Obligate coupling of CFTR pore opening to tight nucleotide-binding domain dimerization
Figures
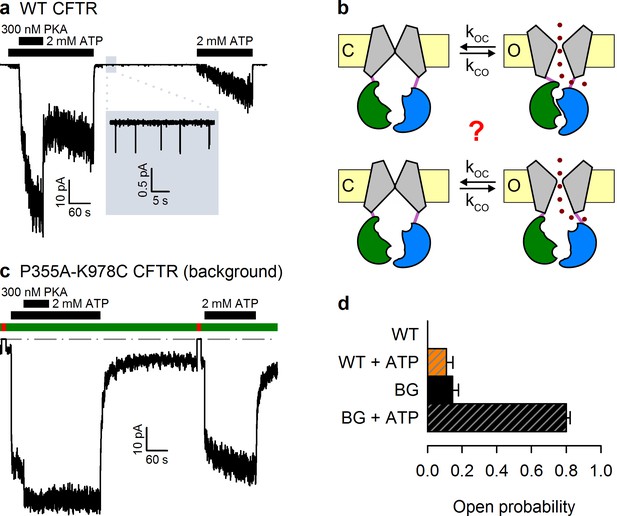
The P355A-K978C double mutation increases both spontaneous and ATP-dependent .
(a,c) Inward macroscopic (a) WT and (c) P355A-K978C CFTR currents at −80 mV, and their dependence on ATP and PKA (black bars). In (a) a 30 s segment of spontaneous current (gray box) is magnified in the inset. In (c) bath chloride (green bars) was repeatedly replaced by gluconate (red bars) to determine baseline current (gray dashed line). (b) Two alternative mechanisms of spontaneous gating in cartoon representation. TMDs (gray), TMD-NBD interface formed by intracellular loops (light violet), NBD1 (green), NBD2 (blue), membrane (yellow). (d) Spontaneous (solid bars) and in 2 mM ATP (striped bars) for WT and P355A-K978C ('background', BG) CFTR (n = 5–21).
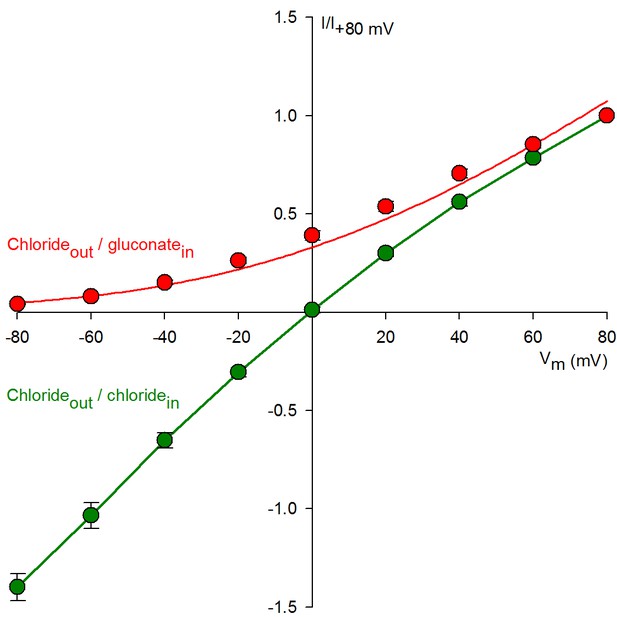
Replacement of bath chloride with gluconate abolishes CFTR currents at -80 mV.
Normalized macroscopic current-voltage relationships of WT CFTR under chloride(out)/chloride(in) (green symbols) and chloride(out)/gluconate(in) (red symbols) ionic conditions (n = 6–9). For both ionic conditions macroscopic steady CFTR currents in 2 mM ATP, and background currents in the absence of ATP, were sampled at membrane voltages ranging from −80 to +80 mV, and ATP-dependent currents normalized to that measured at +80 mV. The Red solid line is a fit to the Goldman-Hodgkin-Katz equation, the green solid line is a linear interpolation between data points. Note mild inward rectification in symmetrical chloride (Cai et al., 2003).
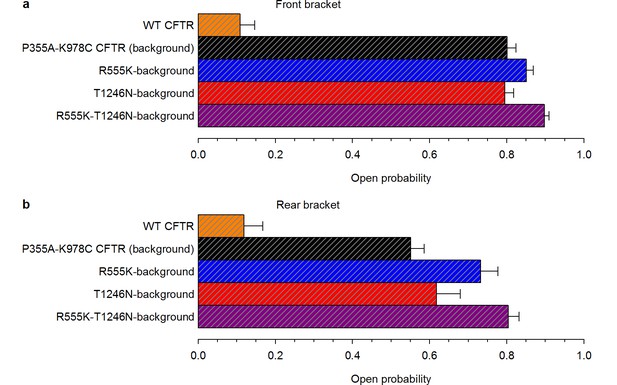
High ATP-dependent due to background mutation facilitates counting channels.
(a–b), Open probabilities in 2 mM ATP, following PKA removal, for WT CFTR and for the four constructs that form the mutant cycle. was estimated from the last ~2-minutes of the 2.5-minute segments of recording in 2 mM ATP, bracketing the 5-minute ATP-free period during which spontaneous activity was sampled (n = 6–17). (a) Front bracket, (b) rear bracket (see Figure 2a–d).
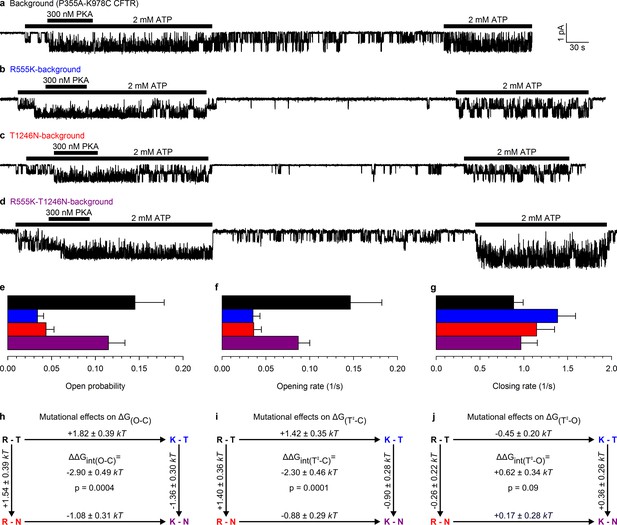
Arg 555 and Thr 1246 become energetically coupled upon spontaneous pore opening.
(a–d), Microscopic currents at −80 mV, and their dependence on ATP and PKA (black bars), for (a) the P355A-K978C background construct, and for this background construct carrying (b) the R555K or (c) T1246N single mutations or (d) the R555K-T1246N double mutation. (e–g), Spontaneous open probabilities (e, n = 19–21), opening rates (f, n = 15–19), and closing rates (g, n = 15–19) of the background construct (black bars), and of constructs carrying additional R555K (blue bars), T1246N (red bars), or R555K-T1246N (violet bars) mutations. (h–j), Thermodynamic mutant cycles showing mutation-induced changes (h) in the stability of the spontaneous open state relative to the closed state, and (i–j) in the height of the activation free energy barriers for spontaneous opening (i) and closure (j). Each corner is represented by the side chains at positions 555 and 1246; , Boltzmann's constant, , absolute temperature.
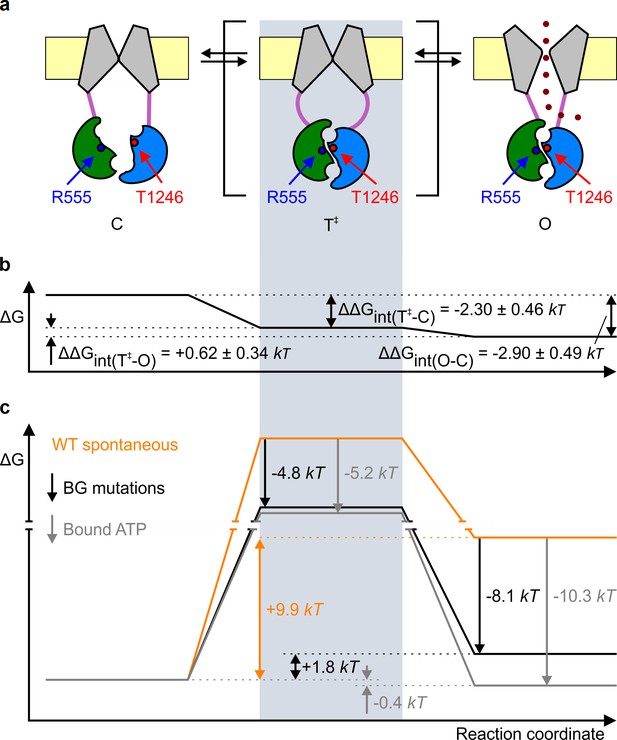
Mechanism of spontaneous pore openings, and energetic effects of bound ATP and gain-of-function mutations.
(a) Cartoon representation of domain organization in closed (), transition (), and open () states, during spontaneous gating. Color coding as in Figure 1b, blue and red circles identify target positions. (b) Changes in energetic coupling between the target positions associated with spontaneous gating in the background construct. (c) Free energy profiles of gating for WT (orange line) and P355A-K978C (black line) CFTR in the absence of ATP, and of hydrolysis-deficient K1250R CFTR in saturating ATP (gray line) (Vergani et al., 2005). Black and gray downward arrows illustrate the energetic effects of the background double mutation and of the presence of ATP bound in both composite sites.





