TRIM28 regulates the nuclear accumulation and toxicity of both alpha-synuclein and tau
Figures
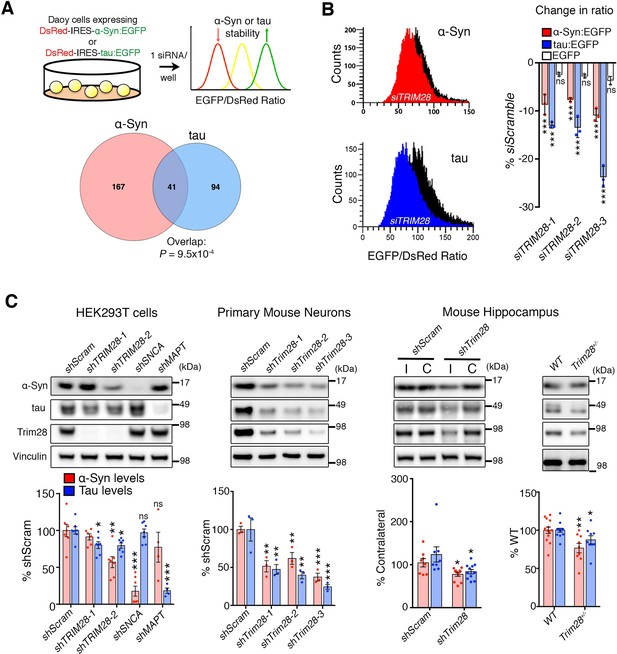
TRIM28 regulates levels of α-Syn and tau.
(A) Schematic of screen approach (see also Figure 1—figure supplement 1). The ratio of either α-Syn:EGFP/DsRed or tau:EGFP/DsRed was measured using an arrayed siRNA library covering 2607 siRNAs in biological triplicates. Venn diagram shows significant overlapping hits from both screens. (B) Representative traces for α-Syn:EGFP/DsRed and tau:EGFP/DsRed ratios: the black curve represents a control condition (siScramble), while the red and blue curves represent siTRIM28 in the α-Syn and tau cell lines, respectively. Quantified ratiometric scores for three independent siRNAs against TRIM28 (siTRIM28-1,-2 and -3) is presented on the right. (C) Effects of different shRNAs targeting TRIM28 on endogenous α-Syn and tau in HEK293T cells (left panel, shTRIM28-1 and shTRIM28-2), primary mouse neurons (middle panel, shTrim28-1,1,-2, and -3) and in adult mouse hippocampus (right panel, where ‘I’ denotes the injection side [ipsilateral] and ‘C’ denotes the uninjected side [contralateral, an internal control]). Rightmost panel depicts the effect of the loss of one allele of Trim28 in the mouse brain. Data are presented as mean ± s.e.m. for each group. In A, p=9.5 × 10–4, hypergeometric test; in B, n = 3 per cell line, *** denotes p<0.001, One-Way ANOVA followed by Dunnet’s multiple comparison test; in c, n = 4–13 condition, *, **, *** and ns denote p<0.05, p<0.01, p<0.001 and p>0.05, respectively, One-way ANOVA followed by Holm-Sidak post-hoc test in two leftmost panels and Student’s t-test in two rightmost panels. Full statistical analyses for all figures are presented as Supplementary file 1.
-
Figure 1—source data 1
List of modifier genes identified in convergent screens.
Genes screened as well as the individual Z-Scores for the average of triplicate readings are presented for each siRNA/cell line in adjacent columns. Top modifiers retrieved from the primary screens meeting criteria enumerated in Online Methods. Pink column depicts screen done in DsRed-IRES-α-Syn:EGFP cells and a blue column depicts screen done in DsRed-IRES-tau:EGFP cells. Overlapping hits between both screens are indicated in grey. Validation Results from Figure 1—figure supplement 2 summarized in the last columns with legend for color-coded significant changes presented at the bottom.
- https://doi.org/10.7554/eLife.19809.004
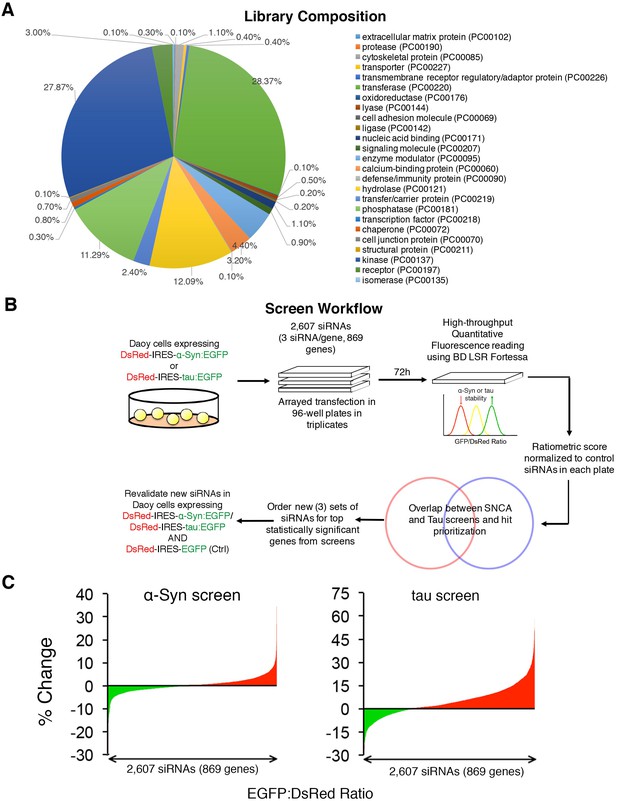
Convergent screens targeting the steady state levels of α-Syn and tau identify common modifiers.
(A) siRNA library composition as determined using Panther gene ontology analysis – Protein function (http://www.pantherdb.org/). A full list of the genes tested is presented in Figure 1—source data 1. (B) Screen workflow delineating steps used to narrow down hit lists and find top shared modifiers of α-Syn and tau levels. (C) Raw distribution of all hits in both ratiometric screens (% Change denotes change in EGFP:DsRed ratio compared to scramble siRNAs from each plate).
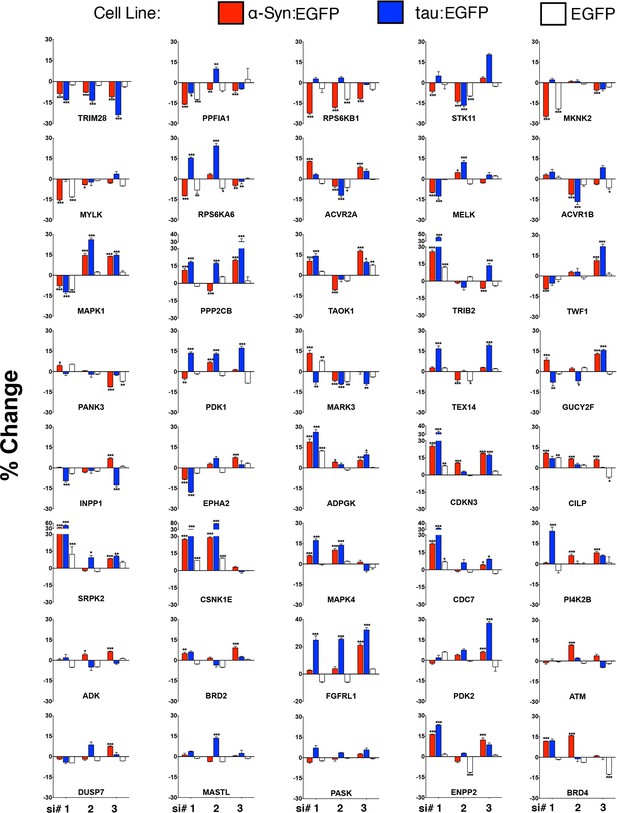
Validation of shared modifiers between α-Syn and tau.
40 shared modifiers from both α-Syn- and tau-based screens were tested using three new siRNAs directed against each target ('si #1, 2, 3'). Changes in EGFP:DsRed levels are presented as a percentage (%) change in relation to Scrambled siRNAs. For each histogram, DsRed-IRES-α-Syn:EGFP, DsRed-IRES-tau:EGFP and DsRed-IRES-EGFP cell lines are represented as red, blue and white bars, respectively. % Change denotes change in EGFP:DsRed ratio compared to scramble siRNAs. Data are presented as mean ± s.e.m. Three independent biological replicates are presented for three independent siRNAs (siRNA codes are listed in Supplementary file 1 for statistical comparison). *, ** and *** denote p<0.05, p<0.01 and p<0.001, respectively, One-Way ANOVA followed by Dunnett’s multiple comparison test. Bars without asterisks denote non-significant differences.
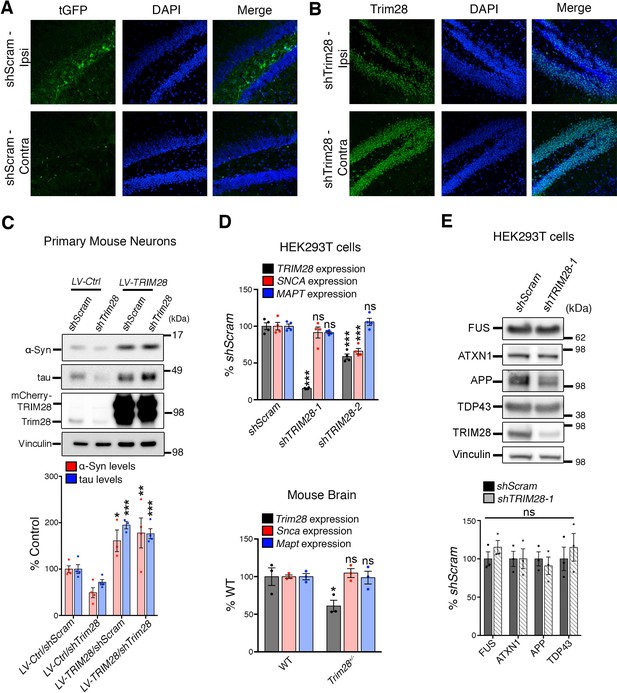
Validation of Trim28 knockdown and lack of effect on other neurodegenerative disease-causing genes.
( A) Confirmation of viral expression was performed in the hippocampus of 12-week-old mice, 14 days after stereotaxic delivery of lentivirus. Cryosections were stained for turbo GFP (tGFP) and DAPI. Note top panels denote side injected with the virus (‘Ipsi’, ipsilateral) and bottom panels, the uninjected side (‘Contra’, contralateral). (B) Confirmation of Trim28 knockdown was performed as in A, but using an antibody directed against Trim28. (C) The effect of Trim28 knockdown on α-Syn and tau in primary neurons can be rescued by overexpressing an shRNA-resistant cDNA of TRIM28, (D) TRIM28 knockdown in human cells does not alter MAPT transcript levels and only mildly affects SNCA expression as assayed by qRT-PCR. Trim28 heterozygous (Trim28+/-) mice have normal levels of Snca and Mapt in their brain. (E) TRIM28 knockdown does not alter protein levels of several other neurodegenerative disease-causing genes. HEK293T cells were infected with virus targeting TRIM28 (shTRIM28-1) or control (non-silencing, shScram) and levels of neurodegenerative disease-causing genes were measured by Western blot and quantified below. Data are presented as mean + s.e.m. In C, n = 3–4 replicates, *, ** and *** denote p<0.05, p<0.01 and p<0.001, respectively, One-Way ANOVA followed Holm-Sidak post-hoc test; in D, n = 3 replicates per group, top panel: *** and ns denote p<0.001 and p>0.05, respectively, One-Way ANOVA followed by Holm-Sidak post-hoc test, bottom panel: * and ns denote p<0.05 and p>0.05, respectively, Student’s t-test; in e, n = 3 replicates per group, ns denotes p>0.05, Student’s t-test.
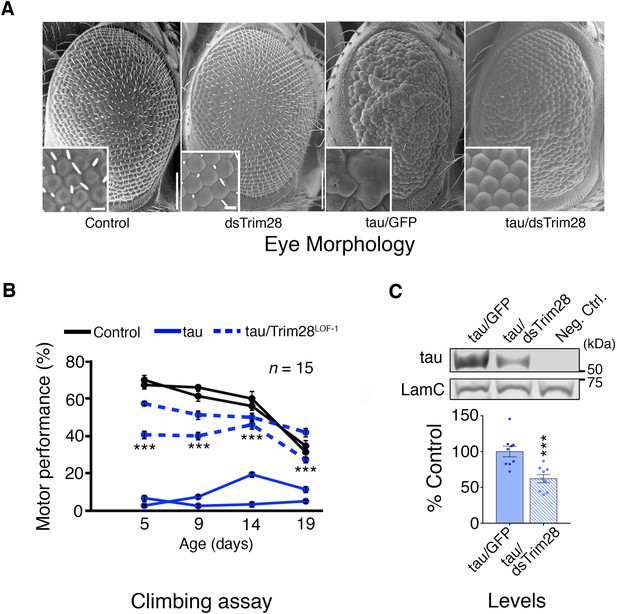
Loss of TRIM28 mitigates tau-mediated neurodegenerative phenotypes in Drosophila.
(A) tau overexpression in the Drosophila eye produces a rough eye phenotype (third panel) compared to negative controls (first panel). Decreasing the levels of Trim28 (dsTrim28) ameliorates this defect (fourth panel). Decreasing Trim28 alone does not result in any overt degenerative phenotypes (second panel). (B) Expression of tau in the Drosophila nervous system (solid blue lines) leads to motor performance deficits that can be quantified in a climbing assay when compared with control flies (black lines). This phenotype is mitigated by partial TRIM28 loss of function (hatched blue lines). Two independent cohorts (15 animals per replicate) are shown per genotype. (C) Western blot images and quantification showing decreased tau levels in the adult Drosophila retina, upon Trim28 knockdown. This reduction of tau protein levels is concordant with the suppression of tau phenotypes shown in A and B. Data are presented as mean ± s.e.m. for each group. In B, n = 15 flies per replicate and 2 replicas per genotype *** denotes p<0.001, Two-Way ANOVA followed by Tukey-Kramer post-hoc test; in C, n = 6 replicates per group, *** denotes p<0.001, Student’s t-test. Scale bars in A: 100 µm (inset 10 µm).
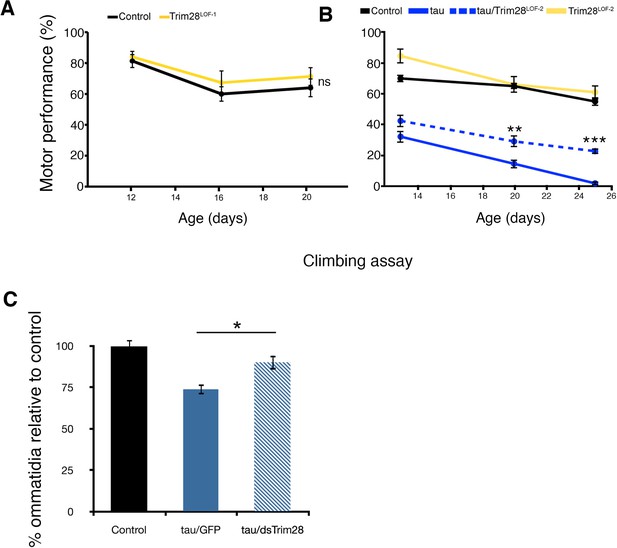
Reduced function of TRIM28 alone does not produce abnormal behavioral phenotypes in Drosophila but rescues tau-mediated degeneration.
(A) expression of a Trim28 loss of function allele in the CNS (Trim28LOF-1, yellow line) does not induce motor performance deficits when compared to control flies (black line). (B) tau-mediated motor phenotypes (solid blue lines) are suppressed by a second loss of function TRIM28 allele (Trim28LOF-2, hatched blue lines). (C) Quantification of ommatidial phenotypes from Figure 2A. Data are presented as mean ± s.e.m. In A, n = 15 flies per group, **, *** and ns denote p<0.01, p<0.001 and p>0.05, respectively, Two-Way ANOVA followed by Tukey-Kramer post-hoc test.
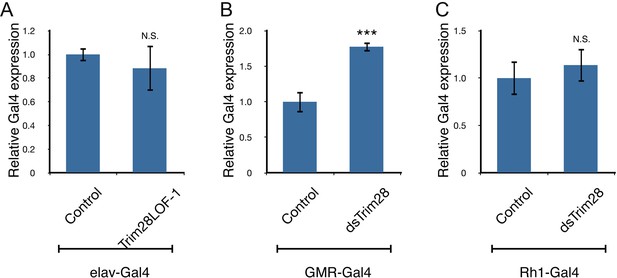
TRIM28 loss does not inhibit Gal4 driver expression.
Levels of Gal4 expression were assayed using qPCR from the three different driver lines: elav-Gal4 (A), GMR-Gal4 (B) and Rh1-Gal4 (C). No significant decreases in expression were observed in the driver expression that could account for the suppression of phenotypes observed in Figure 2 *** and ns denote p<0.001 and p>0.05, respectively, Student’s t-test.
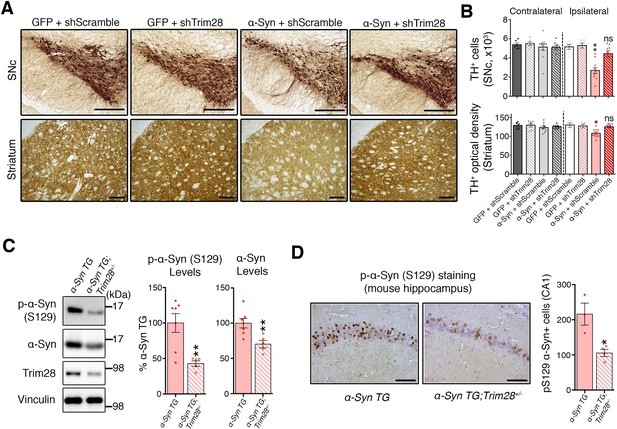
TRIM28 knockdown suppresses α-Syn-mediated neurodegenerative phenotypes in vivo.
(A) Representative photomicrographs of midbrain sections stained for tyrosine hydroxylase (TH) at the level of the Substantia Nigra pars compacta (SNc, top panels) or in the striatum (bottom panels) on the ipsilateral side to the virus injection. (B) Stereological quantification of TH+ cells (top) in the SNc and quantification of optical density of TH+ fibers (bottom) is presented on the right. (C) Western blot analysis of 3.5 month old α-Syn transgenic (TG) mice carrying two (no mark, Trim28+/+) or one (Trim28+/-) copies of Trim28. (D) phosphorylation of α-Syn at serine 129 (pS129) staining at the level of the CA1 in these mice and quantification of positive cell numbers is presented on the right. Data are presented as mean + s.e.m. for each group. In B, n = 5–11 per group, * and ** denote p<0.05 and p<0.01, respectively, One-Way ANOVA followed by Holm-Sidak post-hoc test; in B–D, n = 3–7 mice per group, * and ** denote p<0.05 and p<0.01, respectively, Student’s t-test. Scale bars in A: 400 µm (top panels) 150 µm (bottom panels), C: 50 µm.
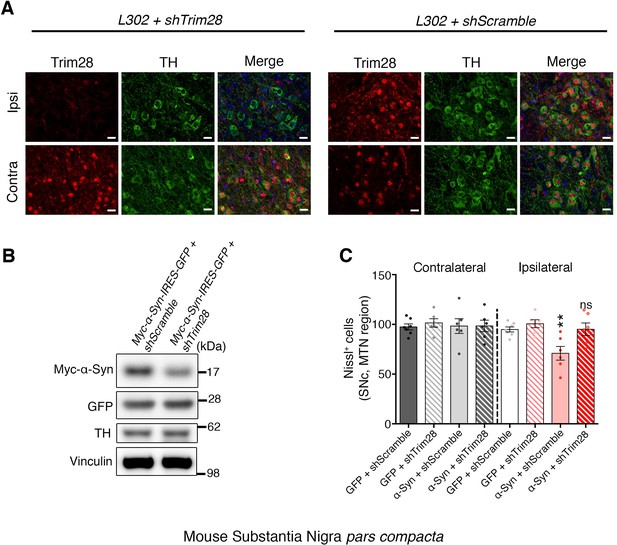
TRIM28 is expressed in the mouse SNc, and can be effectively knocked down in vivo.
(A) On the left, representative immunofluorescence photomicrographs depicting uniform expression of Trim28 (Red) in the Substantia Nigra pars compacta (SNc, stained by TH, Green). Knockdown of Trim28 was confirmed on the side of the injection (Ipsi, top panel) compared to the non-injected side (Contra, bottom panel). (B) Representative western blot of tissue punches from the SNc two weeks following stereotaxic injection showing that Trim28 knockdown downregulates exogenous α-Syn (Myc tagged) but has no effect on the transcription of the viral vector (GFP, expressed under the same promoter as Myc-α-Syn separated by IRES). (C) Nissl staining quantification pertaining to Figure 3B. Nissl positive (Nissl+) cells in the medial terminal nucleus (MTN) region of the SNc were quantified to assess SNc viability. Data are presented as mean ± s.e.m. In B and C, n = 4 animals per condition; in D, n = 6–7 animals per condition, ** and ns denote p<0.01 and p>0.05, respectively, One-Way ANOVA followed by Holm-Sidak post-hoc test. Scale bars in B: 20 µm.
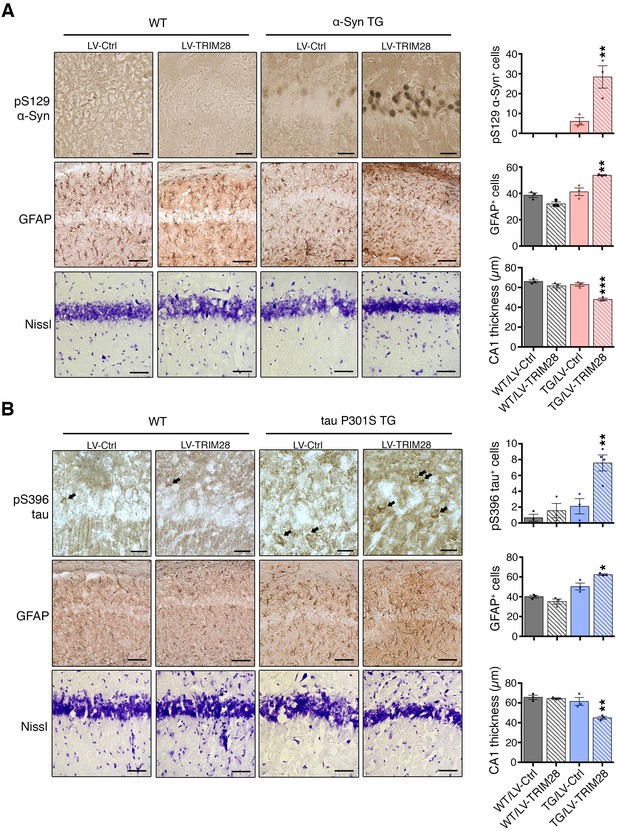
TRIM28 expression worsens histopathology in mouse models of synucleinopathy and tauopathy.
Transgenic mice overexpressing α-Syn (A, mThy-Syn 'Line 61') or P301S tau (B, PS19) were injected at a presymptomatic stage in the hippocampus with lentiviruses expressing TRIM28. Pathological evaluation of phosphorylation of α-Syn at serine 129 (pS129, top panels); Glial Fibrillary Acidic Protein (GFAP, middle panels) as well as Nissl staining (bottom panels) in the CA1 region of α-Syn transgenic (TG) mice (solid bars) and their wild-type littermates (hatched bars) was performed (quantification on the right of each panel sets). Similar pathological evaluation of phosphorylation of tau at serine 396 (pS396 tau); GFAP; as well as Nissl staining in the CA1 region of P301S tau Transgenic mice. Data are represented as mean + s.e.m. In A and B, n = 3 for each genotype and treatment for each experiment, *, ** and *** denote p<0.05, p<0.01 and p<0.001, respectively, One-Way ANOVA followed by Holm-Sidak post-hoc test. Scale bars in A and B: 25 µm (top panels), 100 µm (middle panels) and 50 µm (bottom panels).
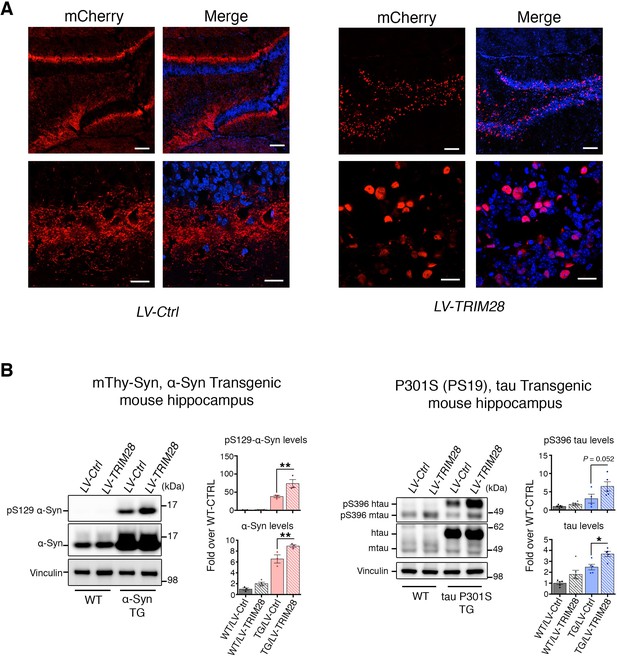
Effective transduction of lentivirus expressing TRIM28 in the hippocampus intensifies biochemical accumulation of α-Syn and tau in prodromal mouse models of synucleinopathy and tauopathy.
(A) Representative photomicrographs of mCherry staining in the hippocampus of a mouse injected with a control lentivirus expressing mCherry (left panel) or TRIM28 fused to mCherry (right panel). (B) Representative western blot of hippocampal punches from α-Syn Transgenic and littermate mice (left panel) and tau P301S transgenic and littermate mice (right panel) injected with lentiviruses overexpressing TRIM28 (or control lentiviruses). Levels of pS129 or total α-Syn (in the case of α-Syn TG mice) or pS396 or total tau (in the case of tau TG mice) are quantified to the right of the blots. Data are represented as mean + s.e.m. In B, n = 3–6 for each genotype and treatment for each experiment, * and ** denote p<0.05 and p<0.01, respectively. Scale bars in A: 100 µm (top panels) and 20 µm (bottom panels).
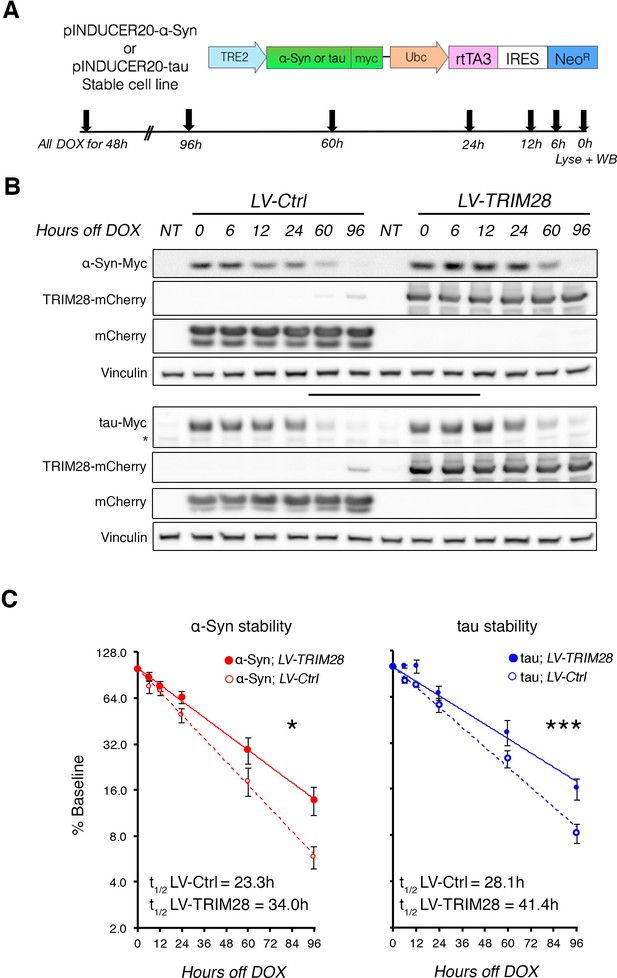
TRIM28 stabilizes α-Syn and tau protein levels.
(A) Schematic of viral vector used to generate stable cell lines (adapted from [Meerbrey et al., 2011]) and assay design. (B) Representative western blots of transgenic α-Syn (top) and tau (bottom) cell lines following different times of doxycycline (DOX) removal; NT denotes non-DOX treated cells; * denotes detection of endogenous tau (not quantified). (C) Quantification of α-Syn and tau stability are presented as a % of baseline and fit to a logarithmic (log2) scale. Dashed lines denote control groups whereas solid lines denote TRIM28 overexpression. Data are presented as mean ± s.e.m. for each group. In C, n = 6 per group, per time point. Protein levels follow a one-phase exponential decay with both curves being significantly different from one another (Comparison of fits, p=0.0111, α-Syn;LV-Ctrl vs. α-Syn;LV-TRIM28; p=0.0003, tau; LV-Ctrl vs. tau;LV-TRIM28).
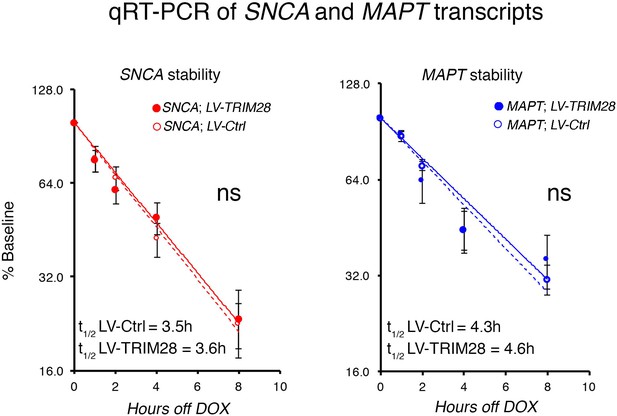
TRIM28 overexpression does not affect SNCA and MAPT RNA stability.
Quantification of SNCA and MAPT levels following indicated doxycycline withdrawal time points reveals expected RNA decay over time, one-phase exponential decay fit to a logarithmic (log2) scale. No significant changes were observed following TRIM28 overexpression (Comparison of fits, ns denotes p>0.05, SNCA;LV-Ctrl vs. SNCA;LV-TRIM28 or MAPT;LV-Ctrl vs. MAPT;LV-TRIM28).
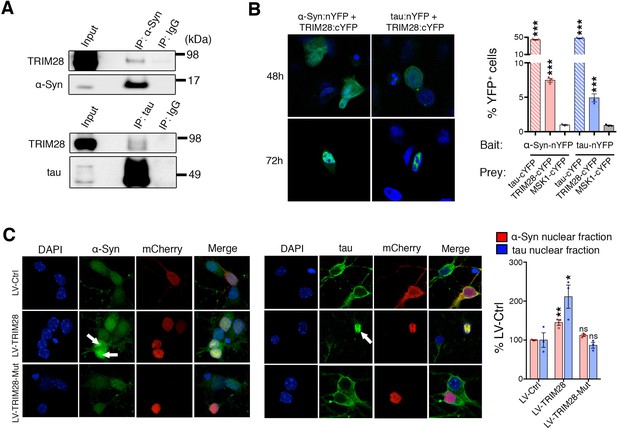
TRIM28 binds to and drives the nuclear localization of α-Syn and tau.
(A) Immunoprecipitation for endogenous α-Syn (top panel) and tau (bottom panel) from HEK293T cells showing the interaction between the former proteins and TRIM28. (B) Bimolecular Fluorescence Complementation studies using stable cell lines expressing either α-Syn or tau fused to an n-Terminal moiety of YFP (α-Syn:nYFP or tau:nYFP, respectively) and TRIM28 (solid), tau (positive control, hatched) or MSK1 (negative control, white) fused to a c-terminal moiety of YFP (TRIM28:cYFP, tau:cYFP and MSK1:cYFP, respectively). Visualization of the epifluorescence generated through protein-protein interaction is depicted in the photomicrographs and quantified by flow cytometry on the right. Note the nuclear accumulation of the interacting proteins 72 hr following transfection. (C) Primary mouse neurons infected with lentiviruses harboring TRIM28, TRIM28 E3 ligase mutant and control were stained for endogenous α-Syn (left panel), and tau in (middle panel) and the relative nuclear fraction of each was quantified (right panel). Arrows point to endogenous α-Syn and tau in the nucleus. Data are represented as mean + s.e.m. In B, n = 3 per group and *** denotes p<0.001, One-Way ANOVA followed by Holm-Sidak post-hoc test; in C, n = 3 per group and *, ** and ns denote p<0.05, p<0.01 and p>0.05, respectively, One-Way ANOVA followed by Holm-Sidak post-hoc test.
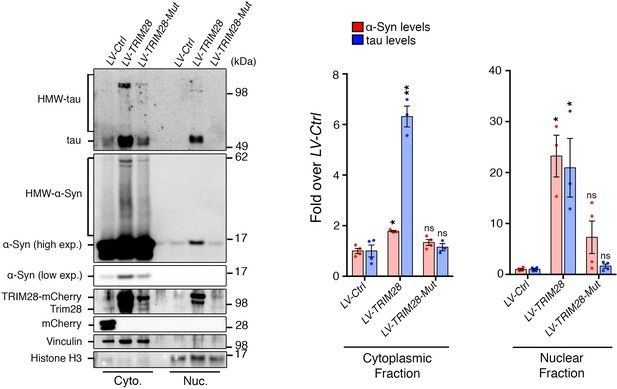
TRIM28 promotes the nuclear localization and accumulation of α-Syn and tau.
Primary mouse neurons infected with lentiviruses harboring TRIM28, TRIM28-Mut or a control mCherry fluorophore were biochemically fractionated into cytoplasmic (‘Cyto.’) and nuclear (‘Nuc.’) components. Relative α-Syn and tau were measured both in the cytoplasm and nucleus in each condition. Notably, the TRIM28-E3 mutant construct consistently exhibited diminished levels. Data are presented as mean + s.e.m. n = 3–4 replicates per condition. *, ** and ns denote p<0.05, p<0.01 and p>0.05, respectively, One-Way ANOVA followed by Holm-Sidak post-hoc test.
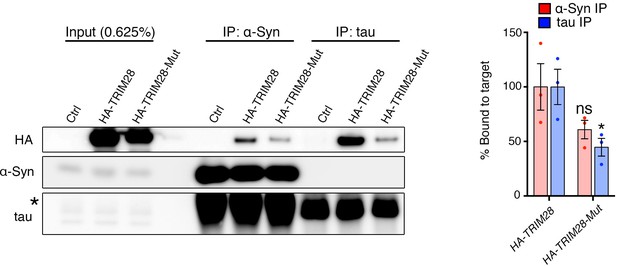
TRIM28 catalytic mutant retains some binding capacity to α-Syn and tau.
(A) α-Syn and tau were immunoprecipitated from HEK293T cells transfected with HA-tagged wild-type TRIM28 or its E3 ligase mutant (C65A/C68A) counterpart. * denotes IgG heavy chain immunoreactivity. (B) Quantification of the relative amount of HA-TRIM28 (or its mutant) bound to α-Syn and tau reveals a decrease in binding affinity in the catalytic mutant. * and ns denote p<0.05 and p>0.05, respectively, Student’s t-test.
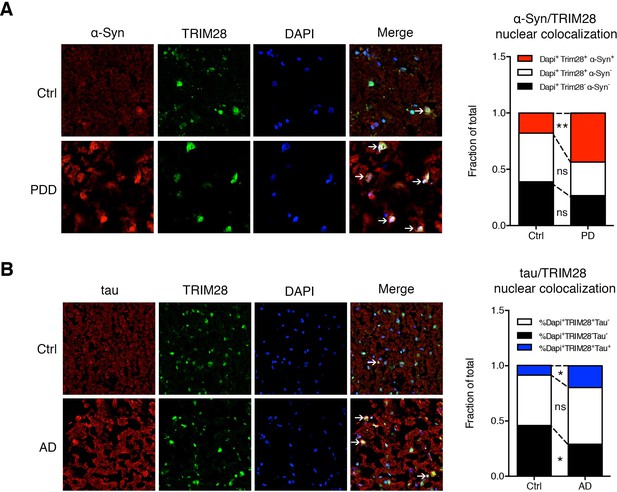
α-Syn and tau colocalize with TRIM28 in the nucleus in cases of synucleinopathy and tauopathy.
(A) Representative photomicrographs of the medial frontal gyrus of cases of PDD and age-matched controls stained for α-Syn, TRIM28 and DAPI. The relative proportion of DAPI positive cells were quantified and are presented as the fraction of total nuclei counted. (B) Representative photomicrographs as in A but for cases of AD and respective age-matched controls, quantification on the right. Data are represented as a fraction of total. In A, n = 4 per post-mortem group, ** and ns denote p<0.01 and p>0.05, respectively, Student’s t-test; in B, n = 5 per post-mortem group, * and ns denote p<0.05 and p>0.05, respectively, Student’s t-test.
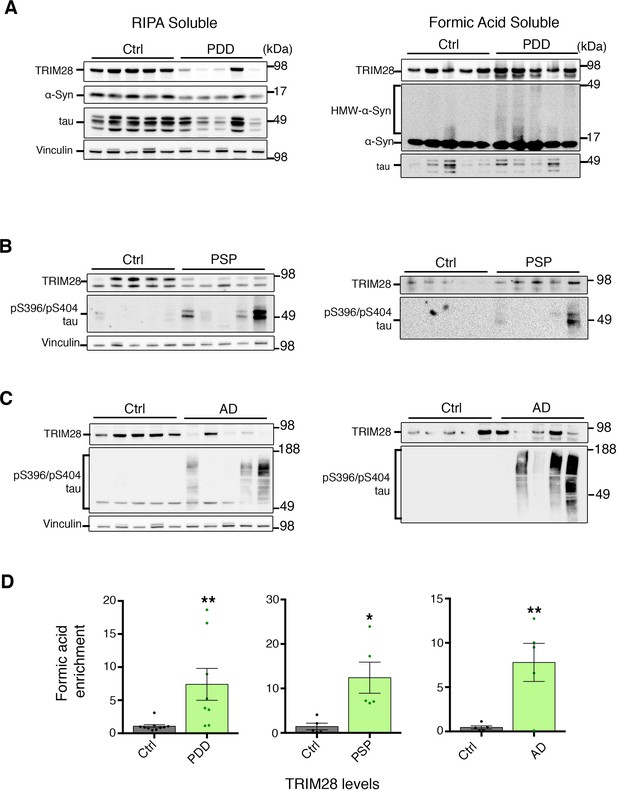
TRIM28 levels are deregulated in human cases of synucleinopathy and tauopathy.
(A) Medial frontal gyrus (MFG) samples from post-mortem Parkinson’s disease with dementia (PDD) or age-matched control cases sequentially lysed in RIPA buffer (left panel) followed by Formic Acid (right panel). (B) Pons samples from Progressive supranuclear palsy (PSP) and control cases prepared as in A. (C) MFG samples from Alzheimer’s disease (AD) and Control cases prepared as in A. (D) Quantification of the relative amount of insoluble – FA enriched – TRIM28 relative to RIPA-soluble TRIM28. Left, middle and right panels denote quantification of PDD, AD and PSP cases, respectively. Data are presented as mean ± s.e.m. In D, n = 5–10 cases per group, * and ** denote p<0.05 and p<0.01, respectively, Student’s t-test with Welch’s correction.
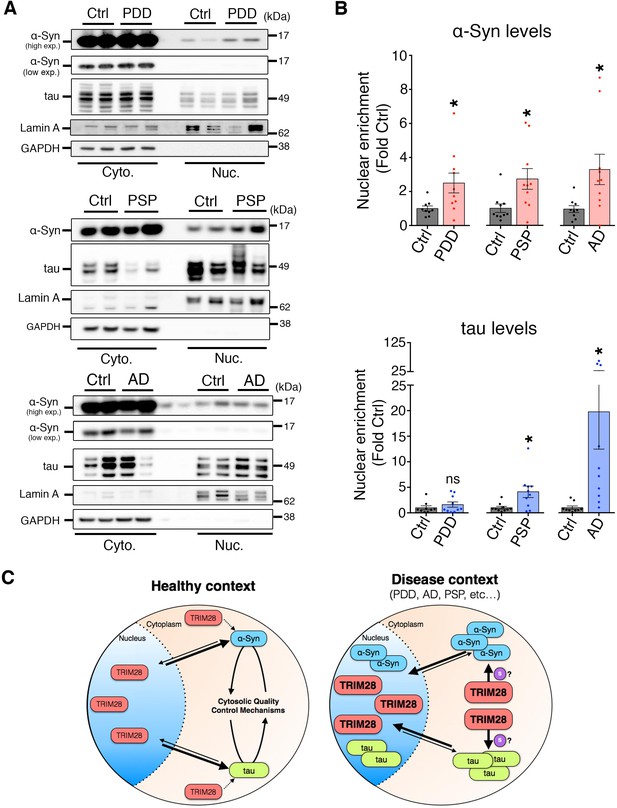
α-Syn and tau accumulate in the nucleus in cases of synucleinopathy and tauopathy.
(A) Quantitative assessment of nuclear and cytoplasmic localization of α-Syn and tau in the pons of post-mortem cases of PDD (top panel), PSP (middle panel) and AD (bottom panel) compared to age-matched/tissue-matched controls. (B) Quantification of relative nuclear enrichment of α-Syn (top) and tau (bottom). (C) Model: In normal conditions, TRIM28 resides primarily in the nucleus, whereas α-Syn and tau perform their respective physiological functions in the cytoplasm and their levels are tightly controlled by cytosolic quality control mechanisms; in disease conditions the levels of α-Syn and tau as well as TRIM28 are abnormally high. TRIM28 mediates the nuclear accumulation, potentially via SUMOylation (denoted by the ‘S’) of α-Syn and tau where they become toxic. Data are presented as mean ± s.e.m. In B, n = 10 cases per condition. * and ns denote p<0.05 and p>0.05, respectively, Student’s t-test with Welch’s correction.
Videos
Representative video.
Partial loss of TRIM28 function in fruit flies overexpressing tau rescues motor behavior in the climbing assay. Video recording shows the three groups of animals tested in Figure 2B. The number of fruit flies climbing up 9 cm (dotted line) in 15 s was recorded.
Additional files
-
Supplementary file 1
Summary of all statistics used throughout the manuscript.
Statistical summary of each figure, including comparisons between each group. Figure in question is in column A. Assay and quantitative measurement from that figure are in columns B and C. Statistical test employed and sample size (n) are in D and E. Statistical comparison is in F. F values, t values (when applicable) and degrees of freedom are present in column G. Calculated p value is presented in column H.
- https://doi.org/10.7554/eLife.19809.024
-
Supplementary file 2
Summary of reagents used throughout the manuscript.
First tab shows antibodies used including concentration and application. Second tab shows shRNA sequences used. Third tab shows Plasmids used and cloning approach if applicable. Fourth tab shows qRT-PCR primers used.
- https://doi.org/10.7554/eLife.19809.025
-
Supplementary file 3
Summary of post-mortem cases used.
Demographics, pathological assessment, post-mortem delay and tissue studied are presented.
- https://doi.org/10.7554/eLife.19809.026





