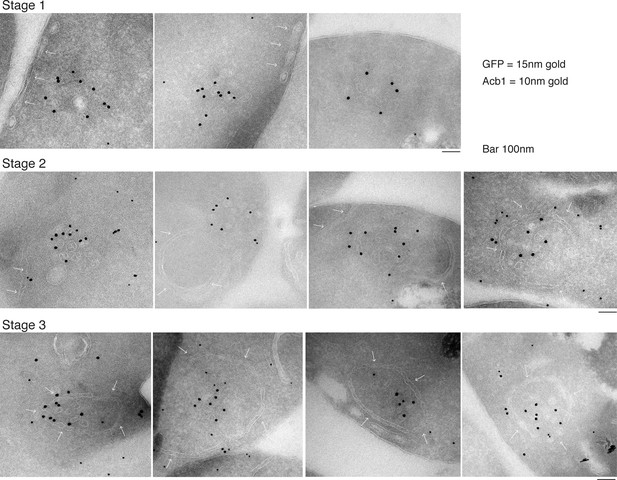
Immunoelectron microscopy identifies Acb1 in stable CUPS.
Grh1-2xGFP expressing cells were starved for 2.5 hr and processed for immunogold labeling and electron microscopy (see Materials and methods). GFP = 15 nm gold, Acb1 = 10 nm gold. Stage 1 – Grh1 labels tubulo-vesicular structure. Stage 2 – A sheet/saccule approaches and initiates engulfment of the Grh1 labelled structure (white arrows). Stage 3 – The Grh1 structure is completely engulfed and contains Acb1 – mature or stable CUPS.
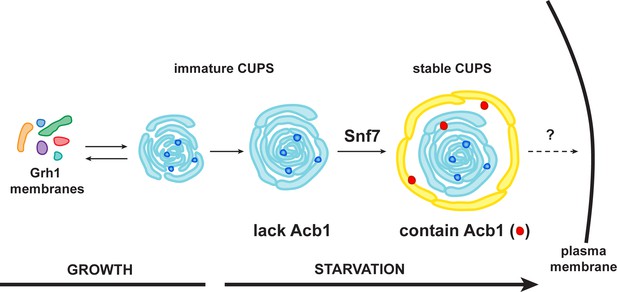
A schematic presentation of steps in Acb1 secretion.
Grh1 containing vesicles and tubules assemble into foci that are clearly visible as one to two spots per cell. This collection of membranes are formed and consumed constitutively during growth (immature CUPS). However, upon shifting cells to starvation medium, the immature CUPS become encased in a saccule (yellow membrane) by an Snf7 mediated reaction. We call this membrane-bounded compartment 'stable CUPS'. This stable form of CUPS is in fact found to contain Acb1. Stable CUPS are quite different from a standard MVB. The stable CUPS are double membrane bounded and contain internal membranes of different sizes and shapes. An MVB on the other hand, is composed of a single bilayer containing uniformed sized vesicles. The key difference also is that Vps4 is required for the formation of an MVB, but not for the Snf7 dependent engulfment of Grh1 containing immature CUPS. The stable CUPS then release Acb1 to the exterior of the cells.





