Mitochondrial Bol1 and Bol3 function as assembly factors for specific iron-sulfur proteins
Figures
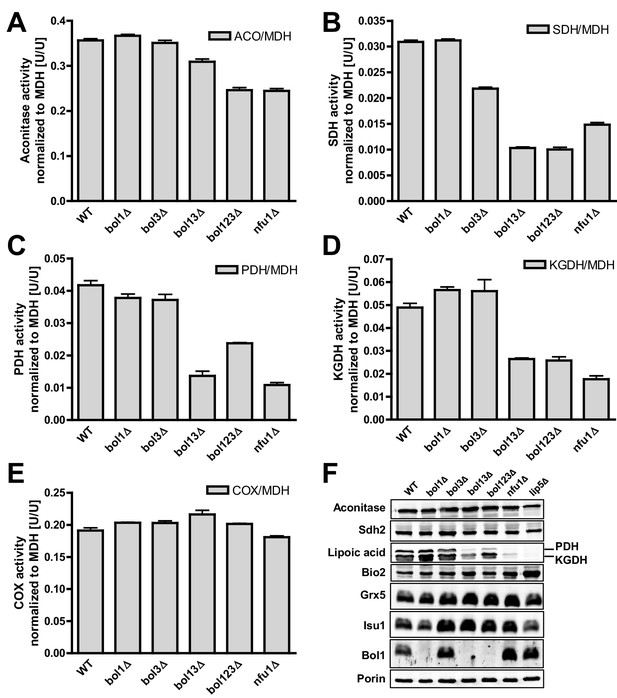
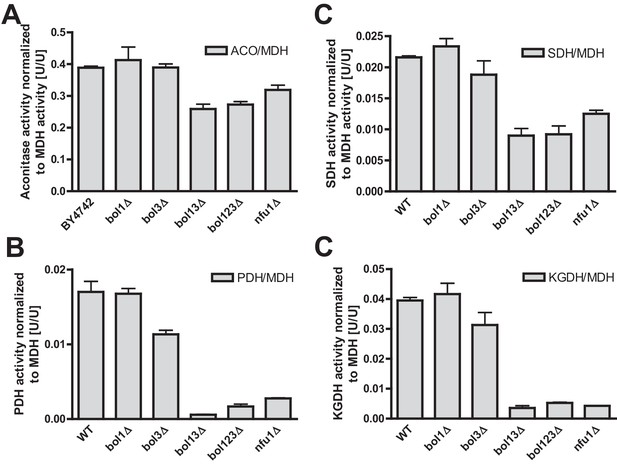
Deficiency of both mitochondrial Bol proteins causes defects in a subset of mitochondrial [4Fe-4S] enzymes.
Wild-type (WT; strain BY4742), and the indicated BOL and NFU1 deletion yeast strains were grown in minimal medium containing 2% galactose supplemented with 50 µM ferric ammonium citrate and used for the preparation of mitochondria. Mitochondrial extracts were assayed for specific activities of (A) aconitase (ACO), (B) succinate dehydrogenase (SDH), (C) pyruvate dehydrogenase (PDH), (D) 2-ketoglutarate dehydrogenase (KGDH), and (E) cytochrome c oxidase (COX). Values were normalized to those of malate dehydrogenase (MDH). Error bars indicate the SEM (n≥4). (F) Mitochondrial extracts were subjected to TCA precipitation and the levels of the indicated proteins and of lipoic acid attached to E2 subunits of PDH and KGDH were determined by immunostaining. Staining for porin served as a loading control.
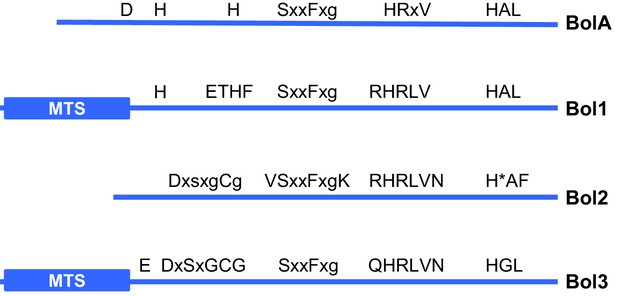
Cartoon of bacterial BolA and eukaryotic Bol1, Bol2 and Bol3 proteins.
Conserved amino acid sequence elements that characterize the various members of the BOLA protein family are shown. A conserved His (H*) in Bol2 serves as a Fe/S cluster ligand in a hetero-dimeric complex with Grx3 (Li et al., 2011). MTS; mitochondrial targeting sequence.
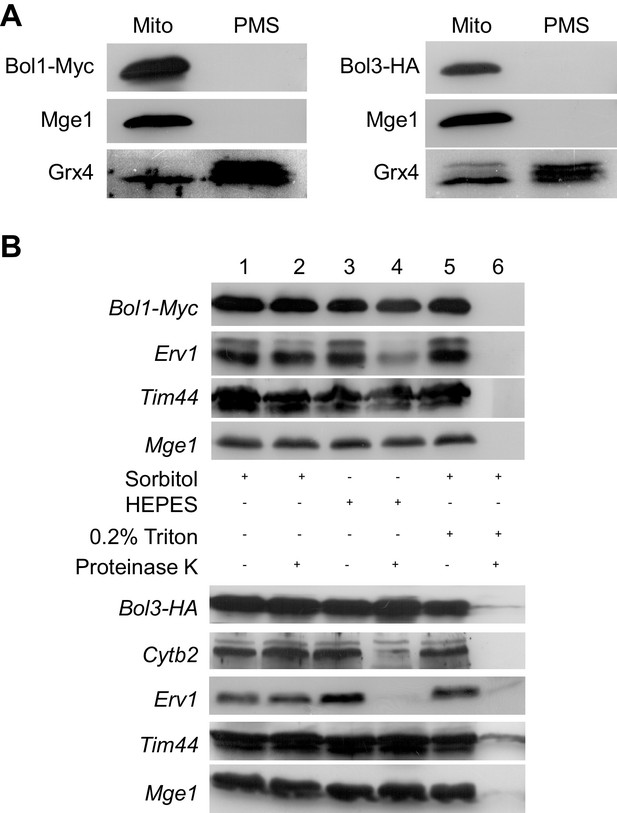
Bol1 and Bol3 are proteins of the mitochondrial matrix.
(A) Wild-type yeast cells (W303) expressing Myc-tagged Bol1 (vector p424-MET25; Supplementary file 1B) or HA-tagged Bol3 (from vector p425-TDH3) were grown in SD medium, and mitochondria (Mito) and post-mitochondrial supernatant (PMS) fractions were isolated. Immunostaining was performed using antisera raised against the mitochondrial matrix protein Mge1, cytosolic Grx4 (also recognizing Grx3), and monoclonal anti-Myc (A14, Santa Cruz) or anti-HA. (B) Wild-type yeast cells (W303) expressing Bol1-Myc or Bol3-HA were grown in SD medium. Isolated mitochondria were either left intact (sorbitol), hypotonically swollen by tenfold dilution in HEPES buffer, or lysed with 0.2% Triton X-100 detergent (Diekert et al., 2001). Samples were incubated in the presence or absence of 100 µg/ml proteinase K for 20 min on ice, and proteinase K was inactivated by PMSF. After TCA precipitation, samples were analyzed by immunostaining for the Myc-tag of Bol1, the HA-tag of Bol3, the intermembrane space proteins Erv1 and cytochrome b2, and the matrix proteins Tim44 and Mge1.
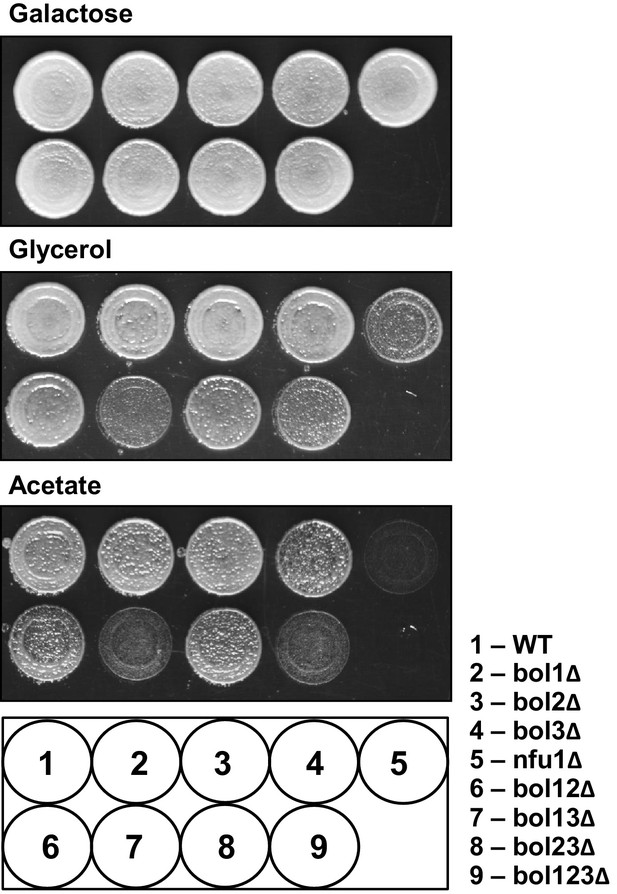
The growth behavior of the various BOL gene deletion strains in comparison to NFU1 deletion cells.
The indicated yeast strains were grown overnight on minimal medium with 2% glucose as carbon source. Cells were diluted to OD600 = 0.5 in water and spotted on rich medium (YP) agar plates with 2% galactose, 3% glycerol or 2% acetate as indicated. Plates were incubated at 30°C for 3 days. WT, wild-type.
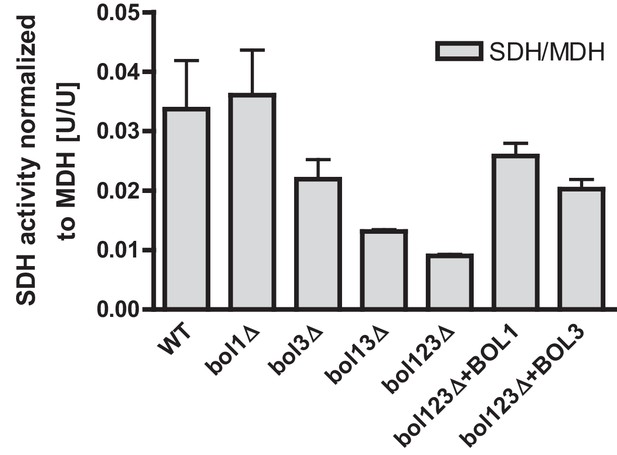
Specific rescue of succinate dehydrogenase activities in BOL gene deletion cells by both mitochondrial Bol proteins.
Wild-type (WT; strain BY4742) and the indicated BOL deletion strains were transformed with vector p416-MET25 lacking (empty) or overexpressing BOL1 or BOL3 as indicated. Cells were grown in minimal medium with 2% glucose without uracil and used for preparation of mitochondria. Mitochondrial extracts were assayed for the specific activity of succinate dehydrogenase (SDH) and normalized to malate dehydrogenase (MDH) activities. Error bars indicate the SEM (n≥4).
Fe/S enzyme activity defects in cells lacking Bol1-Bol3 after growth in lactate medium.
Wild-type (WT; strain BY4742), the indicated BOL and NFU1 deletion yeast strains were grown in lactate medium and used for the preparation of mitochondria. Mitochondrial extracts were assayed for specific activities of (A) aconitase (ACO), (B) succinate dehydrogenase (SDH), (C) pyruvate dehydrogenase (PDH), and (D) 2-ketoglutarate dehydrogenase (KGDH). Values were normalized to those of malate dehydrogenase (MDH). Error bars indicate the SEM (n≥4).
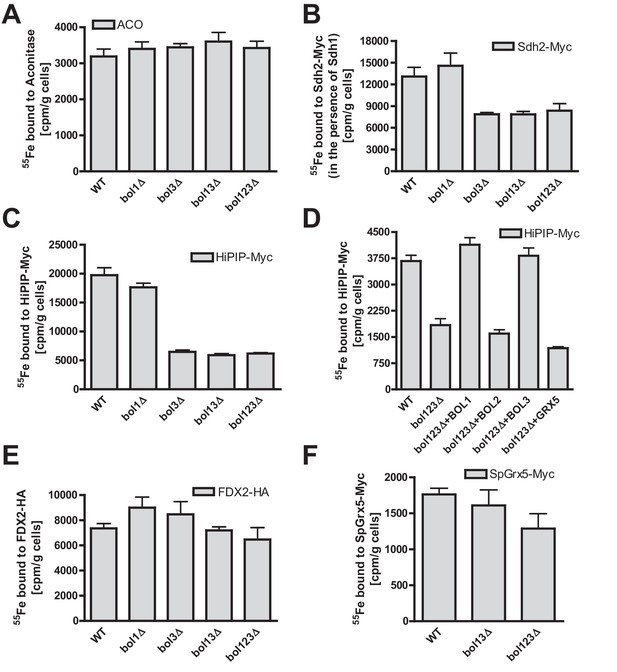
Bol1-Bol3 are required for de novo Fe/S cluster incorporation into specific mitochondrial [4Fe-4S] but not [2Fe-2S] proteins.
(A–F) Wild-type (WT, strain BY4742) and the indicated BOL deletion strains were transformed with vectors overproducing (B) Sdh2-Myc and Sdh1, (C and D) HiPIP-Myc, (E) human FDX2-HA, or (F) Schizosaccharomyces pombe (Sp) Grx5-Myc. In part D, bol123Δ cells were additionally transformed with p414-MET25 lacking or containing BOL1, BOL2, or BOL3 genes or p424-TDH3-GRX5. Cells were grown overnight in iron-poor SD medium and radiolabeled with 10 µCi 55Fe for 2 hr. The overproduced proteins were immunoprecipitated from cell extracts with specific antibodies. The amount of co-precipitated 55Fe was quantified by scintillation counting. Error bars indicate the SEM (n≥4).
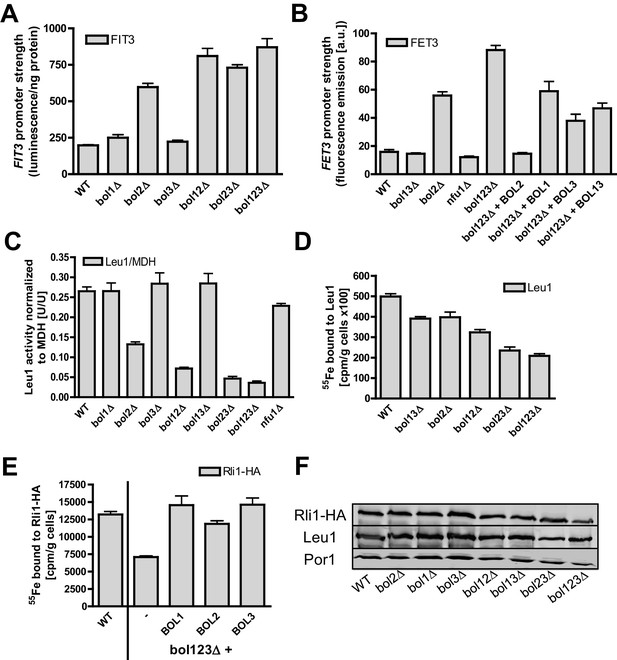
Mitochondrial Bol1-Bol3 and cytosolic Bol2 are not involved in cytosolic Fe/S protein biogenesis.
Wild-type (WT, strain BY4742) and the indicated BOL deletion strains harboring vector (A) pFIT3-Luc2 or (B) pFET3-GFP were cultivated in iron-replete medium to mid-log phase and the activities of the FIT3 and FET3 promoters, respectively, were determined. In (B) the bol123Δ cells were also transformed with centromeric plasmids producing the indicated Bol proteins. Abbreviation: Bol13; Bol1 plus Bol3. (C) Leu1 (relative to MDH) activities were determined in the indicated deletion stains cultivated in iron-replete medium. (D) The indicated BOL deletion stains were radiolabeled with 55Fe, and the Leu1-bound radioactivity was determined by immunoprecipitation with α-Leu1 antibodies followed by scintillation counting. (E) 55Fe incorporation into Rli1-HA was determined accordingly using WT and bol123Δ cells transformed with centromeric plasmids producing the three Bol proteins as indicated or with the empty vector (-). (F) Representative immunoblots determining the protein levels of Leu1 (D) and Rli1-HA (E) in the indicated strains. Porin served as a loading control. Error bars indicate the SEM (n≥4).
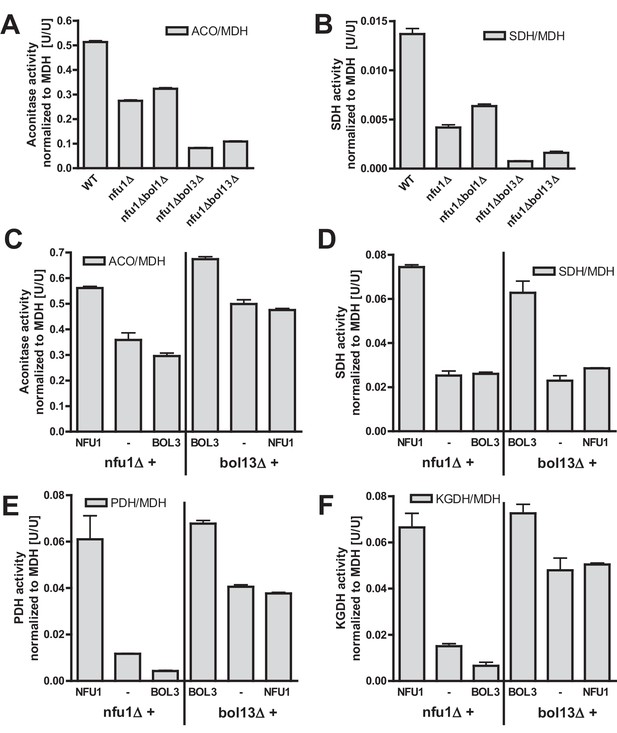
Bol1-Bol3 and Nfu1 cannot functionally replace each other in Fe/S protein maturation.
(A–B) Wild-type (WT, strain BY4742) and the indicated deletion strains were grown in minimal medium containing 2% glucose and used for the preparation of mitochondria. Mitochondrial extracts were assayed for the indicated specific enzyme activities as described in Figure 1. (C–F) The indicated deletion strains were transformed with vector p416-MET25 lacking (empty) or containing BOL3 or NFU1 as indicated. Cells were grown in minimal medium containing 2% galactose and used for the preparation of mitochondria. Mitochondrial extracts were assayed for the indicated enzyme activities as described in Figure 1. Error bars indicate the SEM (n≥4).
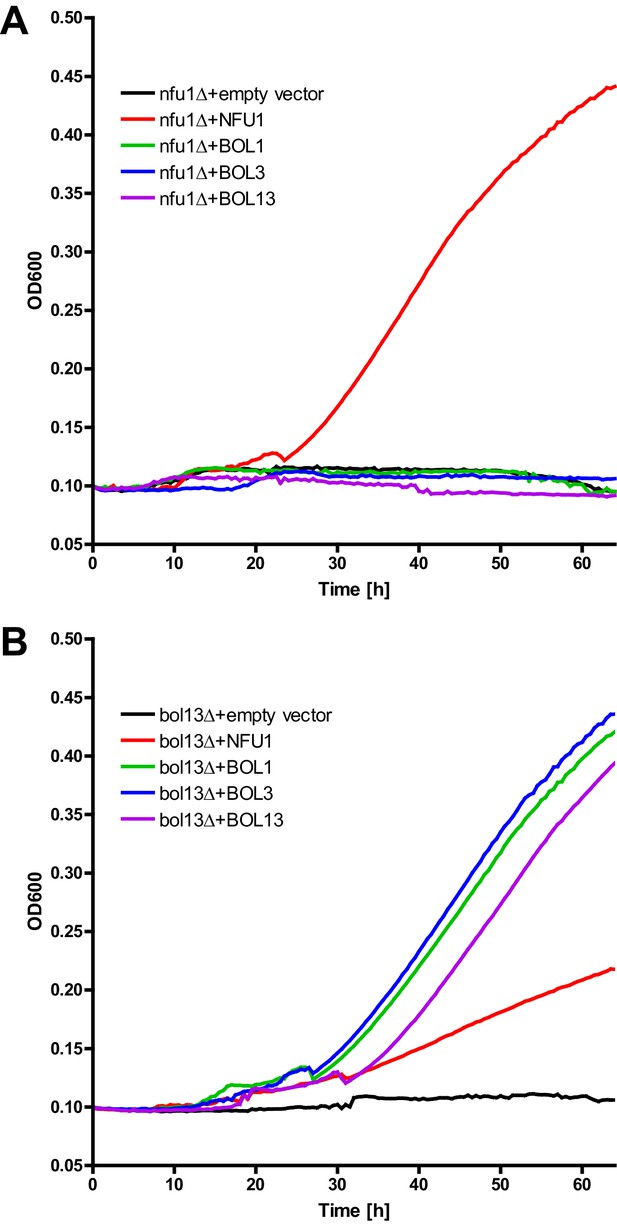
Growth complementation test of nfu1Δ and bol13Δ cells.
(A) nfu1Δ and (B) bol13Δ cells were transformed with vector p414-MET25 containing no gene (empty), BOL1, BOL3 or BOL1-BOL3 (BOL13) or p416-MET25-NFU1 as indicated. Cells were grown overnight on minimal medium without uracil containing 2% glucose as a carbon source. Cells were diluted to OD600 of 0.1 in 500 µl of liquid minimal medium without uracil containing 2% acetate as sole carbon source, and put into a 48 well plate. Plates were incubated at 30°C for 3 days with shaking, and the OD600 was measured by a TECAN plate reader every 30 min.
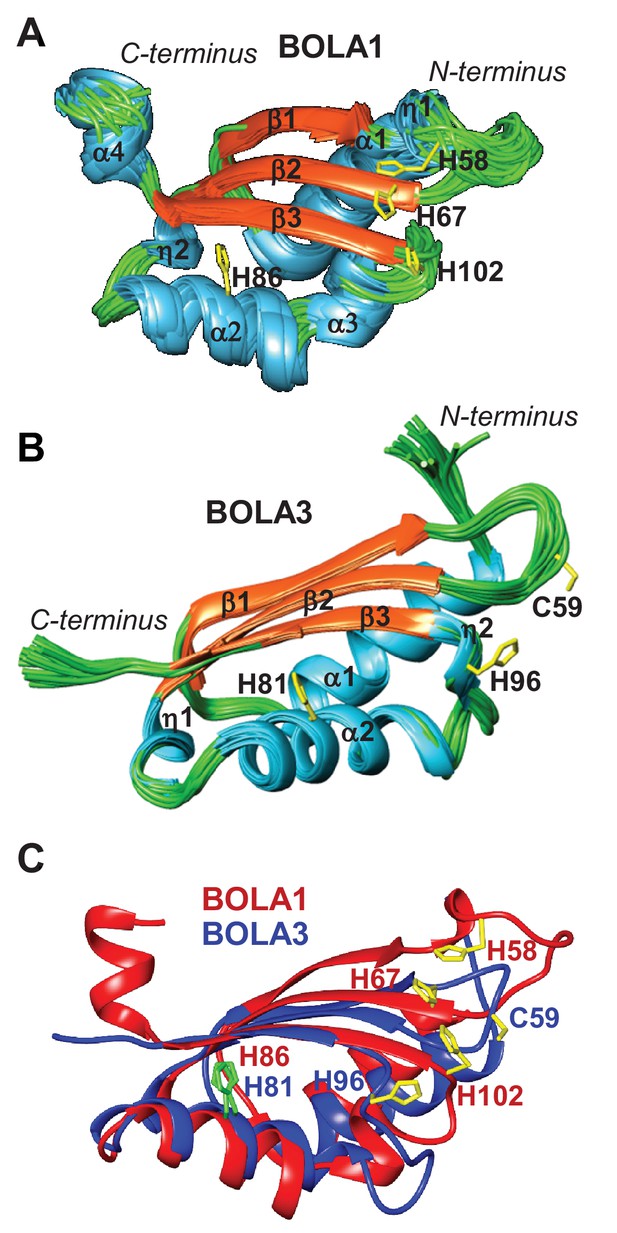
NMR solution structures of human BOLA1 and BOLA3.
The structures of (A) BOLA1 and (B) BOLA3 were solved by solution NMR. Residues His58, His67 and His102 are conserved within the eukaryotic BOLA1 proteins, and residues Cys59 and His96 are conserved within the BOLA3 proteins. (C) Backbone superimposition of BOLA1 (red) and BOLA3 (blue) structures depicting conserved His and Cys residues in both proteins. Yellow sticks represent potential Fe/S cluster ligands, and green sticks indicate other conserved His residues.
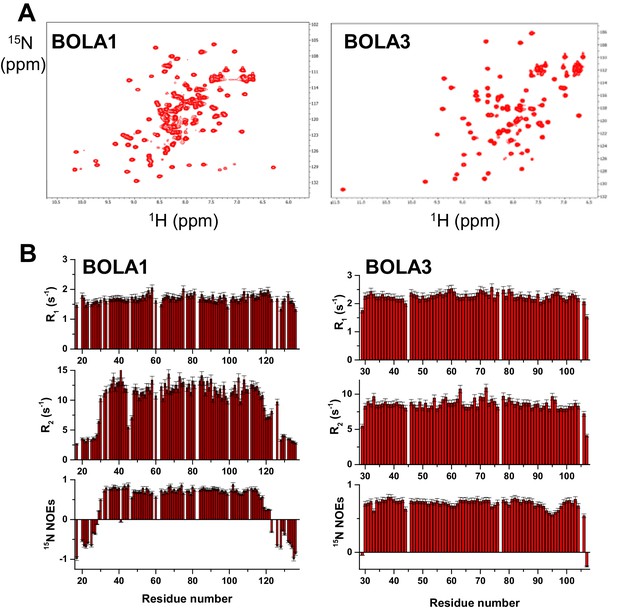
Structural and dynamic properties of BOLA1 and BOLA3 by solution NMR.
(A) 2D 1H-15N HSQC spectra of 15N-labeled BOLA1 and BOLA3. (B) 15N R1, R2 relaxation rates and 15N(1H) NOE values per residue of BOLA1 and BOLA3 obtained at 500 MHz and 298 K. The proteins were in buffer N with 10% (v/v) D2O). The 1H-15N HSQC spectra of the apo forms of BOLA3 and BOLA1 showed well-dispersed resonances indicative of essentially folded proteins. The overall topology of the solution structure of human BOLA3 is α1β1β2η1α2η2β3 (h: 310-helix), in which β1 and β2 are antiparallel, and β3 is parallel to β2 (Figure 5). All α-helices are on the same side of the β-sheet and the 310-helix located between α2 and β3 contains the His96 residue that is invariant in all prokaryotic and eukaryotic BolA homologues (Li and Outten, 2012). A cysteine residue (Cys59), typically conserved in Bol3 homologues, is located close to the invariant His96 residue in an extended loop between β1 and β2. The two residues are not in the proper orientation to coordinate a [2Fe-2S] cluster (Sγ of Cys59 is at an average distance of ~8 Å from Nε2 of His96). The overall topology of the solution structure of BOLA1 is similar to that of BOLA3, with the exception of the presence, in BOLA1, of short helices in the loop regions and at the C-terminus (α1η1β1β2η2α2α3β3α4 topology) (Figure 5). In addition, at both termini BOLA1 has ca. 15 residues that are unstructured and whose backbone NH resonances are clustered in the central region of the 1H-15N HSQC spectrum (part B). The three His residues typically conserved in Bol1 homologues are located in two spatially close regions (Figure 5). Although they are close to each other, it is not possible to predict which ones may be involved in [2Fe-2S] cluster coordination. Information on backbone motions and on the protein oligomerization state of BOLA1 and BOLA3 were obtained through 15N R1, R2, heteronuclear 15N(1H)-NOEs NMR experiments. The 15N backbone relaxation properties showed that the N and C termini of BOLA1 are highly flexible, as about 15 to 20 residues on each terminus have negative 15N NOE values, while the folded domain is essentially rigid. BOLA3 is a rigid molecule, with the exception of the first detected residue at the N terminus, the last two residues at the C terminus and residues around invariant His96. All of them experience fast backbone motions in the ns-ps time scale, as indicated by their low or negative 15N NOE values.

Multi-sequence alignment of the mitochondrial Bol proteins.
Multalin (Corpet, 1988) was used to generate a multi-sequence alignment of Bol1- and Bol3-like proteins from fungi and various higher eukaryotes. The conserved His and Cys residues are indicated. The numbers refer to the residues of the human mBols. Two His (58 and 67) in BOLA1 are candidates for the structural counterpart of Cys59 of BOLA3.
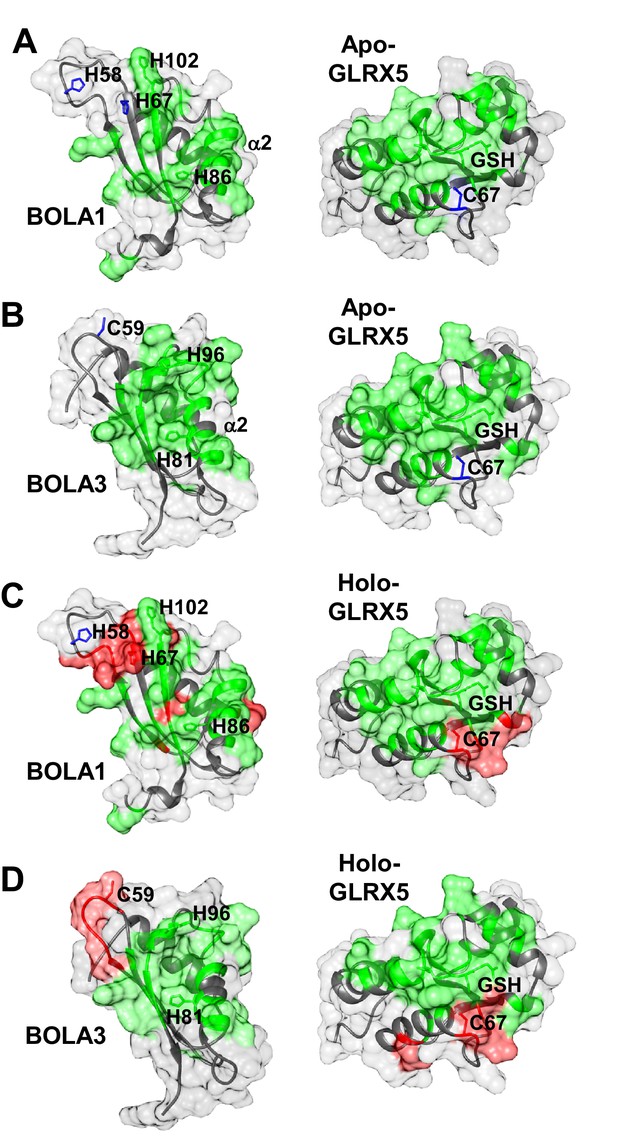
Structural basis of the interaction between human apo- and holo-GLRX5 with the BOLA proteins.
(A and B) Backbone chemical shift differences, obtained from a comparison of the 1H-15N HSQC spectra of apo-GLRX5 or BOLA proteins with that of (A) BOLA1-apo-GLRX5 or (B) BOLA3-apo-GLRX5 (1:1 mixture in buffer N), were mapped on the solution structures of the proteins. (C and D) Backbone chemical shift differences, obtained from a comparison of the 1H-15N HSQC spectrum of apo-GLXR5 or BOLA proteins with that of (C) BOLA1-apo-GLRX5 or (D) BOLA3-apo-GLRX5 (1:1 mixture in buffer N) chemically reconstituted with a [2Fe-2S] cluster, were mapped on the solution structures of the proteins. Green regions show residues with significant chemical shift changes (that is both in terms of chemical shift and broadening beyond detection effects) observed upon formation of the apo- or holo-complexes. Red areas depict those residues additionally affected upon holo-complex formation. Critical residues and GLRX5-bound GSH are depicted as sticks.
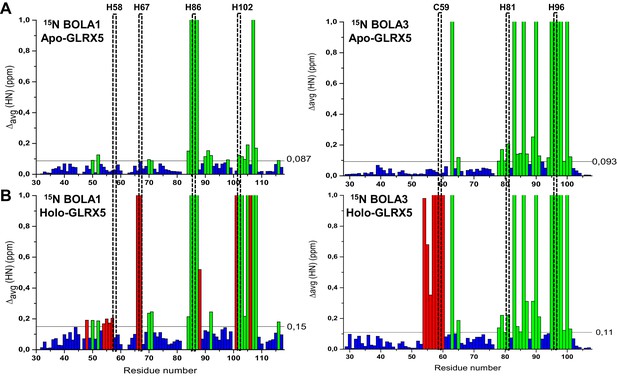
Chemical shift changes upon complex formation between GLRX5 and BOLA proteins monitoring backbone chemical shift changes of BOLA1 and BOLA3.
Backbone weighted average chemical shift differences Δavg(HN) obtained by comparing 1H-15N HSQC map of (A) 15N-labeled BOLA1 or BOLA3 with an equimolar mixture of 15N-labeled BOLA1 or BOLA3 with unlabeled apo-GLRX5, and of (B) 15N-labeled BOLA1 or BOLA3 with an equimolar mixture of 15N-labeled BOLA1 or BOLA3 chemically reconstituted with unlabeled GLRX5 to generate a [2Fe-2S] cluster (buffer N). Bars with Δavg(HN) = 1 indicate residues whose backbone NH signals broaden beyond detection. The indicated thresholds (obtained by averaging Δavg(HN) values plus 1σ) were used to define significant chemical shift differences. Green bars show significant chemical shift changes (that is both chemical shift differences and broadening beyond detection effects, respectively) in the apo interactions, and red bars those additionally occurring upon holo complex formation. The position of conserved residues is highlighted.
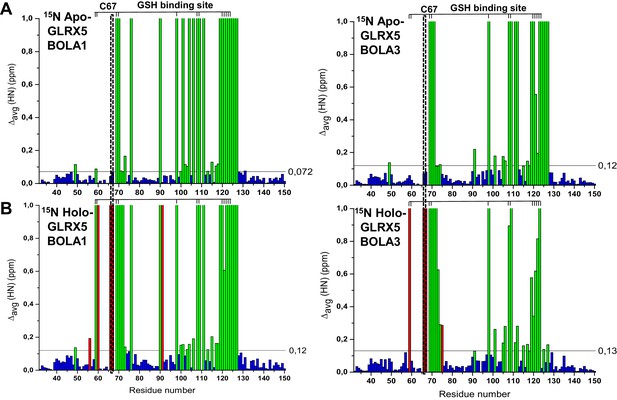
Chemical shift changes upon complex formation between GLRX5 and BOLA proteins, monitoring backbone chemical shift changes of GLRX5.
Backbone weighted average chemical shift differences Δavg(HN) obtained by comparing 1H-15N HSQC map of (A) 15N-labeled apo-GLRX5 with that of a 1:1 mixture of 15N-labeled apo-GLRX5 and unlabeled BOLA1 or BOLA3, and of (B) 15N-labeled apo-GLRX5 with that of a 1:1 mixture of 15N-labeled apo-GLRX5 with unlabeled BOLA1 or BOLA3 after chemical reconstitution of a [2Fe-2S] cluster in buffer N. The data are presented as in Figure 6—figure supplement 1. The position of the conserved Cys67 and of the residues of the GSH binding site are highlighted.
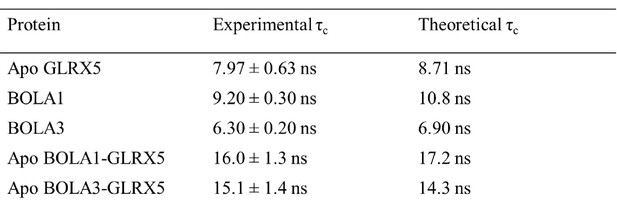
Experimental and predicted rotational correlation times (τC).
For the isolated proteins the τC values were obtained by the HYDRONMR program. For the apo-complexes the τC values were calculated from the sum of the τC values of the two isolated proteins.
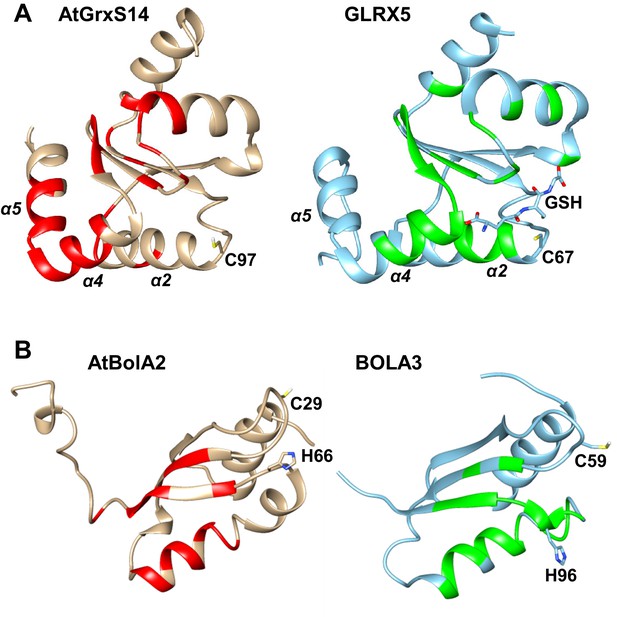
Comparison of the apo-AtGrxS14-AtBolA2 complex with apo-GLRX5/BOLA3.
Significant chemical shift changes (red and green for A. thaliana and human proteins, respectively) are mapped on the structures of (A) apo-AtGrxS14 and apo-GLRX5 upon the interaction with AtBolA2 and human BOLA3, respectively. Vice versa, part (B) shows the significant chemical shift changes of AtBolA2 and human BOLA3 upon interaction with AtGrxS14 and GLRX5, respectively. Conserved residues and the GLRX5-bound GSH are highlighted as sticks.
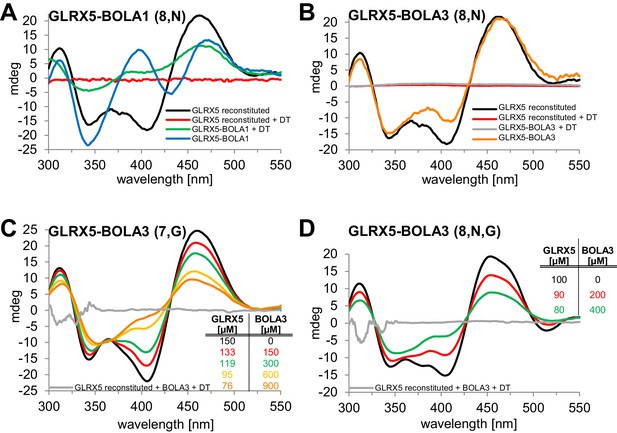
Human BOLA1 but not BOLA3 stabilizes the [2Fe-2S] cluster of holo-GLRX5 upon heterodimer formation.
(A–B) Chemical reconstitution of Fe/S clusters in buffer R was performed with GLRX5 in the absence or presence of stoichiometric amounts of (A) BOLA1 or (B) BOLA3, and CD spectra were monitored under anaerobic conditions. Additional spectra were recorded after addition of 2 mM dithionite (DT). (C–D) Chemically reconstituted GLRX5 was titrated with the indicated concentrations of BOLA3 in (C) buffer N or (D) buffer P, and CD spectra (corrected for dilution) were recorded under anaerobic conditions. Abbreviations for varied buffer conditions: 7, 8: pH; N, 150 mM NaCl; G, 5 mM GSH.
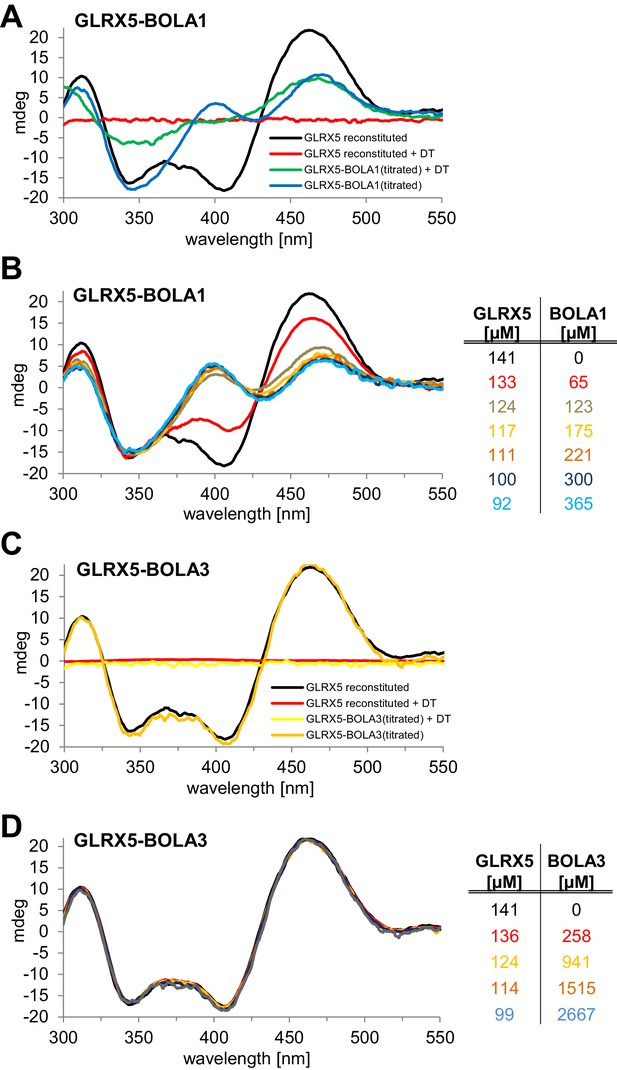
Stoichiometric hetero-complex formation of human holo-GLRX5 with BOLA1 results in characteristic CD spectral changes indicating shared binding of the [2Fe-2S] cluster.
(A,C) Chemically reconstituted GLRX5 was mixed in buffer R (lacking additional GSH) with a stoichiometric amount of (A) BOLA1 or (C) BOLA3, and CD spectra were recorded under anaerobic conditions. Additional spectra were recorded after addition of 2 mM dithionite (DT). (B, D) Chemically reconstituted GLRX5 was titrated with the indicated concentrations of (B) BOLA1 or (D) BOLA3, and CD spectra (corrected for dilution) were recorded under anaerobic conditions.
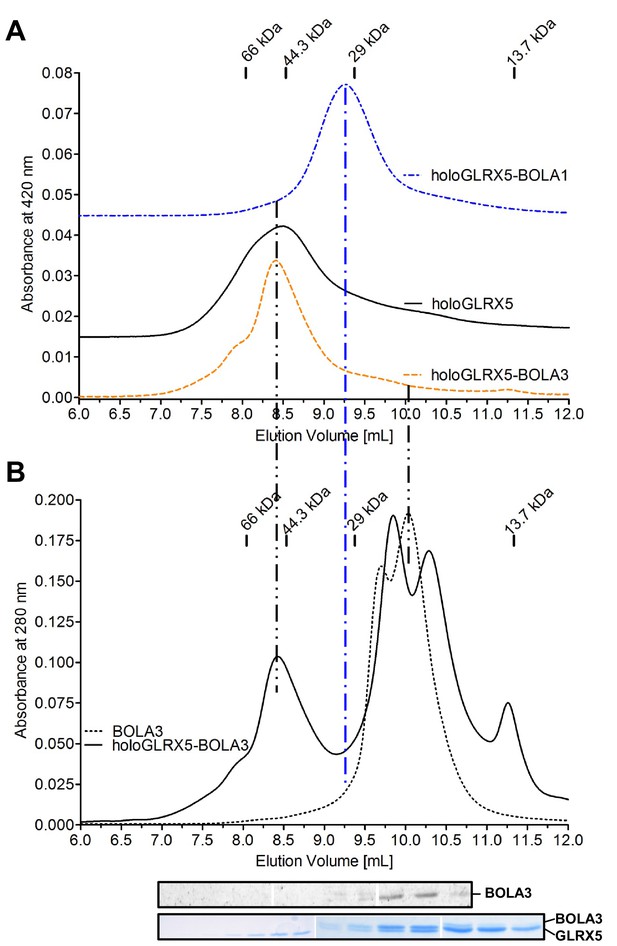
Gel filtration verifies the complex formation between human holo-GLRX5 and BOLA1.
(A) The gel filtration elution profile of co-reconstituted equimolar holo-GLRX5-BOLA3, holo-GLRX5 (offset by OD 0.015), or co-reconstituted equimolar GLRX5-BOLA1 (offset by OD 0.045) was recorded at 420 nm in buffer R. Elution positions of molecular marker proteins are indicated in kDa. (B) As a control, the elution behavior of the holo-GLRX5+BOLA3 mixture or BOLA3 alone were recorded at 280 nm in parallel to part A. Fractions of the holo-GLRX5+BOLA3 mixture were analyzed for GLRX5 and BOLA3 by Coomassie and immunostaining (bottom part).
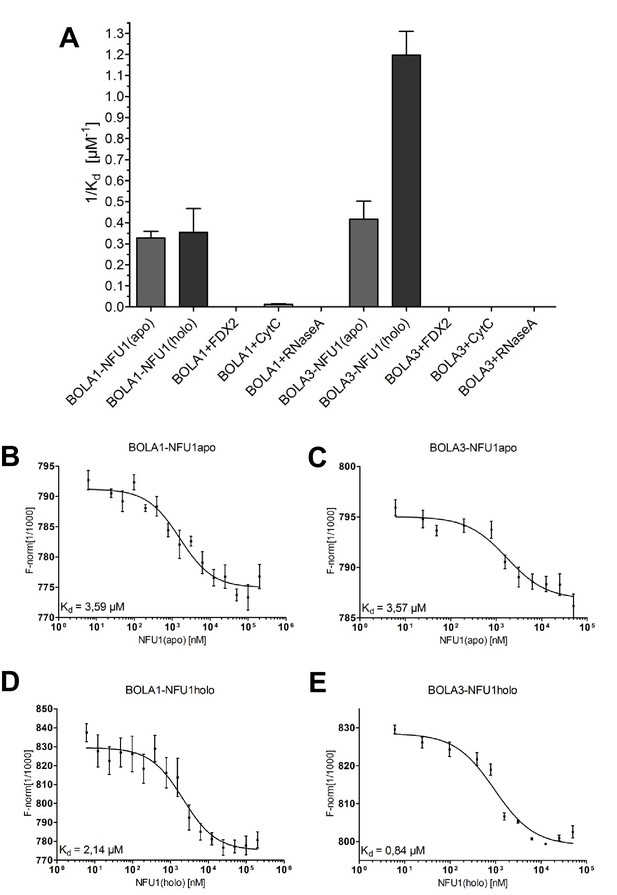
Preferential binding of human BOLA3 to the holo-form of NFU1.
(A) NFU1 was used in either apo- or holo-form and mixed at increasing concentrations with 200 nM fluorescently labeled BOLA1 or BOLA3. Microscale thermophoresis (MST) analyses were performed under anaerobic conditions, and dissociation constants (Kd) were determined. Control experiments were performed with human [2Fe-2S] ferredoxin FDX2, cytochrome c, and RNase A. Error bars indicate the SD (n = 3). (B–E) Examples for original MST data.
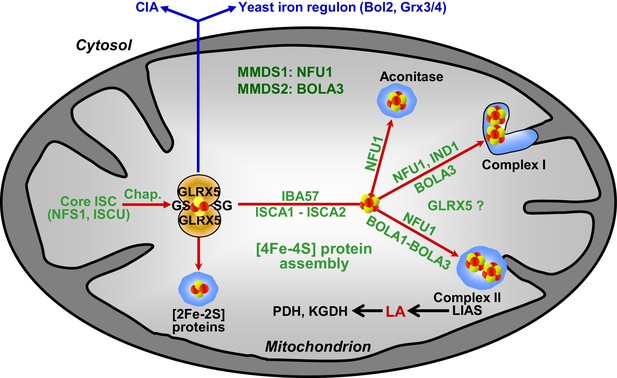
Working model for the role of mitochondrial BOLA proteins as specific ISC assembly factors in the late phase of mitochondrial Fe/S protein biogenesis.
Members of the core ISC assembly machinery including the sulfur donor NFS1, the scaffold protein ISCU, and dedicated chaperones (Chap.) mediate the assembly of a transiently bound, glutathione (GS)-coordinated [2Fe-2S] cluster on the monothiol glutaredoxin GLRX5 which is essential for [2Fe-2S] protein maturation, cytosolic Fe/S protein assembly (CIA), and cellular iron regulation in yeast. With the help of ISCA1, ISCA2, and IBA57, the GLRX5-bound [2Fe-2S] cluster is converted into a [4Fe-4S] type. BOLA1 and BOLA3 together with NFU1 and IND1 function in a specific assembly of mitochondrial [4Fe-4S] proteins as indicated. The central target of the BOLA proteins is the Fe/S protein lipoic acid (LA) synthase (LIAS). Its product is used as a cofactor of five mitochondrial enzymes including pyruvate dehydrogenase (PDH) and 2-ketoglutarate dehydrogenase (KGDH). Based on the hetero-complex formation with the BOLA proteins an additional function of GLRX5 in this late phase of mitochondrial Fe/S protein assembly is likely. Mutations in human NFU1 and BOLA3 cause multiple mitochondrial dysfunction syndromes (MMDS; for review see [Beilschmidt and Puccio, 2014; Stehling et al., 2014]).
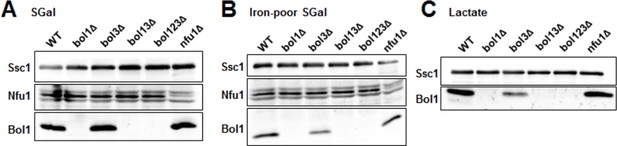
Late ISC protein levels in various yeast strains.
Wild-type (WT; BY4742), indicated BOL deletion strains and nfu1Δ cells were grown in (A) minimal medium containing 2% galactose supplemented with 50 µM ferric ammonium citrate, (B) iron-poor minimal medium containing 2% galactose or (C) lactate medium. Mitochondria were isolated and the indicated protein levels were determined by immunostaining. The Hsp70 protein Ssc1 served as a loading control. As shown below (Author response image 2), wild-type levels of Bol3 were not detectable by our antibody precluding analysis of this protein.
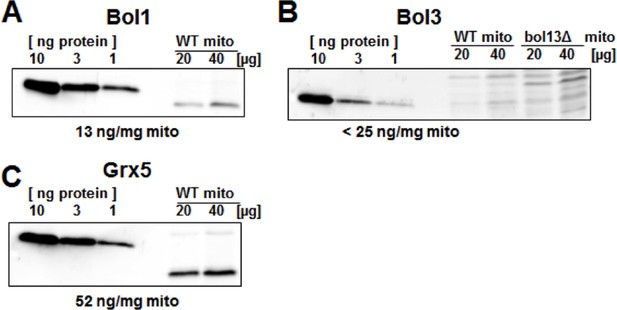
Protein levels of Bol1, Bol3 and Grx5 in yeast mitochondria.
Wild-type (WT; BY4742) and bol13Δ cells were grown in minimal medium containing 2% galactose supplemented with 50 µM ferric ammonium citrate and used for isolation of mitochondria (mito). Mitochondrial proteins and the indicated amounts of purified (A) Bol1, (B) Bol3, and (C) Grx5 were analyzed by immunostaining with specific antibodies. Densitometry of the bands was used to calculate the proteins levels (in ng per mg mitochondria). Since our antibody did not detect wild-type amounts of Bol3 in mitochondria (B), only an upper limit of Bol3 could be calculated. The different gel mobility of purified and mitochondrial Bol1 and Grx5 is explained by a His-tag on purified proteins.
Additional files
-
Supplementary file 1
Yeast strains and plasmids used in this study.
(A) Yeast strains used in this study. Gene disruptions and promoter exchanges were generated by PCR-based gene replacement and verified by PCR as described previously (Gueldener et al., 2002; Mühlenhoff et al., 2002). Yeast cells were transformed by the lithium acetate method (Gietz and Woods, 2002). In some cells, the TRP1 gene was disrupted by a natNT2 cassette (Janke et al., 2004) in order utilize plasmids with the TPR1 marker. (B) Plasmid constructs used in this study. The plasmids were constructed inserting the indicated genes into vector. The amino acid residues of the encoded proteins and the hexa-histidinyl tag (6xHis) are indicated.
- https://doi.org/10.7554/eLife.16673.026





