Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival
Figures
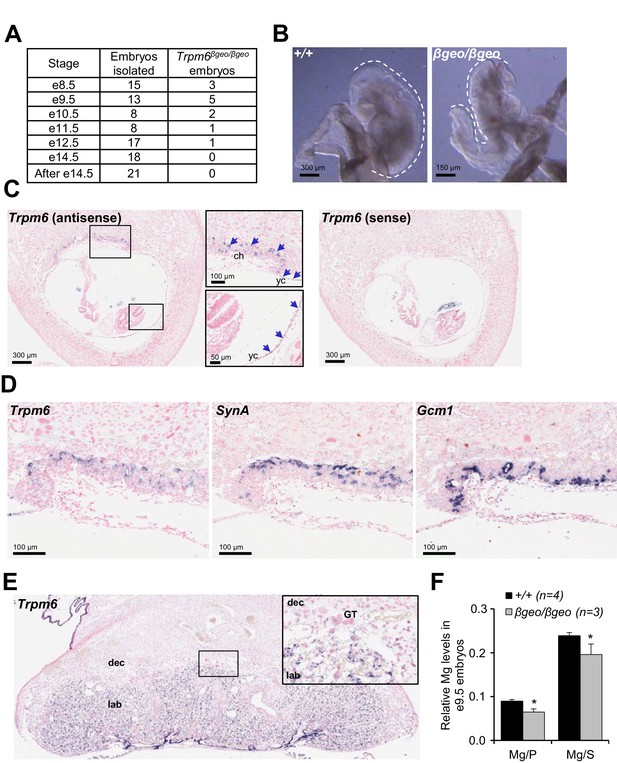
Assessment of Trpm6 function in extraembryonic tissues.
(A) Survival of Trpm6βgeo/βgeo embryos obtained from Trpm6βgeo/+ intercrosses. (B) Representative images of e9.5 Trpm6+/+ (+/+, n = 13) and Trpm6βgeo/βgeo (βgeo/βgeo, n = 5) embryos from dataset in (A). Dashed lines underline C-shaped versus S-shaped morphology of Trpm6+/+ and Trpm6βgeo/βgeo embryos, respectively. (C) ISH on serial paraffin sections obtained from wildtype n = 5 e8.5 fetus using antisense (left) and sense (right) probes for Trpm6. Boxes indicate the positions of the magnified images of the chorion (ch) and yolk sac (yc). Arrows indicate Trpm6-positive cells in the developing labyrinth (chorion) and the endoderm layer in the visceral yolk sac. (D) ISH on serial paraffin sections of wildtype e8.5 placenta using DIG-labelled probes for Trpm6 (left), SynA (middle) and Gcm1 (right), respectively. Note: Trpm6 expression was restricted to cells positive for SynA, a marker of SynT-I, and absent in cells expressing Gcm1, a marker of SynT-II. Representative images of n = 2 independent tissues are shown. (E) ISH of WT e14.5 placenta with the antisense Trpm6 probe. The box indicates the position of the magnified image. The Trpm6 signal is restricted to the labyrinth (lab) and not detectable in the decidua (dec) and trophoblast giant cells (GT). Representative images of n = 8 independent placentas are shown. (F) Mg2+ levels in e9.5 Trpm6+/+ (n = 4) and Trpm6βgeo/βgeo (n = 3) embryos. Distal segments of the embryos were used for genotyping, and the remaining parts were analysed by ICP-MS. Elementary magnesium (Mg) contents were normalized to phosphorus (P) and sulfur (S) levels represented as mean±SEM. *-p≤0.05 (Student’s t-test).
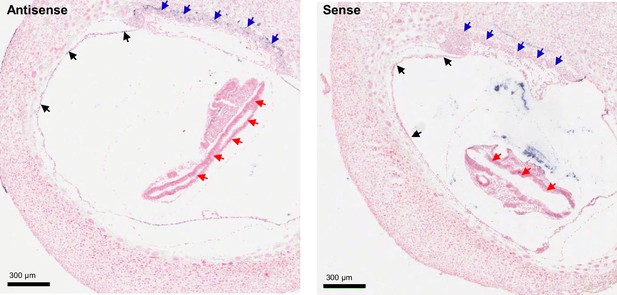
ISH on serial paraffin sections obtained from wildtype e8.5 fetus using antisense (left) and sense (right) probes for Trpm6.
Arrows indicate Trpm6-positive cells in the chorion (blue arrows) and the endoderm layer in the visceral yolk sac (black arrows) stained only by the antisense probe. Note: Trpm6 was not detectable in embryonic tissues including the neural tube (red arrows).
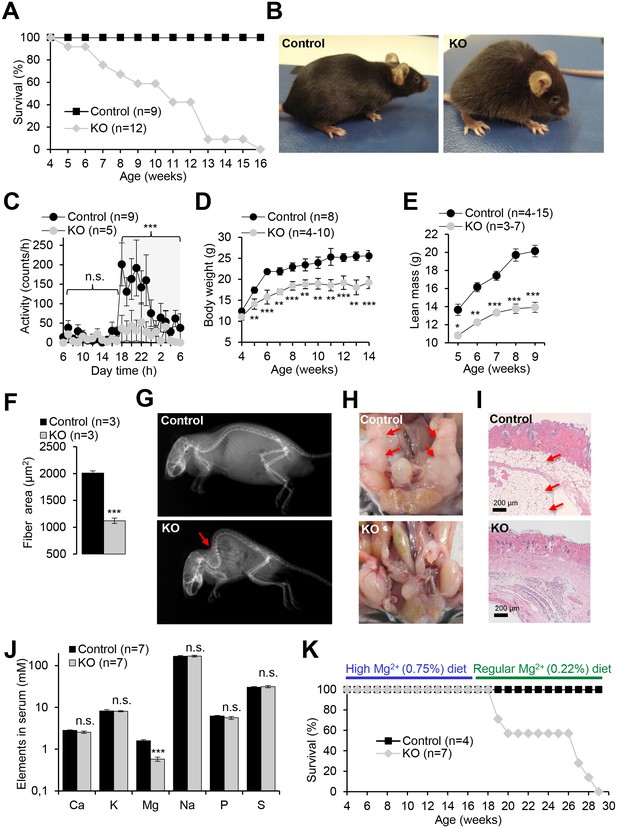
Pathophysiological changes displayed by Trpm6-deficient adult mice.
Unless stated otherwise, 10–12 week-old Trpm6fl/+ (Control) and Trpm6Δ17/Δ17;Sox2-Cre (KO) littermates were studied. (A–E) Mice were examined for survival rate (A), overall physical appearance (B), day/night activity of 8 week-old individuals (C), growth rate (D) and lean mass (E). (F) Fibre size of the gastrocnemius muscle after hematoxylin-eosin staining. (G) X-ray images of mice. The red arrow indicates the characteristic skeletal deformation (kyphosis) observed in Trpm6-deficient mice. (H) Assessment of abdominal fat. Arrows indicate fat deposits observed only in control mice. (I) H and E staining of paraffin skin sections. Arrows indicate a layer of fat cells present only in control mice. Histological analysis was performed with three animals per group resulting in similar observations. (J) The levels of main elements in the serum of 8 week-old mice assessed by ICP-MS. (K) The survival rate of mice maintained on high Mg2+ (0.75%) and regular (0.22%) chows. Data are represented as mean±SEM. ***-p≤0.001; **-p≤0.01; *-p≤0.05; n.s. – not significantly different (Student’s t-test); n – number of mice examined.
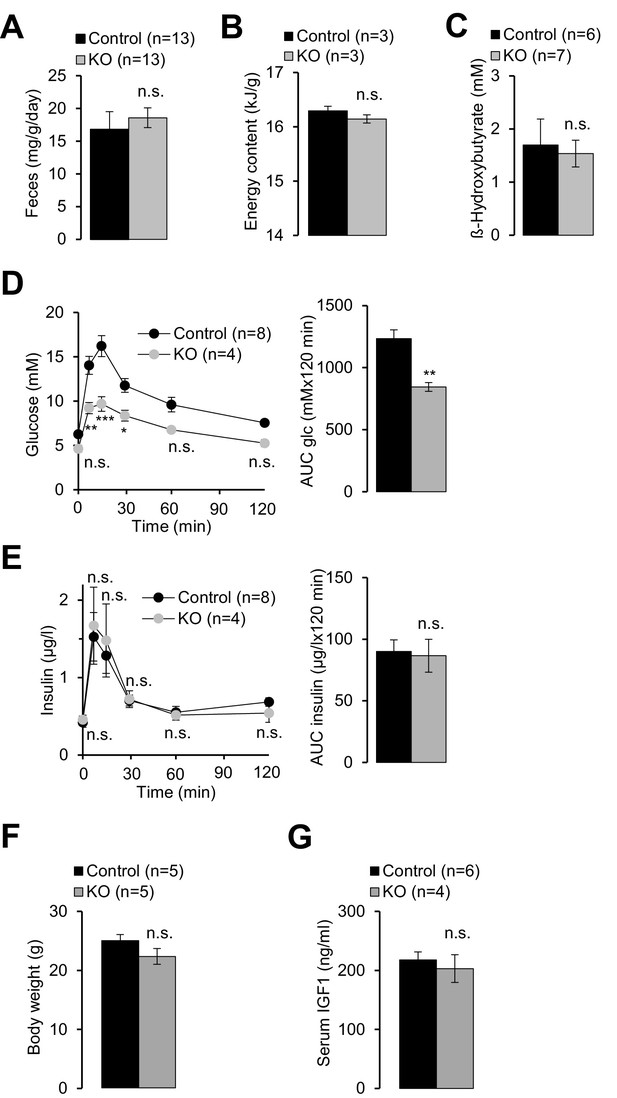
Examination of energy balance in Trpm6-deficient mice.
Eight week-old Trpm6fl/+ (Control) and Trpm6Δ17/Δ17;Sox2-Cre (KO) littermates were evaluated for (A) chow intake as assessed by feces production, (B) energy content of feces studied by bomb calorimetry and () serum levels of β-hydroxybutyrate. Glucose (D) and insulin levels (E) in the serum of mice subjected to a glucose tolerance test. Note: mutant mice had lower peripheral glucose concentrations than controls despite of a similar amount of insulin released, thus reflecting increased insulin sensitivity. Data are represented as mean±SEM. ***-p≤0.001; **-p≤0.01; *-p≤0.05; n.s. – not significantly different (Student’s t-test); n – number of mice examined.
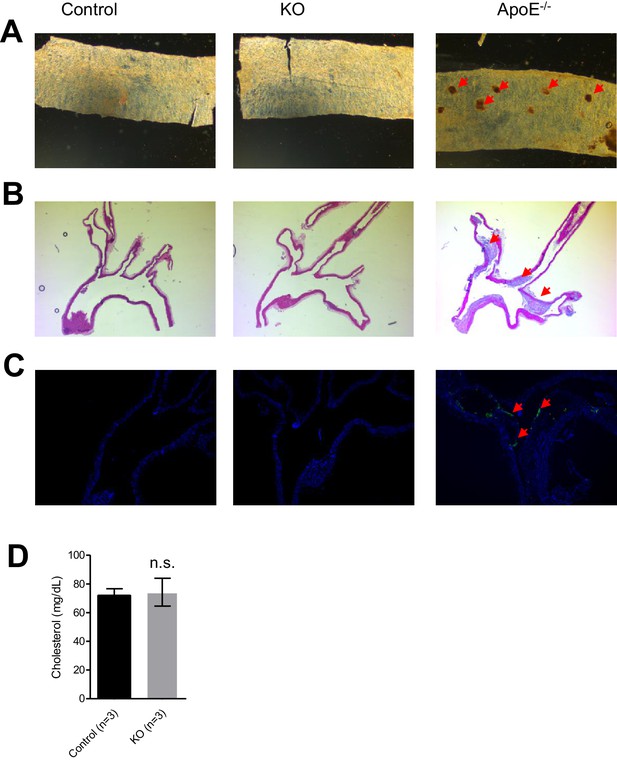
Evaluation of atherosclerosis development in Trpm6-deficient mice.
An assessment of 8 week-old control (Control, n = 3) and Trpm6-deficient (KO, n = 3) with ApoE-/- mice (as a positive control, n = 1) using en-face thoracal aorta preparation and Oil-Red O staining (A) and hematoxylin-eosin staining (B), Mac2-staining (green) (C) of aortic arches. Representative images are shown. Arrows indicate atherosclerosis lesions observed only in ApoE-/- mice. (D) Plasma cholesterol levels (mean±SEM) of control and Trpm6-deficient mice. n.s. – not significantly different (Student’s t-test); n – number of mice examined.
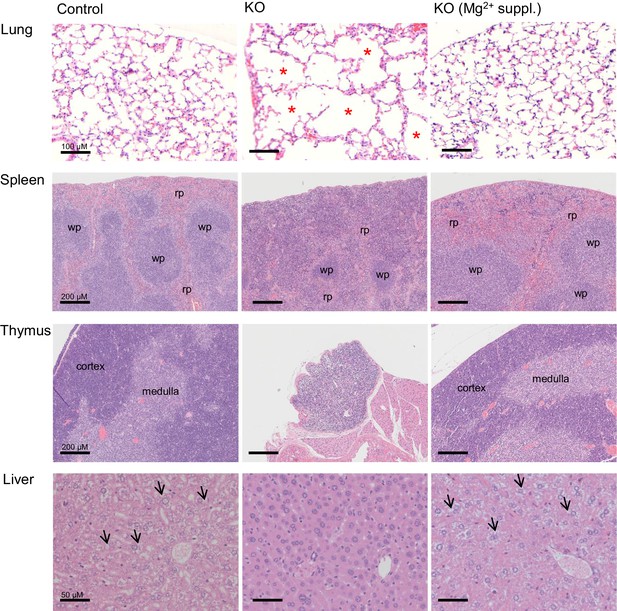
Histology of internal organs of Trpm6-deficient mice.
Hematoxylin-eosin staining of paraffin embedded tissue sections of 12–13 week-old control (Control) and Trpm6-deficient (KO) mice maintained either on regular (0.22% Mg2+) or Mg2+ supplemented (0.75% Mg2+) chows. Trpm6-deficient mice maintained on the regular diet showed marked airspace enlargement (indicated by stars) mimicking lung emphysema, distortion of splenic red pulp (rp)/ white pulp (wp) microarchitecture, thymic atrophy, and reduction of intracellular glycogen in hepatocytes (indicated by arrows). Histological analysis was performed with three animals per group resulting in similar observations.
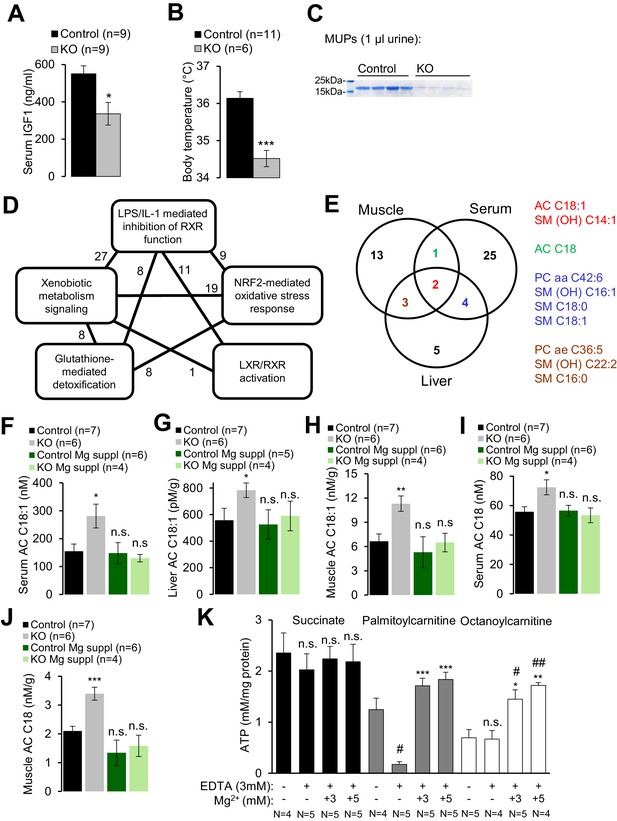
Assessment of metabolic profiles of Trpm6-deficient mice.
(A–C) 8 week-old Trpm6fl/+ (Control) and Trpm6Δ17/Δ17;Sox2-Cre (KO) littermates were evaluated for (A) serum IGF1, (B) body temperature, (C) urinary MUPs content in individual mice. Data are represented as mean±SEM. ***-p≤0.001; *-p≤0.05 (Student’s t-test); n – number of mice examined. (D) IPA analysis of genome-wide hepatic transcriptome profiling of Trpm6-deficient (n = 3) vs control (n = 4) littermates. The diagram shows the top 5 of IPA Canonical Pathways significantly changed in mutant mice (Supplementary file 2). Numbers of the commonly changed transcripts are indicated close to the lines connecting the pathways. (E) Venn diagram for sets of metabolites significantly changed (FDR p≤0.05) in serum, liver and gastrocnemius muscle Trpm6-deficient (n = 6) vs control (n = 8) littermates (Supplementary file 3). Commonly changed metabolites are listed in different colours as outlined in the Venn diagram. (F–J) Levels of AC C18:1 (F–H) and AC C18 (I–J) examined in the serum (F, I), liver (G) and gastrocnemius muscle (H, J) of Trpm6-deficient and control mice. Data are represented as mean±SEM. ***-p≤0.001; **-p≤0.01; *-p≤0.05; n.s. – not significantly different to control group maintained on a regular Mg2+ diet (one-way ANOVA); n – number of mice examined. (K) ATP production by mitochondria isolated from the liver of wildtype C57BL/6 mice with succinate, palmitoylcarnitine or octanoylcarnitine as energy sources. ATP levels were determined after 30 min incubation of untreated (-) or treated (+) mitochondria by EDTA with or without Mg2+. Data are represented as mean±SEM of 4–5 independent isolations (N). ##-p≤0.01; #-p≤0.05 significantly different to the control group; ***-p≤0.001; **-p≤0.01; *-p≤0.05 significantly different to the EDTA treated group (Student’s t-test). n.s. – not significantly different.
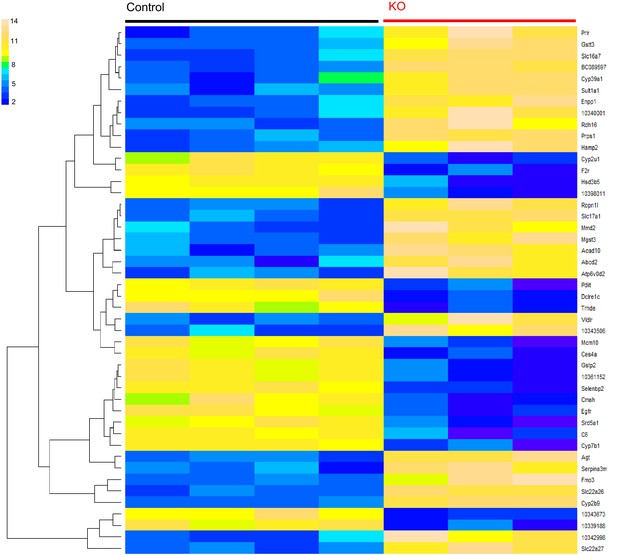
Gene expression profiling of Trpm6-deficient and control mice.
Heatmap diagram of differently expressed genes with FDR p≤0.1 in the liver of 12–13 week-old control (Trpm6fl/+, n = 4) vs Trpm6-deficient (Trpm6Δ17/Δ17;Sox2-Cre, n = 3) male littermates. n – number of mice examined.
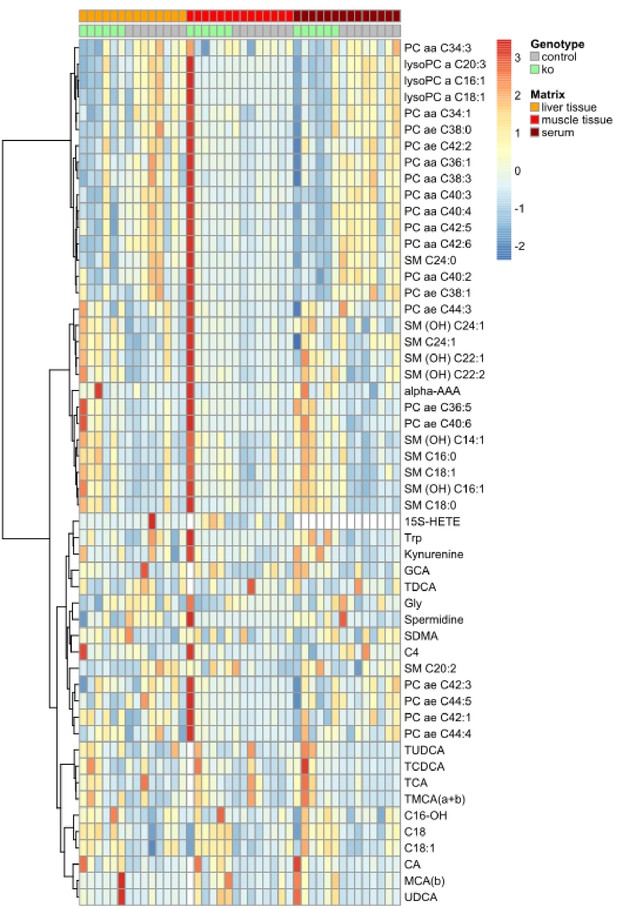
Metabolomic profiling of Trpm6-deficient and control mice.
Profiling of metabolites in the serum, liver and gastrocnemius muscle samples from 8–10 week-old control (Trpm6fl/+, n = 8) and KO (Trpm6Δ17/Δ17;Sox2-Cre, n = 6) male littermates were studied for a panel of 237 metabolites outlined in Supplementary file 3. The heatmap diagram shows concentration levels scaled to zero mean and unit standard deviation of significantly changed metabolites for control and KO genotypes in a colour-coded way. n – number of mice examined.
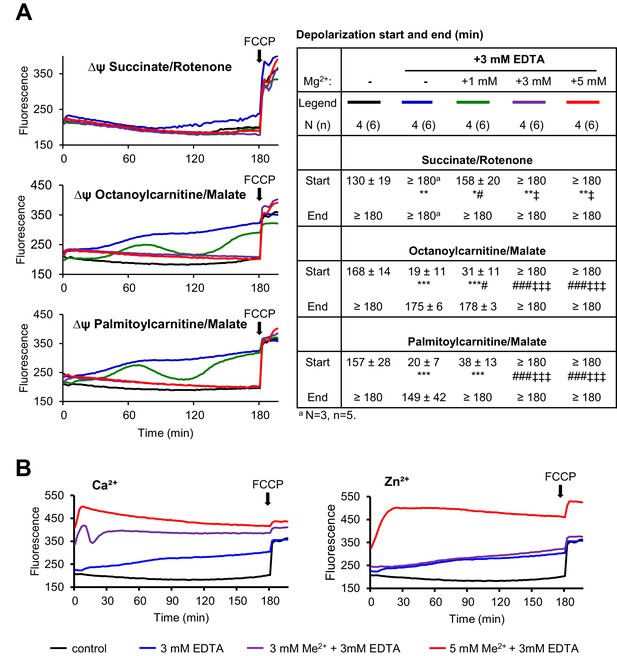
Assessment of the membrane potential (Δψm) in isolated mitochondria.
(A) Mitochondria isolated from the liver of wildtype C57BL/6 mice were incubated (30 min) in the presence of succinate/rotenone, octanoylcarnitine/malate or palmitoylcarnitine/malate as energy source. The left panel shows representative measurements of Δψm using Rh123 probe. As a positive control, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) was added at the end of recording to induce breakdown of Δψm. The right panel shows the calculated start- and endpoints of Δψm elicited by the application of 3 mM EDTA in the absence or presence of 1–5 mM Mg2+ for traces shown in left panel. Data are represented as mean±SD. N – independent mitochondria isolations; n – independent measurements. ***-p≤0.001, **-p≤0.01, *-p≤0.05 significant to the untreated (control) group; ###-p≤0.001, ##-p≤0.01, #-p≤0.05 significant to 3 mM EDTA + 0 mM Mg2+ group; ‡‡‡-p≤0.001, ‡‡-p≤0.01, ‡-p≤0.05 significant to 3 mM EDTA + 1 mM Mg2+ group (Student’s t-test). (B) Representative measurements of ψm in the presence of 3 mM EDTA with/without 3–5 mM Ca2+ or Zn2+ performed analogously to (A). Three independent experiments showed similar results.
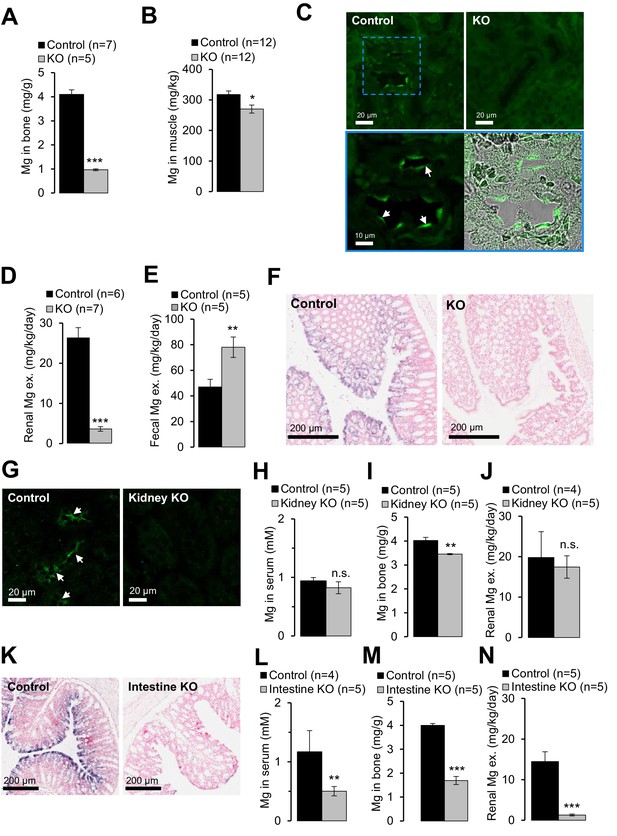
Examining of Mg2+ balance in Trpm6-deficient adult mice.
(A–F) Assessment of 8 week-old Trpm6fl/+ (Control) and Trpm6Δ17/Δ17;Sox2-Cre (KO) littermate males. (A) Mg2+ levels in bones and (B) gastrocnemius muscle assessed by ICP-MS. (C) Immunostaining of kidney cryosections using a TRPM6-specific antibody. Representative images are shown (n = 2 tissues per genotype). The blue square indicates the position of the confocal and differential interference contrast magnified images acquired from control tissue. Arrows indicate labelling of the apical surface of renal tubules. (D) 24 hr urinary and (E) fecal Mg2+ excretion rates. (F) ISH on paraffin sections obtained from the colon of control and Trpm6-deficient mice (n = 2 tissues per genotype). (G–J) Examination of 6 month-old Trpm6fl/+ (Control) and Trpm6Δ17/fl;Ksp-Cre (Kidney KO) littermate males. (G) Immunostaining of TRPM6 in kidney cryosections. Arrows indicate labelling of renal tubules. (H–I) Determination of Mg2+ in serum (H) and bones (I). (J) 24 hr urinary Mg2+ excretion rate. (K–N) Assessment of 6 month-old Trpm6fl/+ (Control) and Trpm6Δ17/fl;Villin1-Cre (Intestine KO) littermate males. (K) ISH on paraffin sections of the colon using a Trpm6-specific probe (n = 2 tissues per genotype). (L, M) Mg2+ levels in the serum (L) and bones (M). (N) 24 hr urinary Mg2+ excretion rate. Data are represented as mean±SEM. ***-p≤0.001; **-p≤0.01; *-p≤0.05; n.s. – not significantly different (Student’s t-test); n – number of mice examined. Histological analysis in (F) and (K) was performed with n = 3 animals per group resulting in similar observations.
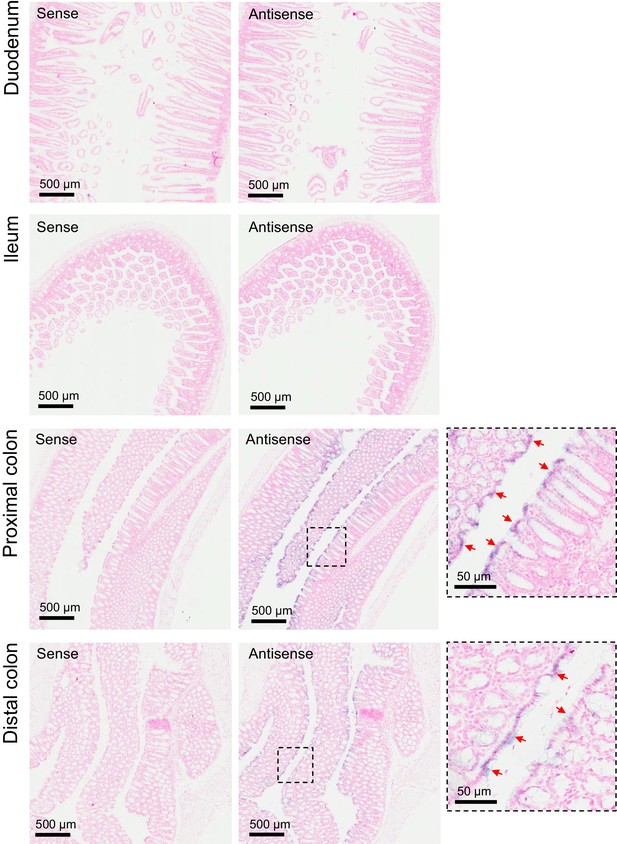
Expression pattern of Trpm6 in the intestine.
ISH on serial paraffin sections obtained from wildtype intestine using sense (left) and antisense (right) DIG-labelled probes for Trpm6. Boxes indicate the positions of the magnified images of the proximal and distal colon. Representative images of n = 3 tissues are shown. Arrows indicate Trpm6-positive cells in the colon. Note: Trpm6 transcripts were not detectable in the duodenum and ileum and specifically present in the absorptive epithelium cells of the proximal and distal colon.
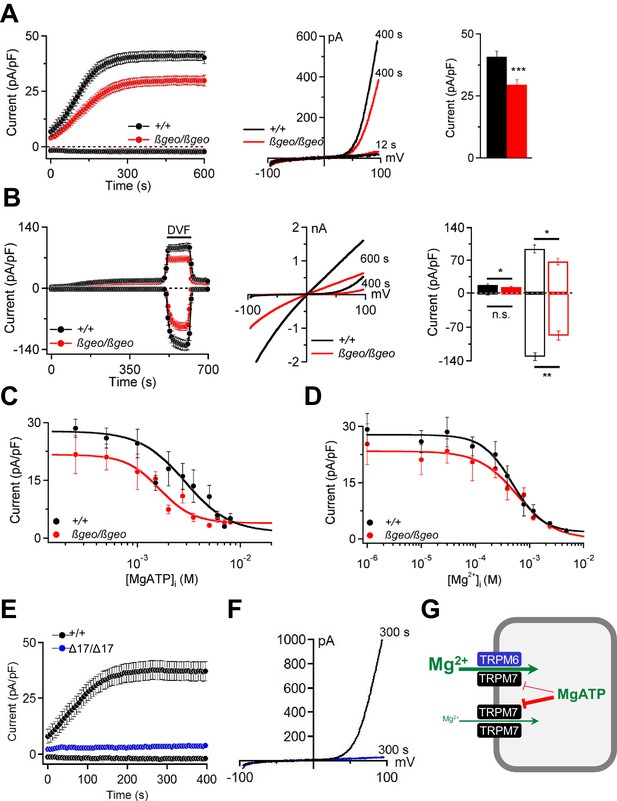
Characterization of TRPM6/M7-like currents in Trpm6- and Trpm7-deficient TS cells.
(A) Left panel: Whole-cell currents measured at −80 mV and +80 mV over time in Trpm6+/+ (n = 22) and Trpm6βgeo/βgeo (n = 22) TS cells. Middle panel: Representative current-voltage relationships obtained at 12 s and 400 s. Right panel: Bar graphs of current amplitudes at +80 mV (400 s). (B) Measurements were performed in control (n = 16) and Trpm6-deficient (n = 14) TS cells analogous to (A) except that the external saline (containing 2 mM Mg2+ and 1 mM Ca2+) was exchanged with divalent-free (DVF) solution (black bar). Right panel shows currents measured before (filled bars) and after application of DVF solution (open bars) at 400 s and 600 s, respectively. (C, D) Dose-dependent inhibition of currents (+80 mV, 400 s) by [MgATP]i and [Mg2+]i, respectively (n = 4–18 cells per concentration). (E, F) Whole-cell currents of Trpm7+/+ (n = 15) and Trpm7Δ17/Δ17 (n = 10) TS cells studied similar to (A, B). Data are represented as mean±SEM. ***-p≤0.001; **-p≤0.01; *-p≤0.05; n.s. – not significantly different (Student’s t-test). n – number of cells examined. (G) A suggested model for the molecular role of TRPM6 in epithelial cells.
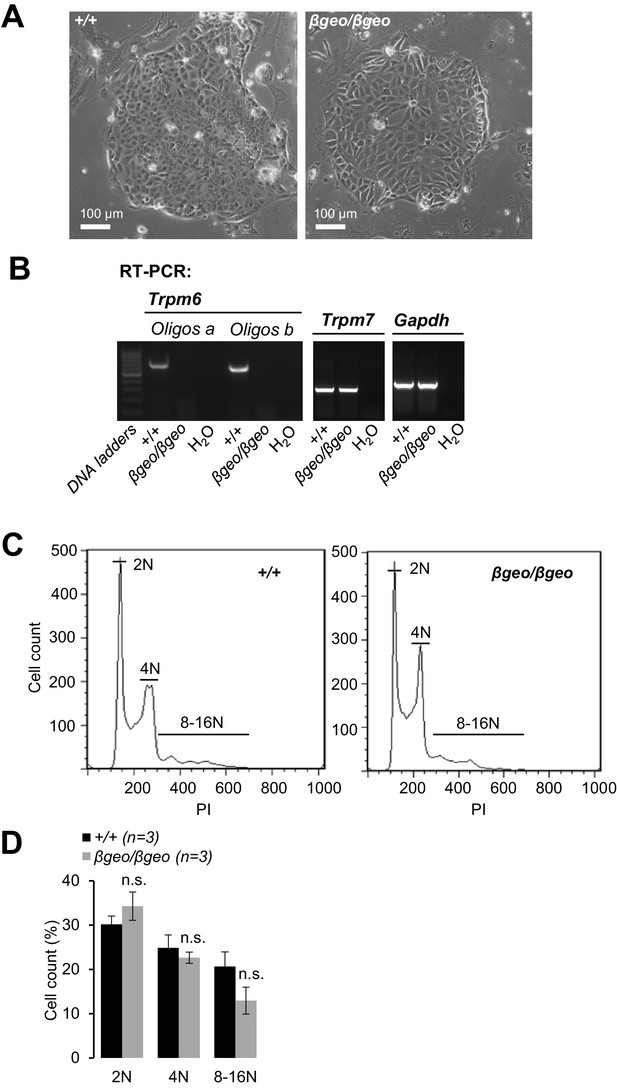
Characterization of Trpm6-deficient TS cells.
(A) Phase-contrast images of Trpm6+/+ (+/+) and Trpm6βgeo/βgeo (βgeo/βgeo) TS cells cultured in a regular cell culture medium. (B) Expression of Trpm6 and Trpm7 in TS cells assessed by RT-PCR. (C, D) Assessment of self-renewal of Trpm6+/+ and Trpm6βgeo/βgeo cells. (C) Diploid (2N), tetraploid (4N), and polyploid (8–16N) DNA content was analysed by flow cytometry of Trpm6+/+ and Trpm6βgeo/βgeo TS cells stained with propidium iodide (PI). (D) Bar graphs showing DNA contents (mean+/-SEM) calculated from three independent experiments outlined in (A). n.s. – not significantly different (Student’s t-test). Note: 2N DNA content (diploid cells in G1), 4N (diploid cells in G2 or tetraploid cells in G1) and 8–16N (spontaneously differentiated polyploid trophoblasts) were not significantly altered in Trpm6-deficient TS cells indicating that self-renewal of Trpm6βgeo/βgeo cells was not affected.
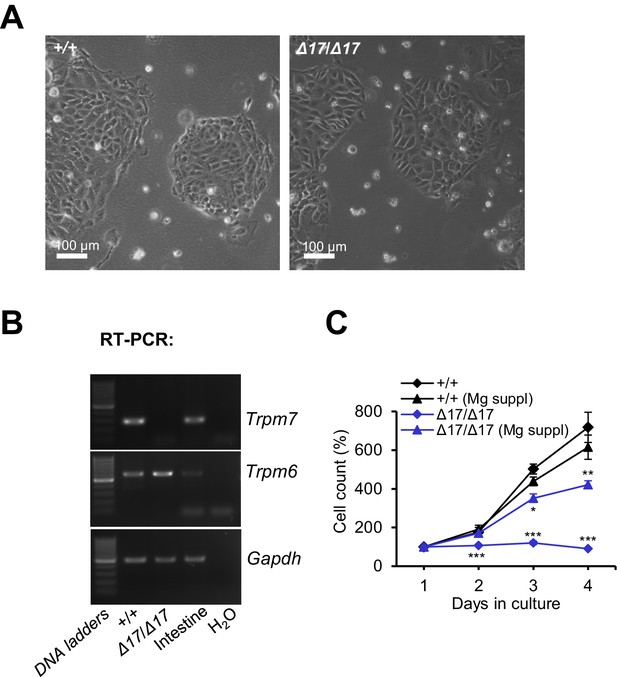
Examination of TS cells deficient in Trpm7.
(A) Phase-contrast images of Trpm7+/+ (+/+) and Trpm7Δ17/Δ17 (Δ17/Δ17) TS cells cultured in a cell culture medium supplemented by 10 mM Mg2+. (B) RT-PCR analysis of Trpm7 and Trpm6 in TS cells and mouse intestine (positive control). (C) Proliferation rate of Trpm7+/+ (+/+) and Trpm7Δ17/Δ17 (Δ17/Δ17) TS cells in standard and Mg2+ (10 mM) supplemented medium. The experiment was repeated three times. Student’s t-test was applied for comparison of Trpm7+/+versus Trpm7Δ17/Δ17 datasets.
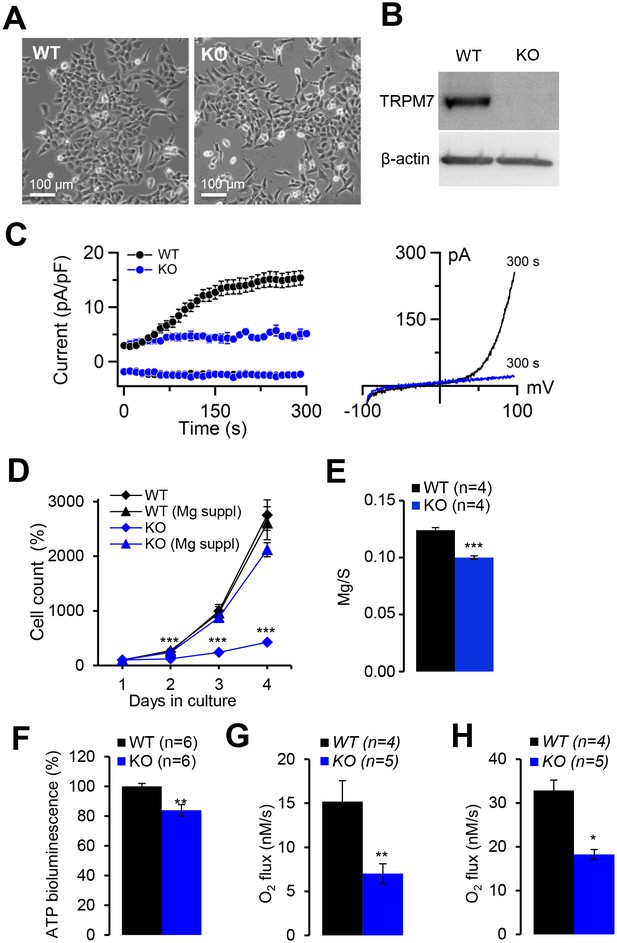
Evaluation of human haploid leukaemia (HAP1) cells deficient in TRPM7.
(A) Phase-contrast images of parental (WT) and TRPM7-deficient (KO) HAP1 cells cultured in a cell culture medium supplemented with 10 mM Mg2+. (B) Western-blot analysis of TRPM7 in WT and KO HAP1 cells. (C) Whole-cell currents in WT and KO HAP1 cells (determined as described in Figure 6). Left panel: currents measured at −80 mV and +80 mV over time in WT (n = 9) and KO (n = 4) HAP1 cells. Data are represented as mean±SEM. Right panel: Representative current-voltage relationships obtained at 300 s. (D) Proliferation rate of WT and KO HAP1 cells either in standard or in Mg2+ (10 mM) supplemented medium. The experiment was repeated three times (n = 3). Data are represented as mean±SEM. Student’s t-test was applied for comparison of the growth rates of WT versus KO cells cultured in standard medium (***-p≤0.001). (E) Determination of total Mg content in WT and KO HAP1 cells. Dried cell pellets (n = 4 for each genotype) were obtained from WT and KO HAP1 cells cultured for 24 hr in standard medium and analysed by ICP-MS. Elementary magnesium (Mg) content was normalized to sulfur (S) levels and represented as mean±SEM. ***-p≤0.001 (Student’s t-test). (F) Assessment of total ATP levels in WT and KO HAP1 cells cultured for 24 hr in standard medium. ATP-induced luminescent of luciferase (CellTiter-Glo2.0 reagent) was normalized to a number of viable cells (Cell Counting Kit-8). The normalized luminescent signal in WT HAP1 cells was designated 100%. The experiment was repeated six times (n = 6). **-p≤0.01 (Student’s t-test). (G–H) Routine respiration rate (G) and maximal respiration rate (H) analysed by Oxygraph-2k in WT and KO HAP1 cells cultured for 24 hr in standard cell culture medium (n – number of independent experiments).
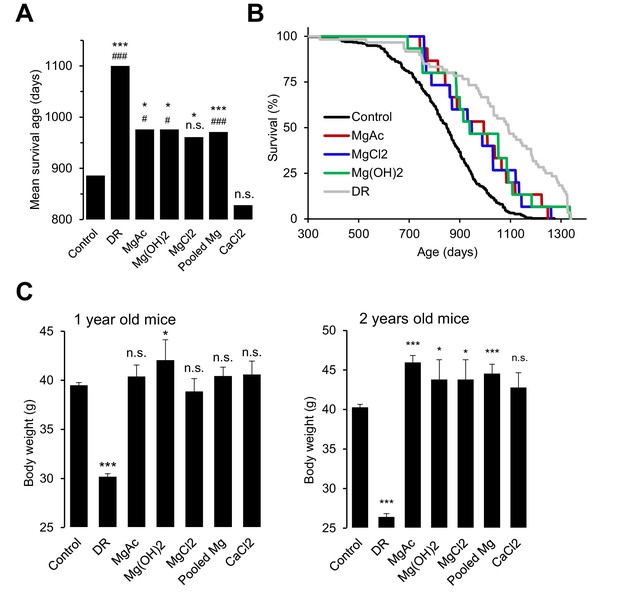
Effects of whole-life Mg2+ dietary treatments on B6C3F1 mouse strain.
(A) Mean survival ages of B6C3F1 mice maintained at a control diet (Control, n = 335), under dietary restriction (DR, n = 60), or supplemented by Mg(CH3COO)2 (MgAc, n = 15), Mg(OH)2 (n = 15), MgCl2 (n = 15) and CaCl2 (n = 15) in drinking water as outlined in Table 2. Pooled Mg shows results for all Mg2+ supplemented mice pooled within a common group (n = 45). The obtained survival distributions were analysed by the MATLAB computing environment to calculate mean lifespans and corresponding P-values: ***-p≤0.001; **-p≤0.01; *-p≤0.05; n.s. – not significantly different. Alternatively, survival data of control mice versus individually treated groups were assessed by log-rank test: ###-p≤0.001; ##-p≤0.01; #-p≤0.05; n.s. – not significantly different. (B) Kaplan-Meier survival distributions of B6C3F1 mice maintained on control diet (Control), Mg2+ supplemented groups (MgAc, MgCl2, Mg(OH)2) or mice under dietary restriction (DR). (C) Body weights (mean+/-SEM) of control and nutritionally fortified mice studied in (A). ***-p≤0.001; **-p≤0.01; *-p≤0.05; n.s. – not significantly different (one-way ANOVA). n – number of mice examined.
Tables
Postnatal survival of the mice with global and tissue-restricted deletions of Trpm6.
Targeted tissue | Breeding strategy | Expected F1 outcome* | Survival of the mutant |
|---|---|---|---|
Constitutive mutagenesis | |||
Whole fetus | ♂Trpm6βgeo/+ x ♀Trpm6βgeo/+ | 25%Trpm6βgeo/βgeo 50% Trpm6βgeo/+ 25% Trpm6+/+ | no |
Whole fetus | ♂Trpm6Δ17/+ x ♀Trpm6 Δ17/+ | 25% Trpm6 Δ17/Δ17 50% Trpm6 Δ17/+ 25% Trpm6+/+ | no |
Whole fetus | ♂Trpm6Δ17/+ x ♀Trpm6βgeo/+ | 25% Trpm6βgeo/Δ17 25% Trpm6βgeo/+ 25% Trpm6Δ17/+ 25% Trpm6+/+ | no |
Conditional mutagenesis using Cre/LoxP system | |||
Epiblast | ♂Trpm6Δ17/+;Sox2-Cre x ♀Trpm6fl/fl | 25% Trpm6Δ17/Δ17;Sox2-Cre 25% Trpm6Δ17/fl 25% Trpm6Δ17/+;Sox2-Cre 25% Trpm6fl/+ | yes |
Intestine | ♂Trpm6Δ17/+;Villin1-Cre x ♀Trpm6fl/fl | 25% Trpm6Δ17/fl;Villin1-Cre† 25% Trpm6Δ17/fl 25% Trpm6fl/+;Villin1-Cre 25% Trpm6fl/+ | yes |
Kidney | ♂Trpm6Δ17/+;Ksp-Cre x ♀Trpm6fl/fl | 25% Trpm6Δ17/fl;Ksp-Cre† 25% Trpm6Δ17/fl 25% Trpm6fl/+;Ksp-Cre 25% Trpm6fl/+ | yes |
-
*Genotypes were determined using genomic DNA extracted from tail fragments.
-
†Individuals were homozygous for Trpm6Δ17 allele in the targeted cells.
Dietary regimes used to maintain B6C3F1 mice.
Experimental group | Chow 5 K54 | Drinking water (ad libitum) | Number of mice |
|---|---|---|---|
Control | ad libitum | Regular water* | 335 |
Dietary restriction | 60% of ad libitum | Regular water | 60 |
Magnesium acetate supplemented | ad libitum | 1 g/l Mg(CH3COO)2.(H2O)4 in regular water | 15 |
Magnesium chloride supplemented | ad libitum | 1 g/l MgCl2 in regular water | 15 |
Magnesium hydroxide supplemented | ad libitum | 14 mg/l Mg(OH)2 in regular water† | 15 |
Calcium chloride supplemented | ad libitum | 0.8 g/l CaCl2 in regular water | 15 |
-
*Regular water contained 0.44 mg/l Mg2+.
-
†Mg(OH)2 concentration was lowered to prevent overall alkalization of the diet.
Additional files
-
Supplementary file 1
Whole genome profiling of hepatic transcripts altered in Trpm6-deficient mice.
Dataset is available in the Dryad Digital Repository (Chubanov and Gudermann, 2016). (1) Genome-wide analysis of hepatic transcriptome in control vs Trpm6-deficient mice. (2) Up- and down-regulated transcripts in the liver of Trpm6-deficient mice with the false discovery rate (FDR) p≤0.1.
- https://doi.org/10.7554/eLife.20914.022
-
Supplementary file 2
Ingenuity Pathway Analysis (IPA) analysis of hepatic transcripts altered in Trpm6-deficient mice.
Dataset is available from the Dryad Digital Repository (Chubanov and Gudermann, 2016). (1) IPA Canonical Pathways representing differentially expressed genes in the liver of Trpm6-deficient mice. (2) IPA Causal Networks for differentially expressed genes in the liver of Trpm6-deficient mice.
- https://doi.org/10.7554/eLife.20914.023
-
Supplementary file 3
Metabolic profiling of the serum, liver and gastrocnemius muscle of Trpm6-deficient mice.
Dataset is available in the Dryad Digital Repository (Chubanov and Gudermann, 2016). (1) Statistical analysis of metabolite measurements in serum samples. (2) Statistical analysis of metabolite measurements in gastrocnemius muscle samples. (3) Statistical analysis of metabolite measurements in liver samples. (4) Metabolites significantly changed in serum samples of Trpm6-deficient mice (FDR p≤0.05). (5) Metabolites significantly changed in gastrocnemius muscle samples of Trpm6-deficient mice (FDR p≤0.05). (6) Metabolites significantly changed in liver samples of Trpm6-deficient mice (FDR p≤0.05). (7) Abbreviations of metabolites.
- https://doi.org/10.7554/eLife.20914.024





