Na+/K+ pump interacts with the h-current to control bursting activity in central pattern generator neurons of leeches
Figures
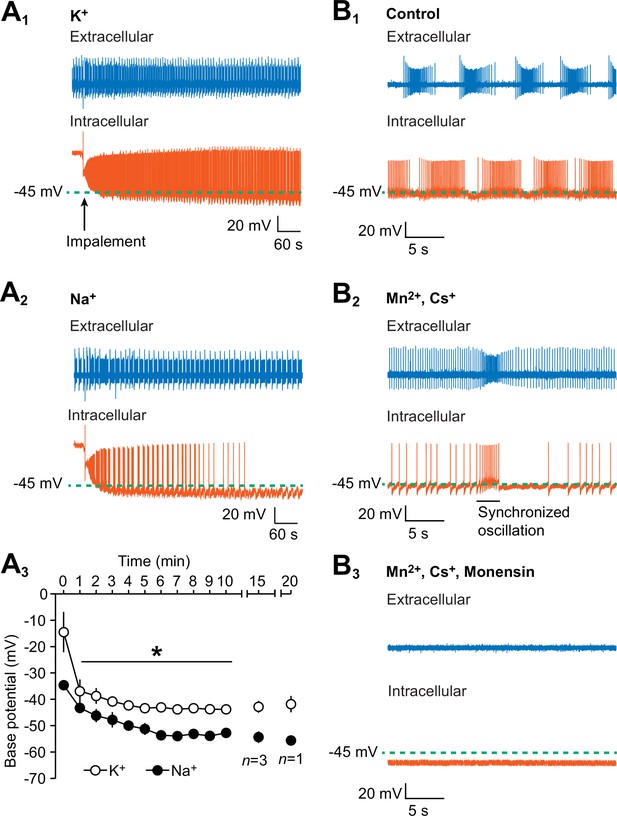
Hyperpolarization of the oscillator heart interneurons and suppression of their spiking activity by intracellular leakage of Na+ from an electrode and by monensin.
(A1) An extracellular (blue) trace of one oscillator heart interneuron and an intracellular (vermilion) trace of a contralateral oscillator heart interneuron that was impaled with a K+-filled intracellular electrode. (A2) Impalement of an oscillator heart interneuron with a Na+-filled electrode gradually suppressed its spiking activity and hyperpolarized the neuron. There was no change in the bursting activity of the extracellularly recorded neurons in the (A1) K+ and (A2) Na+ recordings. (A3) During the first ten minutes, the average base potential of Na+-loaded neurons (closed circles) was significantly more hyperpolarized than the base potential of K+-loaded neurons (open circles). Such differences persisted well into the 15th and 20th minute. The data are represented as mean ± SEM, with the asterisk (*) representing significant differences between the K+ and Na+ base potentials (split-plot ANOVA, F1,8 = 1847.7, p=0.006). (B1) Extracellular (blue) and intracellular (vermilion) traces from a pair of oscillator heart interneurons that were initially bathed in control saline and showed normal alternating bursting. (B2) When the oscillator heart interneurons were bathed in Ca2+-free saline with 2 mM Cs+ and 1.8 mM Mn2+, they produced a more tonic firing pattern that was interspersed with synchronized oscillations. (B3) When the oscillator heart interneurons were subsequently treated with 10 µM monensin in the same Ca2+-free saline, the spiking activity of both oscillator heart interneurons were suppressed and the membrane potential of the intracellularly recoded neuron gradually hyperpolarized.
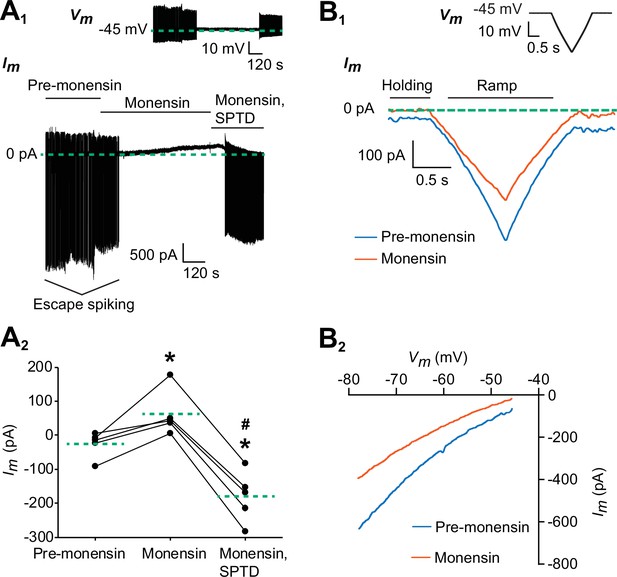
Monensin stimulates the outward Na+/K+ pump current.
(A1) Membrane current trace from an oscillator heart interneuron with its membrane potential (Vm) voltage-clamped at −45 mV (see inset) in Ca2+-free saline with 1.8 mM Mn2+ plus 2 mM Cs. Changes in the neuron’s membrane current (Im) were observed under three experimental treatments: pre-monensin saline for five minutes, 10 µM monensin for 10 min, and 10 µM monensin plus 100 µM strophanthidin (SPTD) for another five minutes. (A2) A scatterplot of membrane currents from five preparations, with each green dashed line representing a mean for each of the three experimental treatments. Monensin induced a significant outward current relative to pre-monensin saline. Monensin plus strophanthidin induced a significant inward current relative to pre-monensin or monensin saline. The asterisks (*) represent significance from the pre-monensin saline whereas the hashtag (#) represents significance from the monensin saline (Tukey’s test, p<0.05 for all tests). (B1) Membrane currents from the same oscillator heart interneuron that was voltage-clamped at −45 mV in the same Ca2+-free saline before and after treatment with 10 µM monensin. Both currents were generated by a slow ramp-clamp protocol, with each voltage ramp running from −45 mV to −80 mV and back (see inset). Compared to the membrane current under pre-monensin saline (blue trace), the membrane current under monensin saline (vermilion trace) shifted outwards across the entire range of negative voltage-ramp values. (B2) Current-voltage relations under pre-monensin (blue line) and monensin (vermilion line) treatments from the same oscillator heart interneuron were generated based on the (B1) voltage-ramp traces.
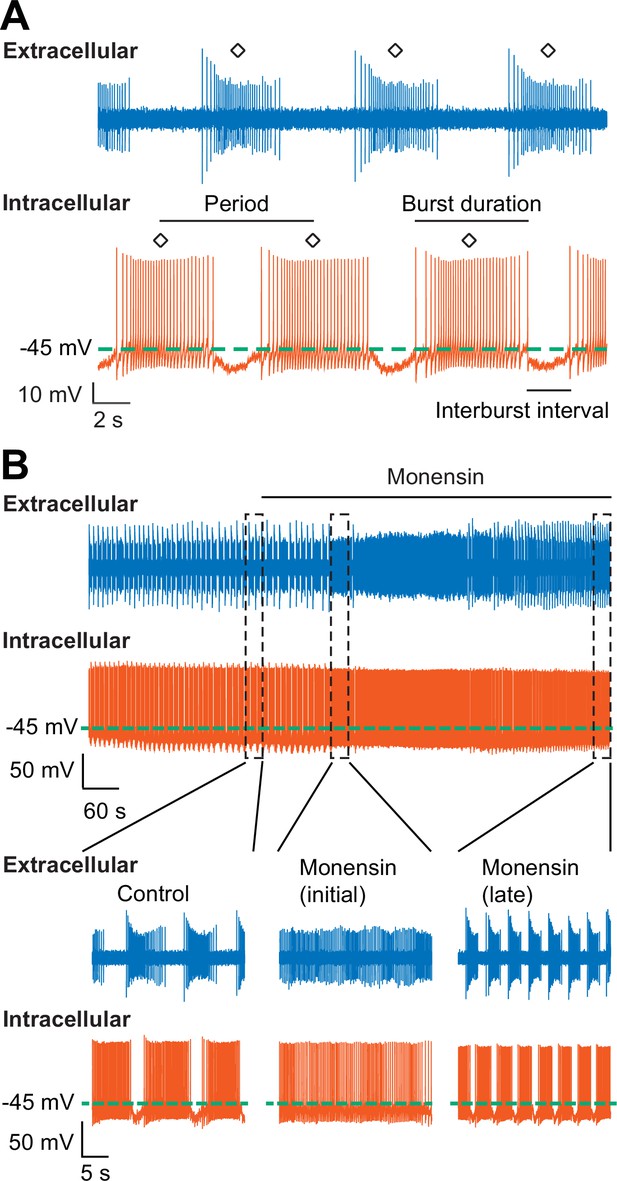
Stimulation of the Na+-K+ pump with monensin speeds up the bursting activity of oscillator heart interneurons as half-center oscillators.
(A) Extracellular (blue) and intracellular (vermilion) traces from a pair of oscillator heart interneurons functioning as a half-center oscillator. Burst characteristics such as the period, burst duration, and interburst interval can be measured from each neuron’s bursting activity. The period was measured from the middle action potential (diamond symbol) of one burst to the middle action potential of the next burst. (B) Extracellular (blue) and intracellular (vermilion) traces from a pair of oscillator heart interneurons. In control saline, both neurons function as a half-center oscillator by firing alternating bursts of action potentials. Adding 10 µM monensin to the saline resulted in initial tonic firing by both neurons followed by alternating bursts of action potentials with a reduced period.
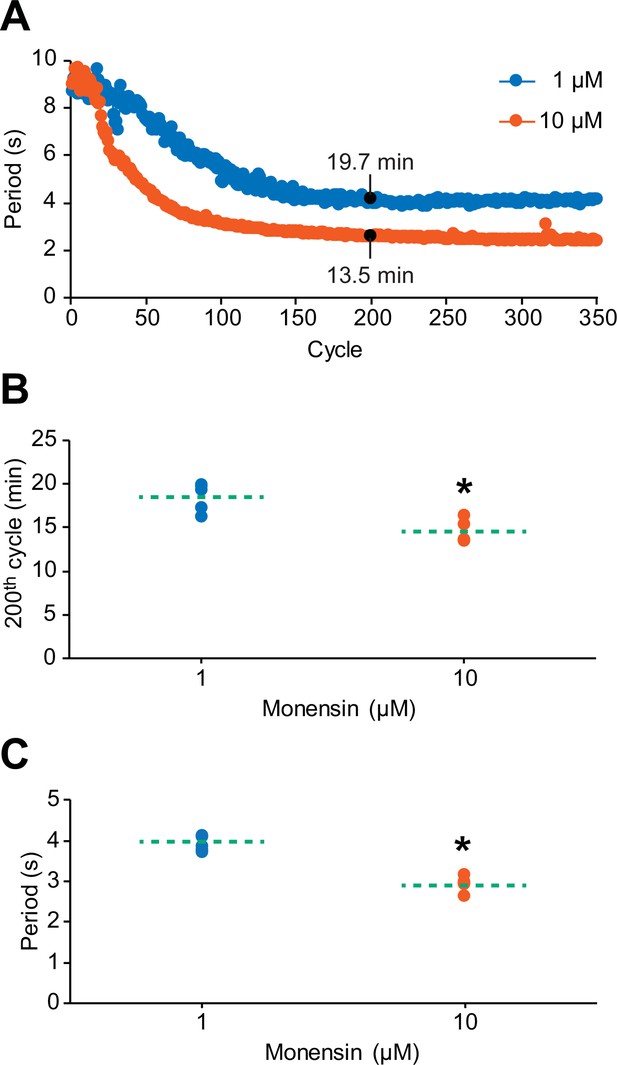
The cycle-to-cycle effects of monensin on the period of oscillator heart interneurons.
(A) Initial application of monensin rapidly shortens the period towards a stable minimum value. The concentration of 10 µM monensin (vermilion line) shortens the period more rapidly than the lower concentration of 1 µM (blue line). The amount of time a period needs to reach its minimum value at the 200th cycle can be measured by summing up all the periods leading up to that 200th cycle. (B) A scatterplot of the amount of time that has passed before the period has reached its value at the 200th cycle in both 1 µM and 10 µM monensin treatments. (C) A scatterplot of the period at the 200th cycle in both 1 µM and 10 µM monensin treatments. The dashed green lines in the scatter plots represent means whereas the asterisk (*) represents significance from control (unpaired t-test, p=0.003).
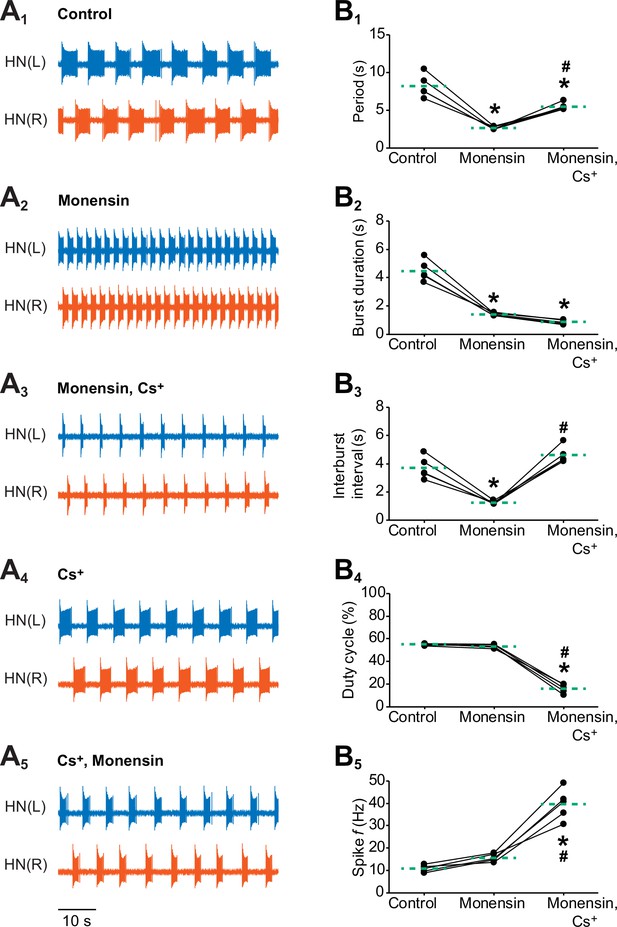
Effects of stimulating the Na+-K+ pump with monensin while blocking the h-current on the burst characteristics of oscillator heart interneurons as half-center oscillators.
Extracellular traces of bursting activity by left (blue) and right (vermilion) oscillator heart interneurons [HN(L) and HN(R) neurons] initially bathed in (A1) control (normal) saline and subsequently treated with (A2) saline that contained 10 µM monensin followed by another treatment with (A3) saline that contained 10 µM monensin plus 2 mM Cs+. Extracellular traces of another pair of oscillator heart interneurons first treated with (A4) saline that contained 2 mM Cs+ followed by another treatment with (A5) saline that contained 2 mM Cs+ plus 10 µM monensin. Scatterplots of the (B1) period, (B2) burst duration, (B3) interburst interval, (B4) duty cycle, and (B5) intraburst spike frequency that were measured from the extracellular traces of five preparations under (A1-3) three experimental treatments. Monensin decreased significantly the (B1) period, (B2) burst duration, and (B3) interburst interval relative to control. The (B4) duty cycle and (B5) intraburst spike frequency were unchanged. Monensin plus Cs+ increased significantly (B1) the period, (B3) interburst interval, and (B5) intraburst spike frequency relative to control. Because the (B2) burst duration under monensin plus Cs+ remained unchanged relative to monensin alone, the (B4) duty cycle decreased significantly under the monensin plus Cs+ saline relative to either control or monensin saline. The dashed green lines represent means whereas asterisks (*) and hashtags (#) represent significance from control and monensin, respectively (Tukey’s test, p<0.05).
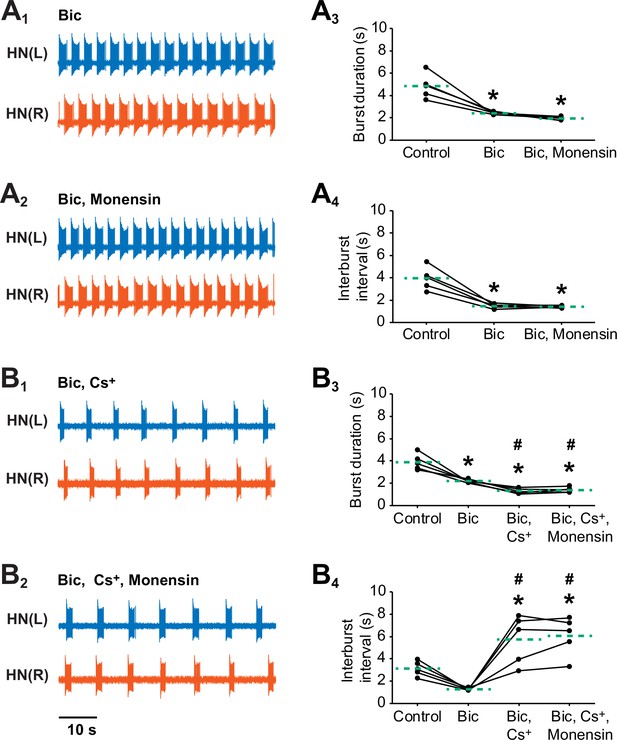
Stimulating the pump with 10 µM monensin in pharmacologically isolated heart interneurons requires h-current to shorten the interburst interval but not to shorten the burst duration.
Extracellular traces from left (blue) and right (vermilion) oscillator heart interneurons [HN(L) and HN(R) neurons] that were pharmacological isolated as bursters by being treated with (A1) saline that contained 500 µM bicuculline (Bic). (A2) The isolated oscillator heart interneurons were then treated with saline that contained 500 µM bicuculline plus 10 µM monensin. Corresponding scatter plots of (A3) burst duration and (A4) interburst interval. Extracellular traces from another pair of isolated oscillator heart interneurons that were treated with (B1) saline that contained 500 µM bicuculline plus 2 mM Cs+ saline followed by followed by another treatment with (B2) saline that contained 500 µM bicuculline, 2 mM Cs+, plus 10 µM monensin saline. Corresponding scatter plots of (B3) burst duration and (B4) interburst interval. The dashed green lines represent means whereas asterisks (*) and hashtags (#) represent significance from control and bicuculline, respectively (Tukey’s test, p<0.05).
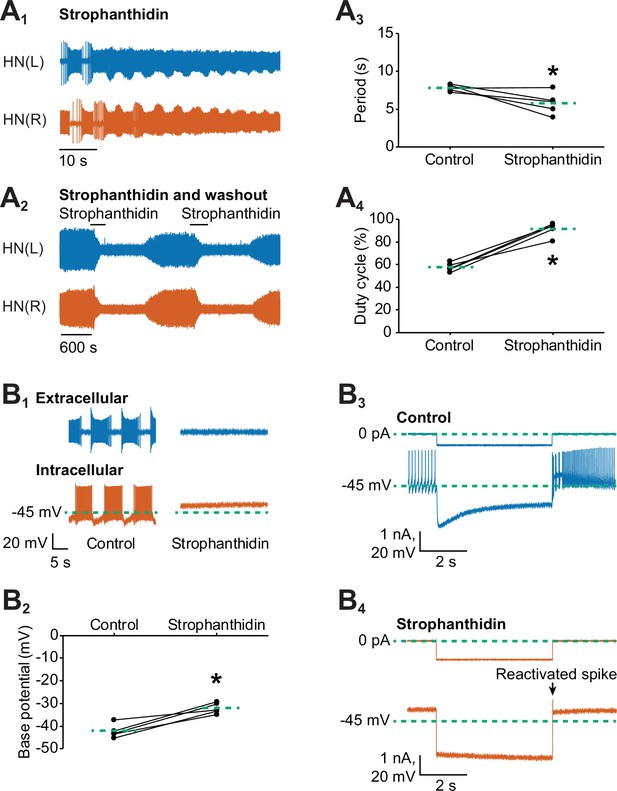
Inhibition of the Na+-K+ pump by applying strophanthidin speeds up the bursting activity of oscillator heart interneurons.
Extracellular traces of bursting activity by left (blue) and right (vermilion) oscillator heart interneurons [HN(L) and HN(R) neurons] functioning as a half-center oscillator in (A1) in saline that contained 100 µM strophanthidin. (A2) Strophanthidin transiently decreased the period but eventually suppressed spiking activity, which was reversible once the neurons were again bathed in normal saline. Corresponding scatterplots of the (A3) period and (A4) duty cycle were measured from the extracellular traces of five preparations under (A1-2) two experimental treatments. (B1) Extracellular and intracellular traces of activity in control saline and strophanthidin saline. (B2) A corresponding scatter plot of the base potential in control saline and strophanthidin saline. (B3) Intracellular traces of current and voltage from an oscillator heart interneuron in normal saline. The neuron was injected with 0.6 nA for 4 s, which hyperpolarized its membrane potential below −60 mV. (B4) In strophanthidin saline, the same neuron was injected with −1 nA for 4 s to hyperpolarize its membrane potential below −60 mV. The dashed green lines in the scatter plots represent means whereas the asterisks (*) represent significance from control (paired t-test, p<0.05 for all tests).
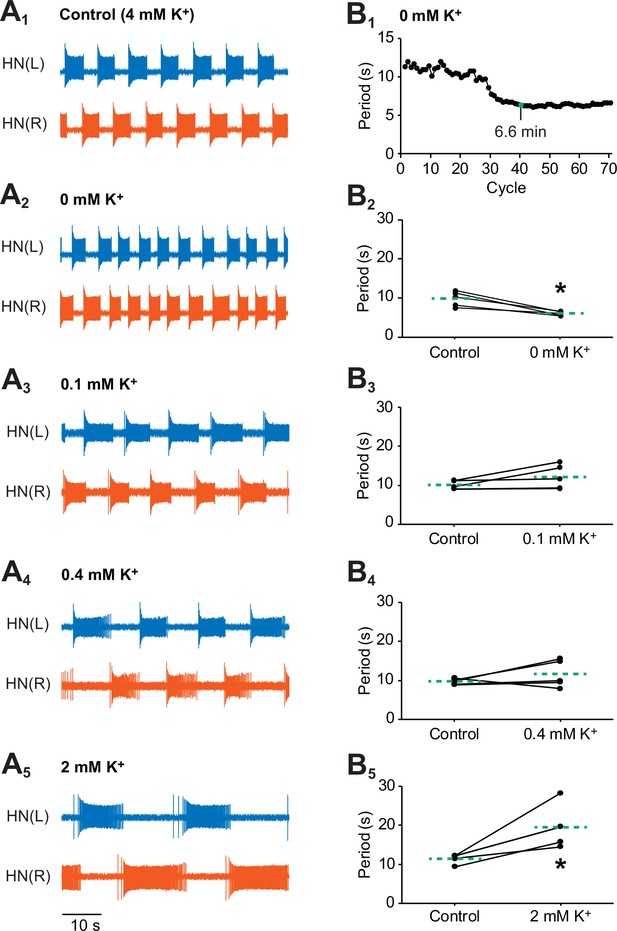
Effects of various concentrations of external K+ on the bursting activity of oscillator heart interneurons.
Extracellular traces of bursting activity by left (blue) and right (vermilion) oscillator heart interneurons [HN(L) and HN(R) neurons] treated with (A1) control (4 mM K+) saline, (A2) 0 mM, (A3) 0.1 mM, (A4) 0.4 mM, and (A4) 2 mM. (B1) A period vs cycle graph of one preparation showing the shortening of the period in K+-free saline over time. Summing up all the periods leading up to the 40th cycle reveals that it takes 6.6 min for the effects of K+-free saline to stabilize. Scatterplots of corresponding periods for (B2) 0 mM, (B3) 0.1 mM, (B3) 0.4 mM, and (B5) 2mM K+ were measured from the extracellular traces of the four groups treated with (A2-5) lower concentrations of external K+. The dashed green lines represent means whereas the asterisks (*) represent significance from control (paired t-test, p<0.05 for all tests).
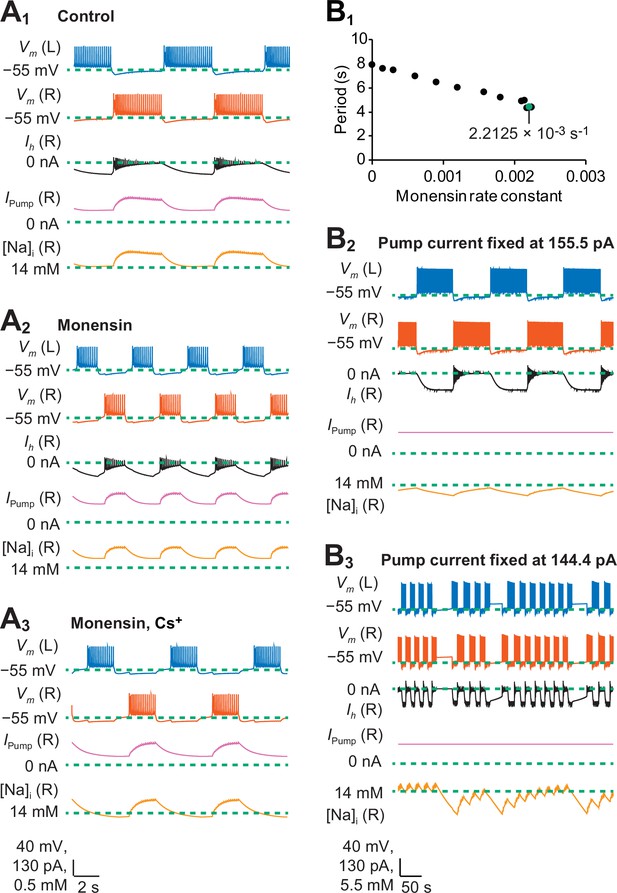
A biophysical model of oscillator heart interneurons that mimics three experimental treatments with monensin.
(A1) Sample traces of simulated activity by oscillator heart interneurons functioning as a half center oscillator in normal saline, which were observed when parameters = 4.9 nS and = 0 s−1. (A2) Simulated activity of oscillator heart interneurons with a Na+/K+ pump stimulated by monensin (monensin saline), observed when = 4.9 nS and = 2.2 × 10−3 s−1. (A3) Simulated activity of a half-center oscillator with blocked h-current and pump stimulated by monensin (monensin plus Cs+ saline), observed when = 0.1 nS and = 1.9 × 10−4 s−1. Sample traces representing membrane potentials (Vm) of both left (L, blue) and right (R, vermilion) oscillator heart interneurons as well as h-current (Ih, yellow), pump current (Ipump, reddish purple), and intracellular Na+ concentration [Na]i (black) belonging to the right oscillator heart interneuron. (B1) A scatterplot depicting incremental shortening of the period as the monensin rate constant increases towards 2.2 × 10−3 s−1 in a model of a half-center oscillator with the h-current present. Spiking activity was suppressed at rate constant values larger than 2.2 × 10−3 s−1. (B2) In a simulation of a half-center oscillator, the average pump current over the entire burst cycle was fixed at 155.5 pA, which resulted in a longer period, burst duration, and interburst interval. Intracellular Na+ concentration [Na]i appeared to increase and decrease more slowly relative to a (A1) normal half-center oscillator with a dynamic pump current. (B3) Fixing the average pump current over the entire burst cycle to 144.4 pA (a value lower than 155.5 pA) produced irregular bouts of bursting.
Tables
Modeled burst characteristics (period, burst duration, interburst interval, and duty cycle) were compared to experimental ranges under three parameter regimes that mimicked our experimental treatments. The control treatment was a normal half-center oscillator. The monensin treatment was a half-center oscillator with a pump stimulated by monensin (M = 2.2 × 10−3 s−1). The monensin plus Cs+ treatment was a half-center oscillator with blocked h-current and a pump stimulated by monensin (M = 1.9 × 10−4 s−1). Asterisks denote out-of-range values.
| Comparison of modelled burst characteristics to experimental ranges under four parameter regimes. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | Period (s) | Burst duration (s) | Interburst interval (s) | Duty cycle (%) | ||||
| Model | Experimental ranges | Model | Experimental ranges | Model | Experimental ranges | Model | Experimental ranges | |
| Control | 8.0 | 4.3-12.3 | 3.7 | 2.6-7.3 | 4.2 | 1.5-6.0 | 47.0 | 46.8-64.7 |
| Monensin | 4.4* | 2.5-4.1 | 1.6 | 1.3-2.3 | 2.8* | 1.1-1.8 | 35.5* | 44.6-59.8 |
| Monensin, Cs+ | 6.6 | 5.9-8.6 | 2.0 | 1.7-4.1 | 4.5 | 3.3-5.5 | 30.9 | 26.6-48.0 |





