Rac1-mediated membrane raft localization of PI3K/p110β is required for its activation by GPCRs or PTEN loss
Figures
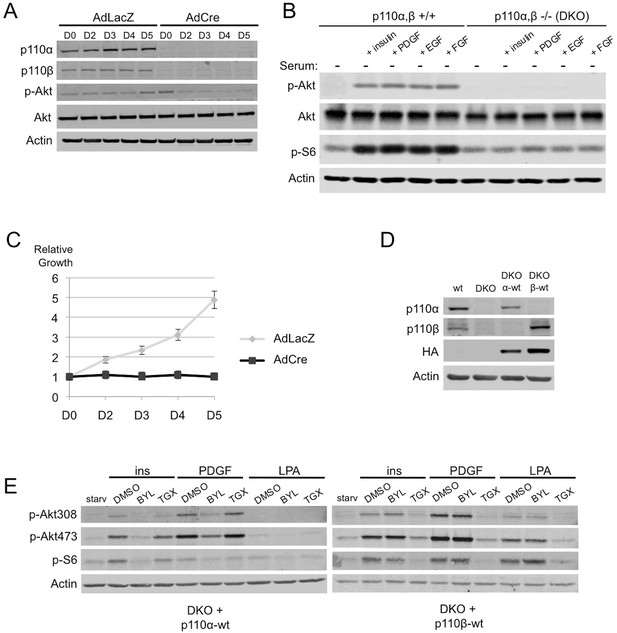
Simultaneous ablation of p110α and p110β disrupts PI3K pathway, which could be reconstituted by membrane microdomain targeting of either isoform in DKO MEFs.
(A) p110αflox/flox; p110βflox/flox MEFs were treated with either AdLacZ or AdCre. 20 hr after the viral transductions, cells were harvested at indicated time points and immunoblots were performed. p-Akt (for T308) immunoblot depicts activation of PI3K pathway. (B) Wt or DKO MEFs were serum starved for 4 hr and stimulated with insulin, PDGF, EGF or FGF. Cells were then harvested and analyzed in immunoblots using the indicated antibodies (T308 for p-Akt and S235/236 for p-S6) to determine the extent of PI3K activation. (C) Proliferation kinetics of AdLacZ or AdCre treated MEFs were determined by crystal violet assays. Error bars depict standard deviation in 3 independent experiments. D denotes days. (D) Wt, DKO, DKO+p110α-wt and DKO+p110β-wt MEFs were analyzed in immunoblots for expression of p110α and p110β with the indicated antibodies. (E) DKO+p110α-wt and DKO+p110β-wt MEFs were starved and stimulated with insulin, PDGF and LPA in the presence of DMSO or isoform specific PI3K inhibitors, BYL-719 (BYL) and TGX-221 (TGX), specific for p110α and p110β, respectively. PI3K signaling efficiency in these cells was determined with p-Akt (for T308 and S473) and p-S6 (for S235/236) immunoblots.
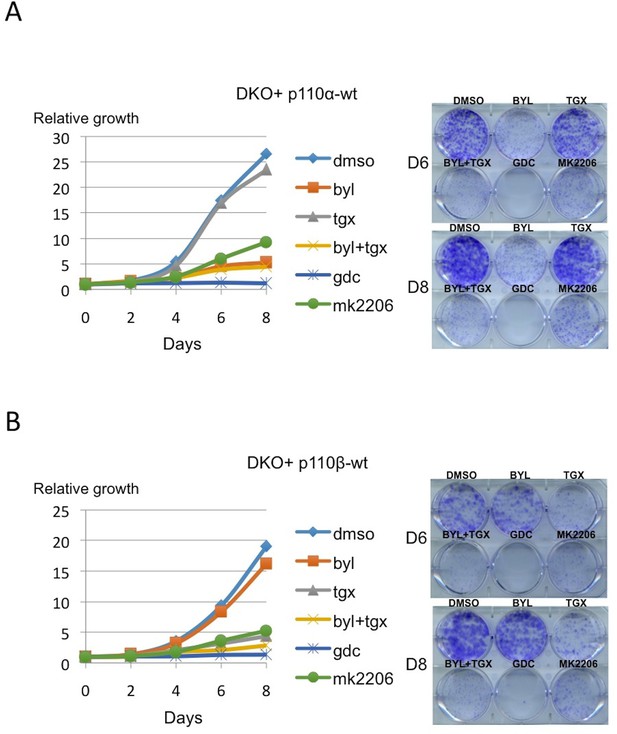
PI3K pathway could be reconstituted by unique expression of either p110α-wt or p110β-wt in DKO MEFs.
(A) On the right, DKO+p110α-wt add-back MEFs were analyzed for proliferation in the presence of DMSO, BYL-719; p110α inhibitor, TGX-221; p110β inhibitor, BYL+TGX, GDC-0941 (GDC); pan-PI3K inhibitor and MK-2206; Akt inhibitor, for six and eight days. On the left, graph depicts mean proliferation of samples in 3 independent experiments. (B) On the right, DKO+p110β-wt add-back MEFs were analyzed for proliferation in the presence of the indicated inhibitors, for six and eight days. On the left, graph depicts mean proliferation of samples in 3 independent experiments.
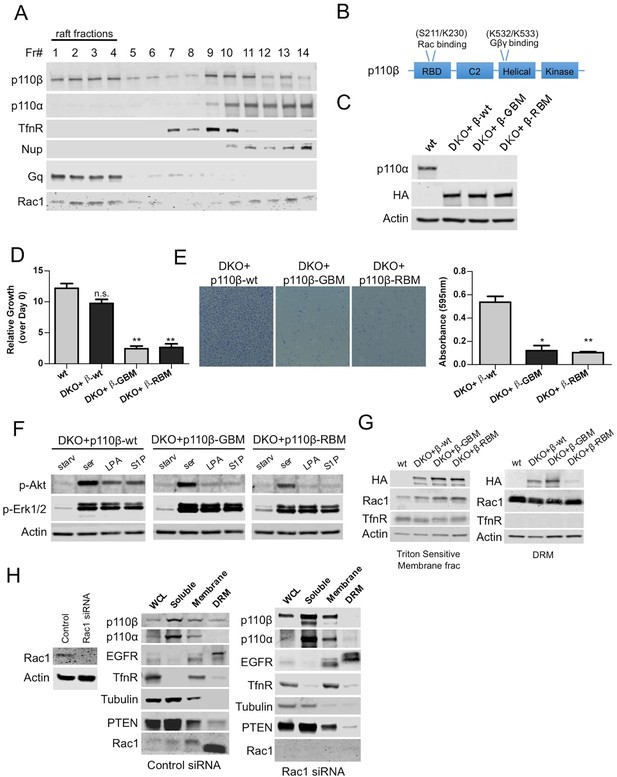
Rac1 binding domain constitutes a raft localization signal for p110β.
(A) Detergent-free fractionation of HMECs on an Opti-prep gradient followed by western blots with the indicated antibodies. TfnR (transferrin receptor); marker for nonraft membranes. Nup (nucleoporin); marker for nuclear membranes. Gq; marker for membrane rafts. (B) Graphical depiction of the functional domains of p110β including the residues responsible for Rac1 and Gβγ interaction. (C) Wt, DKO+p110β-wt, DKO+p110β-Gβγ binding mutant (GBM) and DKO+p110β-Rac1 binding mutant (RBM) MEF samples were processed to determine levels of p110α and p110β expression. (D) The indicated cells were grown in 8% FBS-DMEM and cellular proliferation was assessed after a week. Error bars indicate standard deviation in 3 independent experiments. **p<0.01, n.s. (not significant), p>0.05. (E) The indicated MEFs were analyzed for migration across transwell inserts; cells were detected with crystal violet staining. On the right, MEFs were quantified for migration through the transwell in 3 independent experiments with standard deviation. *p<0.05, **p<0.01. (F) The indicated MEFs were starved and stimulated with serum or GPCR agonists LPA and S1P. p-Akt (for T308) displays level of Akt activation and p-Erk1/2 depicts activation of MAPK pathway. (G) Indicated cells were fractionated into soluble, triton sensitive and triton resistant fractions. Triton soluble and resistant (DRM) fractions were analyzed in immunoblots; anti-HA antibodies were used to visualize the abundance of the p110β variants in those fractions. Anti-Rac1 antibody was used to demonstrate raft enrichment, whereas anti-TfnR immunoblot depicts enrichment of nonraft membranes. Anti-actin serves as loading control. (H) On the left, HMECs transfected with either control or Rac1 specific siRNAs were lysed and processed for western blot. On the right, siRNA treated cells were fractionated. WCL were analyzed to display overall levels of protein expression. Soluble, triton soluble (membrane) and triton resistant membrane fractions (DRM) were analyzed in immunoblots; Anti-Rac1 antibodies were used to assess level of Rac1 knock-down. Anti-EGFR antibodies were used as markers for DRM fractions, whereas anti-TfnR immunoblot depicts enrichment of nonraft membranes. Anti-tubulin immunoblot serves as a marker for soluble fractions.
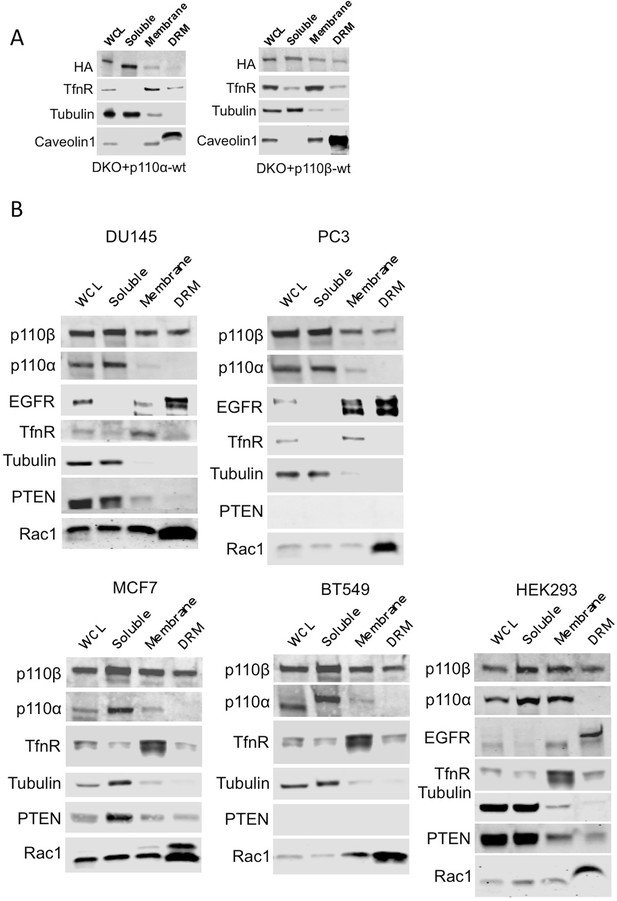
Membrane raft localization of p110β in different cell lines.
(A) DKO+p110α-wt and DKO+p110β-wt MEFs were lysed and fractionated into triton sensitive and triton resistant membrane fractions. Whole cell lysates (WCL) were analyzed to display overall levels of protein expression. Soluble, triton soluble (membrane) and triton resistant membrane fractions (DRM) were analyzed in immunoblots; anti-HA antibodies were used to visualize the abundance of the respective PI3K in fractions. Anti-Caveolin1 antibodies were used as markers for detergent resistant membranes (DRM), whereas anti-TfnR immunoblot depicts enrichment of nonraft membranes. Anti-tubulin immunoblot serves as a marker for soluble fractions. (B) HEK293, DU145, PC3, MCF7 and BT549 cells were lysed and fractionated into triton sensitive and triton resistant membrane fractions. Whole cell lysates (WCL) were analyzed to display overall levels of protein abundance. Soluble, triton sensitive (membrane) and resistant (DRM) membrane fractions were analyzed in immunoblots; anti-EGFR and anti-Rac1 immunoblots were used as markers for detergent resistant membrane (DRM) fractions, whereas anti-TfnR immunoblot depicts enrichment of nonraft plasma membranes. Anti-tubulin antibodies serve as a marker for soluble fractions. Anti-PTEN immunoblots depict PTEN status of the respective cell line.
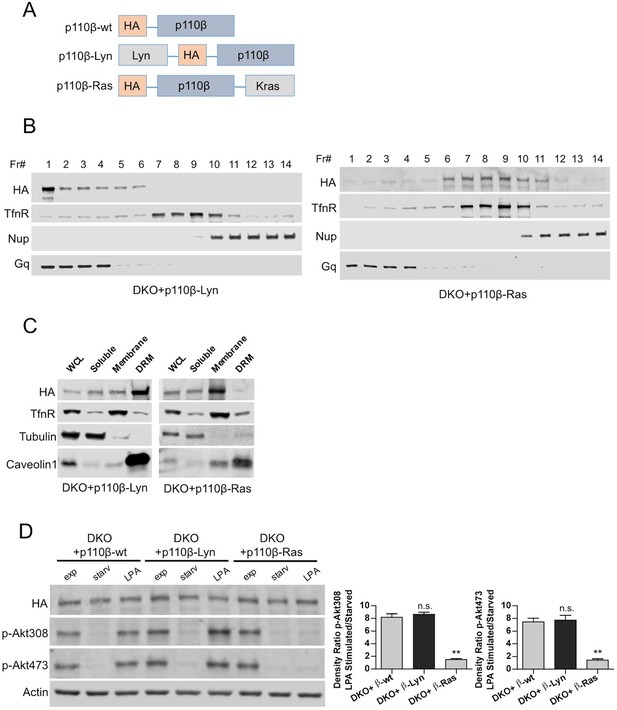
Raft-excluded p110β fails to induce Akt phosphorylation upon GPCR stimulation.
(A) Schematic demonstration of p110β membrane microdomain targeting vectors. (B) Detergent-free fractionation of DKO+p110β-Lyn and DKO+p110β-Ras MEFs on an Opti-prep gradient followed by western blots with the indicated antibodies. TfnR; a marker for nonraft plasma membrane. Nup; a marker for nuclear membranes. Gq; a marker for membrane rafts. (C) The indicated MEFs were lysed and fractionated. WCL were analyzed to display overall levels of protein expression. Soluble, triton soluble (membrane) and resistant membrane fractions (DRM) were analyzed in immunoblots; anti-Caveolin1 antibodies were used as marker for DRM fractions. Anti-tubulin immunoblot serves as a marker for soluble fractions. (D) The indicated add-back MEFs were starved and stimulated with LPA. Anti-HA immunoblot demonstrates levels of exogenous p110β expression whereas anti-p-Akt antibodies (for T308 and S473) mark the activation state of Akt. Anti-actin antibodies were used as loading control. On the right, normalized anti-p-Akt T308 and S473 band intensity quantifications of the samples (mean of 3 independent experiments with standard deviation). Density ratio is the fold change between normalized band intensities of p-Akt signals in LPA stimulated over starved samples. **p<0.01, n.s. p>0.05.
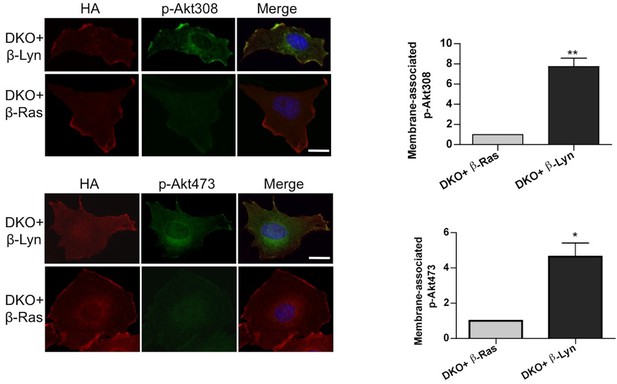
Raft-excluded p110β fails to induce activatory Akt phosphorylation upon GPCR stimulation.
Indicated DKO add-back MEFs were seeded on coverslips, starved and stimulated with LPA; membrane associated Akt phosphorylation at T308 and S473 was detected using anti-p-Akt T308 and p-Akt S473 antibodies (green). Anti-HA antibodies (red) depicted expression levels of the add-back vectors. DNA is shown in blue. Scale bar; 20 µm. On the right, anti-p-Akt T308 and S473 signals on the cell membrane was quantified upon LPA stimulation and the relative corrected total membrane fluorescence was depicted. Results denote mean of 3 independent experiments with standard deviation. *p<0.05, **p<0.01.
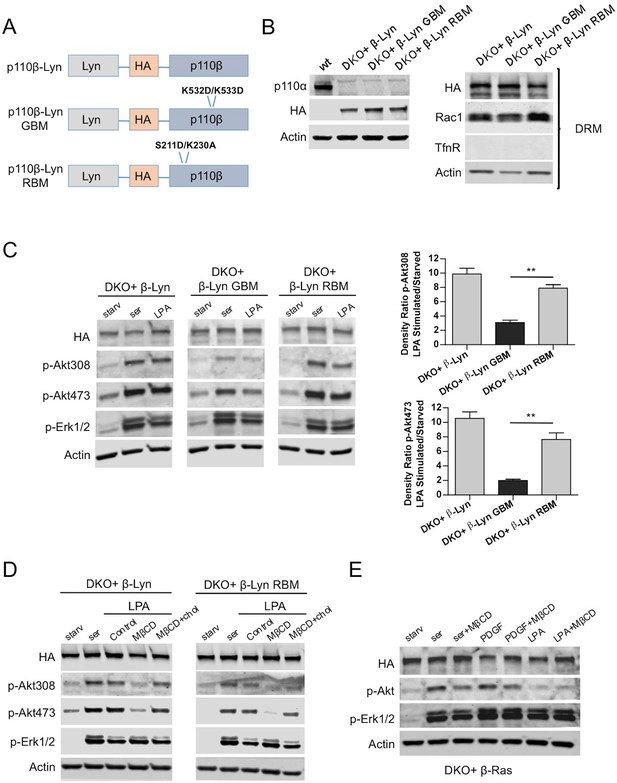
Raft targeting of Rac1-binding deficient p110β rescues Akt activation in GPCR signaling.
(A) Schematic demonstration of p110β-Lyn domain membrane targeting vectors. (B) Lysates from the indicated MEFs were processed and analyzed for expression of p110α and β. On the right, DKO MEFs expressing the indicated p110β alleles were fractionated into soluble, triton sensitive and triton resistant fractions. Triton resistant fractions were analyzed in immunoblots; anti-HA antibodies were used to visualize the abundance of the p110β variants in those fractions. Anti-Rac1 antibody was used to demonstrate raft enrichment, whereas anti-TfnR immunoblot depicts contamination with nonraft membranes. Anti-actin immunoblot serves as loading control. (C) The indicated add-back MEFs were starved and stimulated with serum or LPA. Anti-p-Akt immunoblots on T308 and S473 display level of Akt activation and anti-p-Erk1/2 antibodies (for T202/Y204) depicts activation of MAPK pathway. On the right, density ratios of the normalized fold-increase in baseline Akt phosphorylation at T308 and S473 in starved vs. LPA stimulated states were quantified (mean of 3 independent experiments with standard deviation). **p<0.01. (D) The indicated MEFs were starved and stimulated with either serum or LPA in the presence of MβCD with or without addition of excess cholesterol. Anti-p-Akt immunoblots on T308 and S473 displays level of Akt activation and anti-p-Erk1/2 antibodies (for T202/Y204) depict activation of MAPK pathway (E) DKO+p110β-Ras add-back MEFs were starved and stimulated with serum, PDGF or LPA in the presence or absence of MβCD. Anti-p-Akt (for T308) and anti-p-Erk1/2 (for T202/Y204) immunoblots reveal degree of PI3K and MAPK activation respectively.
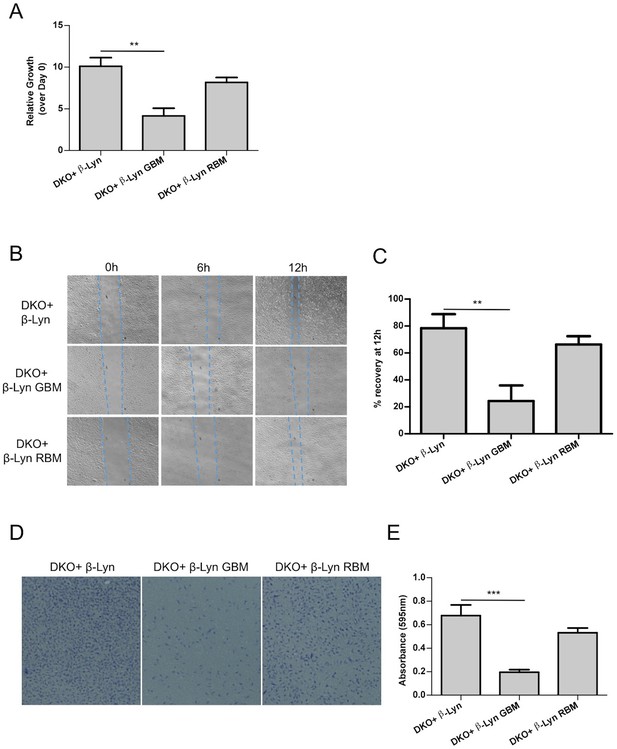
Defects in proliferation and motility are restored upon targeting Rac1-binding deficient p110β to membrane rafts whereas Gβγ interactionremains to be essential for p110β function.
(A) Wt and the indicated DKO add-back MEFs were analyzed for cellular proliferation in crystal violet assays (mean of 3 independent experiments with standard deviation). **p<0.01. (B) Wound healing assays were performed on the indicated MEFs. Images of the cells were captured at the indicated time points; dashed lines represent leading edge of the cells moving into the inflicted wound. (C) Graph depicts the mean percentage of wound healing at the end of 12 hr for cells indicated in (B); in 3 independent experiments with standard deviation. **p<0.01. (D) DKO+p110β-Lyn, DKO+p110β-Lyn GBM and DKO+p110β-Lyn RBM add-back MEFs were analyzed for migration across transwell inserts; cells were detected with crystal violet staining. (E) Cells in (D) were quantified for migration through the transwell in 3 independent experiments, mean absorbance (at 595 nm) with standard deviation is depicted. ***p<0.001.
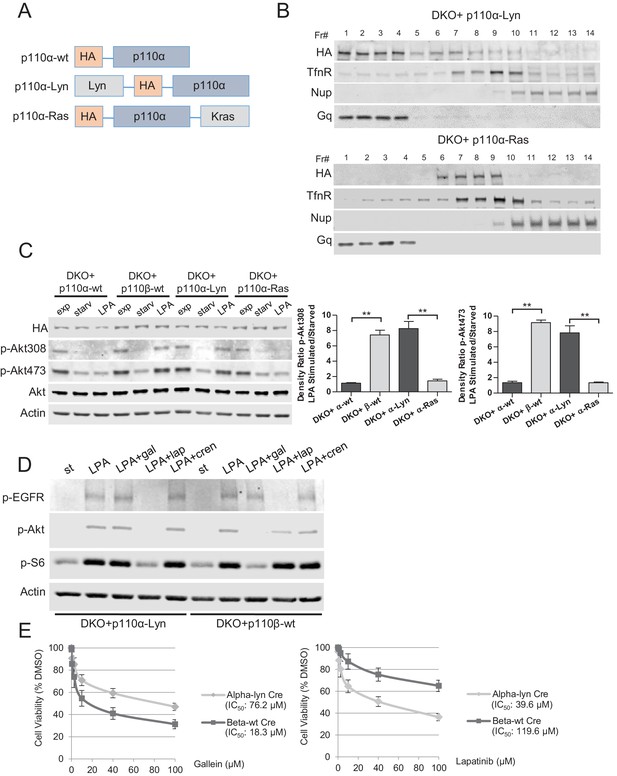
Raft-targeted p110α induces Akt phosphorylation upon GPCR signaling via EGFR activity.
(A) Schematic demonstration of p110α membrane microdomain targeting vectors. (B) Detergent-free fractionation of DKO+p110α-Lyn and DKO+p110α-Ras MEFs on an Opti-prep gradient followed by western blots with the indicated antibodies. TfnR; a marker for nonraft plasma membrane. Nup; a marker for nuclear membranes. Gq; a marker for membrane rafts. (C) The indicated add-back MEFs were starved and stimulated with LPA. Anti-p-Akt antibodies (for T308 and S473) mark the activation state of Akt. Anti-Akt and anti-actin immunoblots were used as loading controls. On the right, normalized density ratios of the mean fold-increase in baseline Akt phosphorylation at T308 and S473 in starved vs. LPA stimulated states. Graphs denote mean of 3 independent experiments with standard deviation. **p<0.01. (D) DKO+p110α-Lyn and DKO+p110β-wt MEFs were starved and stimulated with LPA in the presence of small molecule inhibitors targeting Gβγ, EGFR or PDGFR. Anti-p-EGFR (for Y1068), anti-p-Akt (for T308) and anti-p-S6 (for S235/236) immunoblots depict activation of EGFR, Akt and downstream signaling. Gal denotes gallein, a Gβγ inhibitor; lap depicts lapatinib, an EGFR inhibitor and cren denotes crenolanib, a PDGFR inhibitor. (E) The indicated MEFs were treated with 0, 0.1, 1, 2.5, 10, 40 or 100 μM of lapatinib or gallein in proliferation assays. Cellular growth was assessed after five days in 2% FBS-DMEM. Error bars denote standard deviation in 3 independent experiments.
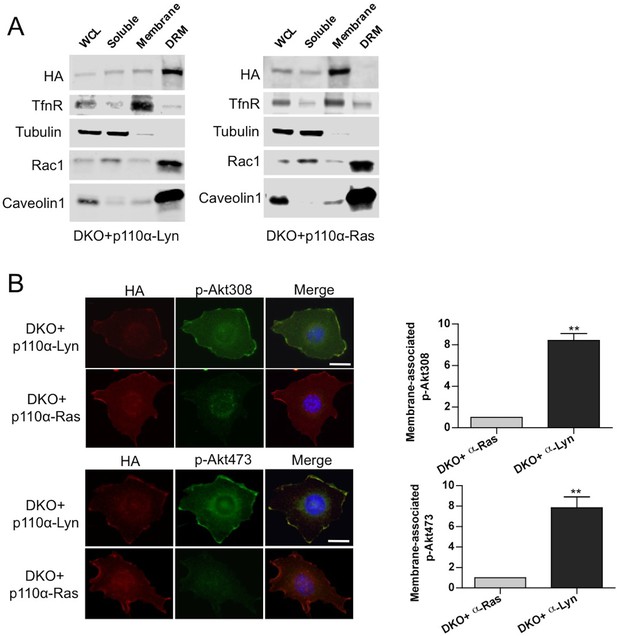
Membrane targeting p110α vectors selectively enrich p110α in the desired microdomain.
(A) DKO+p110α-Lyn and DKO+p110α-Ras MEFs were lysed and fractionated into triton sensitive and triton resistant membrane fractions. WCLs were analyzed to display overall levels of protein expression. Soluble, triton soluble (membrane) and resistant membrane fractions (DRM) were analyzed in immunoblots; anti-Rac1 and anti-Caveolin1 antibodies were used as markers for detergent resistant membrane (DRM) rafts, whereas anti-TfnR immunoblot depicts enrichment of nonraft membranes. Anti-tubulin immunoblot serves as a marker for soluble fractions. (B) The indicated DKO add-back MEFs were seeded on coverslips, starved and stimulated with LPA; membrane associated Akt phosphorylation at T308 and S473 was detected using anti-p-Akt T308 and p-Akt S473 antibodies (green). Anti-HA antibodies (red) depicted expression levels of the add-back vectors. DNA is shown in blue. Scale bar; 20 µm. On the right, anti-p-Akt T308 and S473 signals on the cell membrane was quantified upon LPA stimulation and the relative corrected total membrane fluorescence was depicted. Results denote mean of 3 independent experiments with standard deviation. **p<0.01.
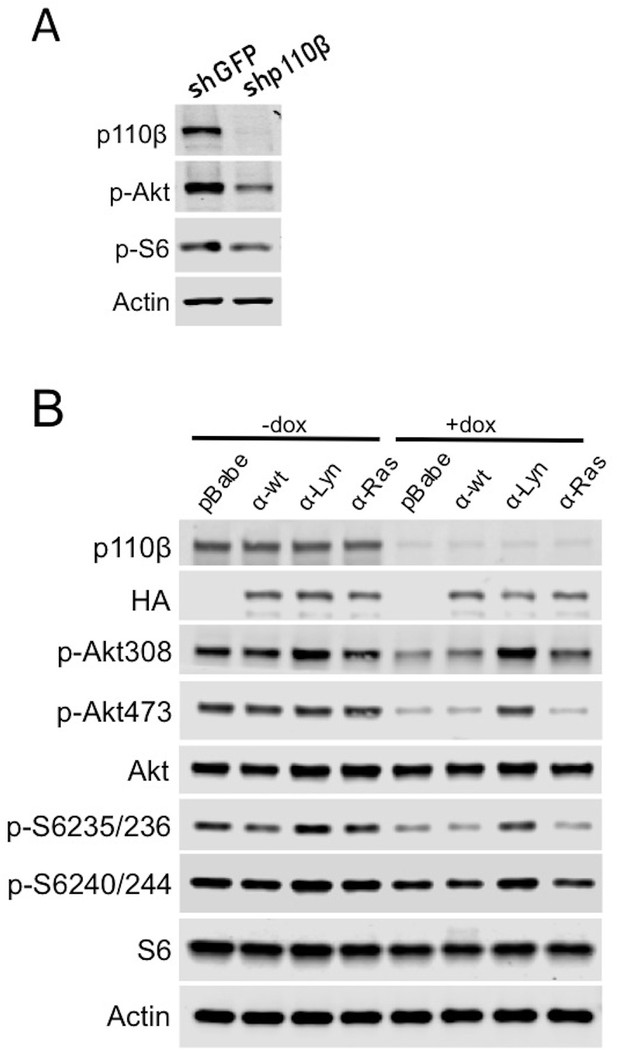
Raft-targeted p110α has redundant functions with wt p110β.
(A) PC3 cells were transduced with PLKO tet.on shGFP and shp110β plasmids and samples were collected 48 hr after dox induction. p110β, p-Akt (for T308) and p-S6 (for S235/236) levels were determined in an immunoblot with the indicated antibodies. (B) PC3 cells transduced with PLKO tet.on shp110β vector were used in exogenous overexpression studies of p110α membrane targeting constructs in the presence or absence of dox. Anti-p-Akt immunoblots (for T308 and S473) depict activation of Akt, whereas anti-p-S6 antibodies (for S235/236 and S240/244) demonstrate level of downstream PI3K activation. Anti-p110β and anti-HA immunoblots determine levels of p110β and exogenously expressed p110α respectively. Akt, S6 and actin immunoblots were used as loading controls.
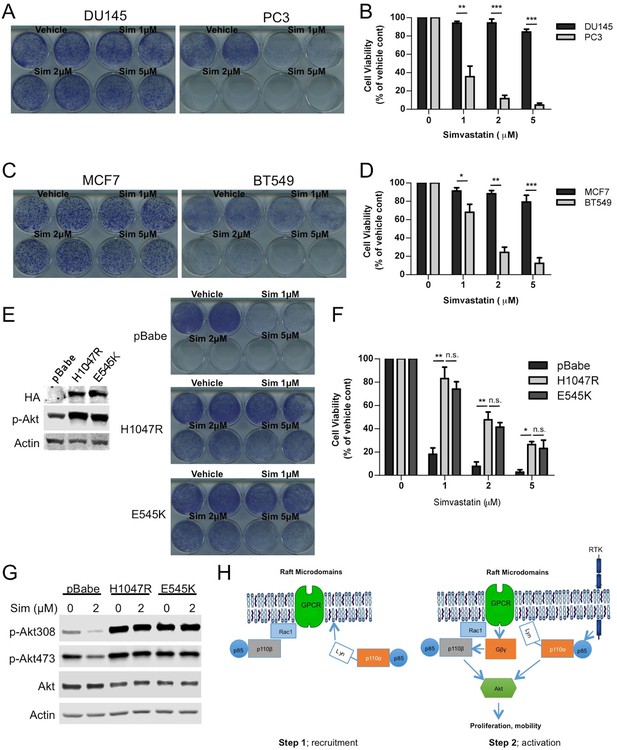
Raft dependent PI3K function is critical for PTEN null cancer cells.
(A) DU145 and PC3 prostate cancer cell lines were seeded on 12-well plates and were treated with indicated doses of simvastatin for a week. Crystal violet assays determined the extent of cell growth. (B) Quantification of cellular proliferation assays in (A), graph displays mean of 3 independent experiments with standard deviation. **p<0.01, ***p<0.001. (C) MCF7 and BT549 breast cancer cell lines were seeded on 12-well plates and were treated with indicated doses of simvastatin for a week. Crystal violet assays determined the extent of cellular growth. (D) Quantification of cellular proliferation assays in (C), graph depicts mean of 3 independent experiments with standard deviation. *p<0.05, **p<0.01, ***p<0.001. (E) PC3 cells expressing empty control plasmid, p110α-H1047R and p110α-E545K vectors processed for western blot analysis with the indicated antibodies. (F) Indicated cells were treated with increasing doses of simvastatin for a week. Crystal violet assays determined the extent of cellular growth. (G) Quantification of cellular proliferation assays in (F), graph depicts mean of 3 independent experiments with standard deviation. *p<0.05, **p<0.01, n.s. p>0.05. (H) Biochemical analysis of PC3 cells in (E) treated with 2 μM simvastatin for 36 hr. Anti-p-Akt immunoblots on T308 and S473 display level of Akt activation. Anti-Akt and anti-actin antibodies were used as loading control. (I) Model representing PI3K signaling downstream of GPCRs in a two-step process. Rac1 mediates raft recruitment of p110β while p110α is elusive from rafts under physiological conditions. Once localized to GPCR signaling permissive membrane microdomains, either p110α or p110β can be activated via distinct mechanisms; p110α activity can be triggered via lateral activation of EGFR by GPCRs, whereas p110β can be activated via the canonical Gβγ pathway.
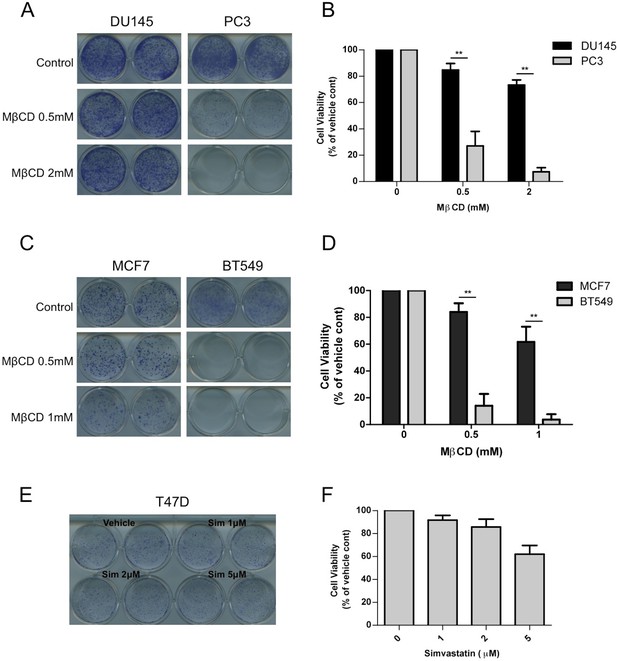
Critical dependency of PTEN null cancer cell lines to raft associated PI3K signaling.
(A) DU145 and PC3 prostate cancer cells were seeded on 12-well plates and treated with indicated doses of MβCD for seven days. Crystal violet assays determined the extent of cell growth. (B) Quantification of cellular proliferation assays in (A), graph displays mean of 3 independent experiments with standard deviation. **p<0.01. (C) MCF7 and PC3 breast cancer cells were seeded on 12-well plates and treated with indicated doses of MβCD for seven days. Crystal violet assays determined the extent of cell growth. (D) Quantification of cellular proliferation assays in (C), graph displays mean of 3 independent experiments with standard deviation. **p<0.01. (E) T47D breast cancer cells were seeded on 12-well plates and were treated with indicated doses of simvastatin for seven days. Crystal violet assays determined the extent of cell growth. (F) Quantification of cellular proliferation assays in (E), graph displays mean of 3 independent experiments with standard deviation.
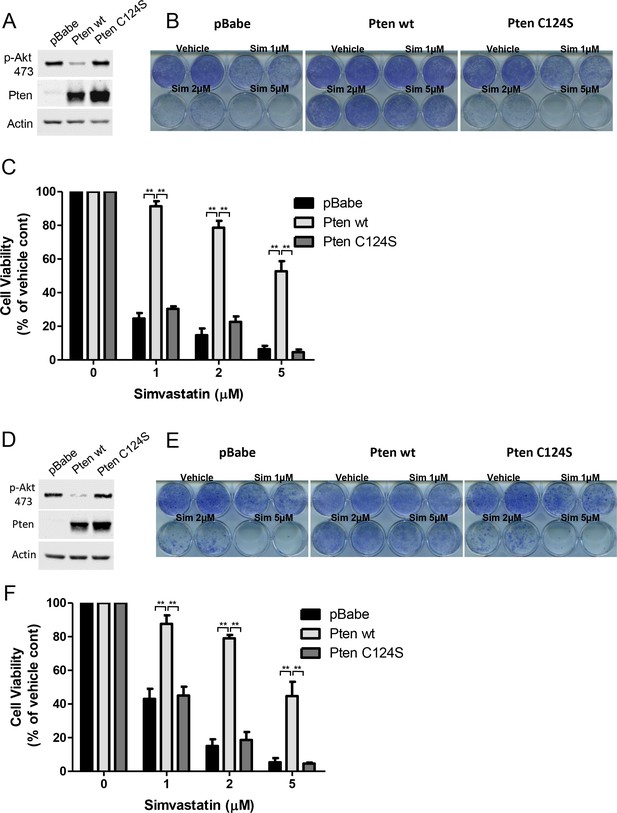
PTEN null cancer lines become sensitized to simvastatin upon re-expression of wild-type PTEN.
(A) PC3 cells expressing empty control plasmid, PTEN wt and PTEN C124S were processed for western blot analysis with the indicated antibodies. (B) PC3 cells in (A) were seeded on 12-well plates and treated with indicated doses of simvastatin for seven days. Crystal violet assays determined the extent of cell growth. (C) Quantification of cellular proliferation assays in (B), graph displays mean of 3 independent experiments with standard deviation. **p<0.01. (D) BT549 cells expressing empty control plasmid, PTEN wt and PTEN C124S were processed for western blot analysis with the indicated antibodies. (E) BT549 cells in (D) were seeded on 12-well plates and treated with indicated doses of simvastatin for seven days. Crystal violet assays determined the extent of cell growth. (F) Quantification of cellular proliferation assays in (E), graph displays mean of 3 independent experiments with standard deviation. **p<0.01.





