Angiopoietin receptor Tie2 is required for vein specification and maintenance via regulating COUP-TFII
Figures
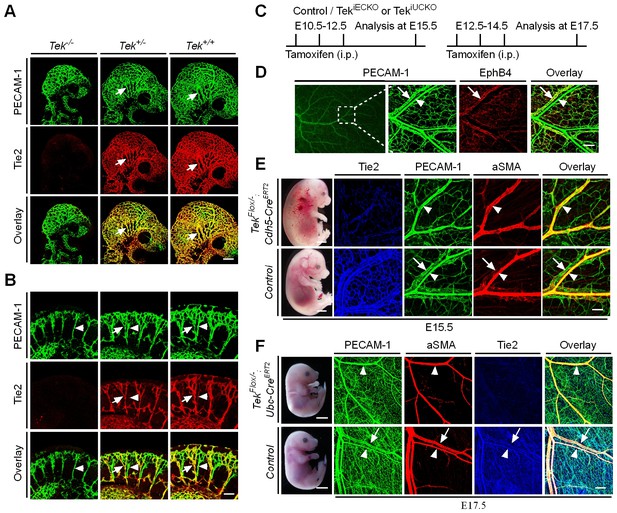
Tie2 insufficiency during embryogenesis arrests venous development.
(A, B) Analysis of blood vessels in head (A, E9.5) and somites (B) by whole-mount immunostaining for PECAM-1 (green) and Tie2 (red). (C) Tamoxifen intraperitoneal (i.p.) administration and analysis scheme. (D–F) Visualization of veins in E15.5 (D, E) or E17.5 (F) skin of wildtype and Tek mutant mice by immunostaining for PECAM-1 (green) and EphB4 or αSMA (red). Arrows point to veins and arrowheads to arteries. The experiments with the ubiquitous or EC-specific Tek deletion were repeated for at least three times. Scale bar: 200 μm in A, D and F (4 mm in F embryos); 100 μm in B and E (2 mm in E embryos).
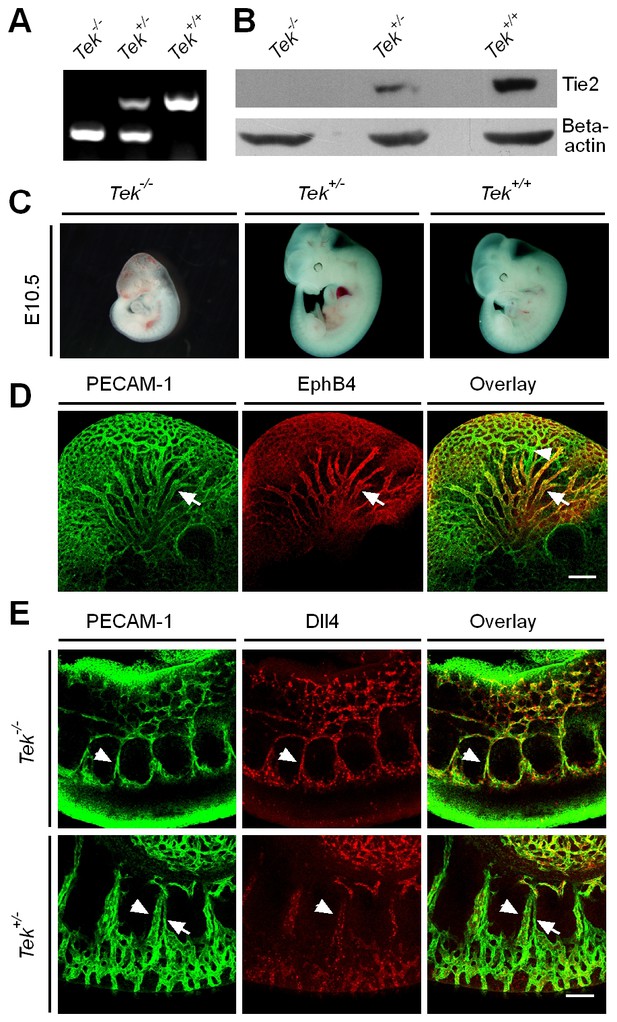
Generation and analysis of Tie2 knockout mice.
(A) PCR genotyping of Tie2 knockout and wild-type alleles. (B) Western blot analysis of Tie2 protein in Tek null, heterozygous and wildtype littermate mice. (C) Tek null mice were smaller, showed bleedings and died at E10.5. (D) Visualization of veins in the head region at E10.5 by whole-mount immunostaining for EphB4 and PECAM-1. (E) Immunostaining for the arterial marker Dll4 and PECAM-1 showed lack of vein formation in somites of Tek null mice at E9.5. Arrows point to veins and arrowheads point to arteries. Scale bar: 200 μm in D, 100 μm in E.
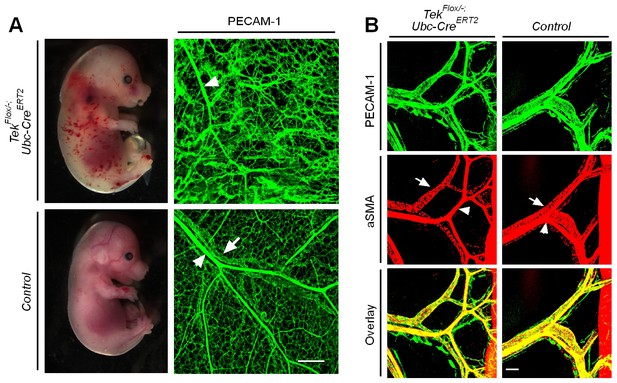
Defective skin vein formation and abnormal arteriovenous alignment in mesenteries of Tek mutant mice (Tek−/iUCKO).
(A) Analysis of skin blood vessels by whole-mount immunostaining for PECAM-1 (green) in Tek−/iUCKO and control mice (E15.5; Tamoxifen treatment from E10.5–12.5). (B) Analysis of mesenteric blood vessels by whole-mount immunostaining for PECAM-1 (green) and αSMA (red) in Tek−/iUCKO and control mice (E17.5; Tamoxifen treatment from E12.5–14.5). In addition to the immunostaining analysis of Tie2 protein, Tie2 deletion efficiency in embryos was also analyzed by real-time RT-PCR. The level of Tie2 mRNA in the lung was 0.25 ± 0.02 in Tek−/iUCKO (n = 4), as compared with that of the heterozygous mice (Tek+/iUCKO: 1.0 ± 0.11, n = 4). Arrowheads point to arteries and arrows to veins. Scale bar: 200 μm in A and B.
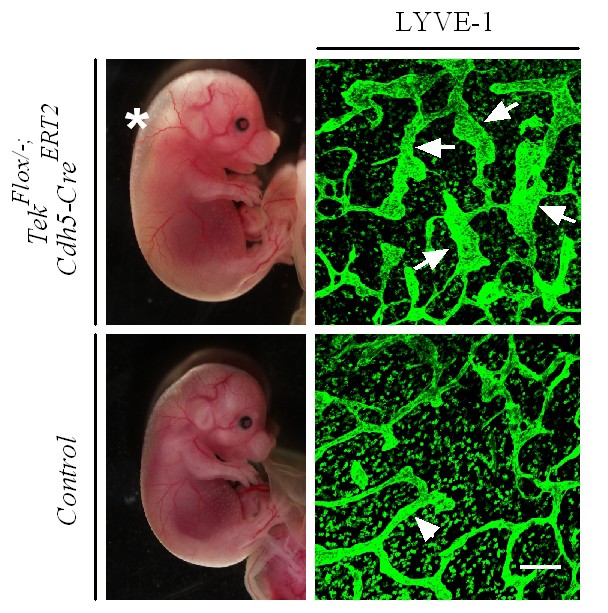
Lymphatic dilation in the skin of mutant mice with Tie2 deletion in vascular endothelial cells.
Analysis of skin lymphatic vessels in Tek−/iECKO mutant and control embryos (E15.5) by immunostaining for LYVE1 (green). Tie2 mutant mice showed subcutaneous edema (asterisk) with the dilation of lymphatic vessels (arrows; arrowheads point to the normal lymphatic vessels in control mice). Scale bar: 200 μm.
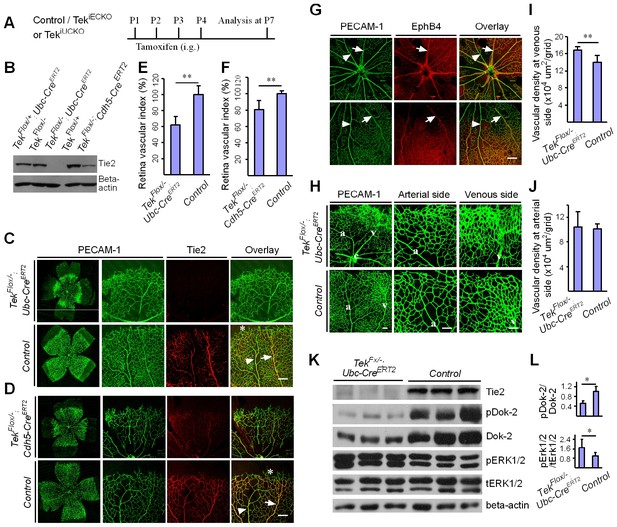
Attenuation of Tie2 expression retards retinal vascularization.
(A) Tamoxifen intragastric (i.g.) administration and analysis scheme. (B–D) The deletion efficiency of Tie2 was examined by Western blot analysis (B) and immunostaining (C, D) for PECAM-1 (green) and Tie2 (red) of tissues from Tek deleted and littermate control mice at P7. Note that Tie2 expression is downregulated in tip endothelial cells (asterisk in C and D) and also low in newly formed retinal arteries (arrowhead in C and D) when compared with veins (arrow in C and D). (E, F) Quantification of the vascularization index (ratio of vascularized area to total retina area normalized against the littermate controls) after ubiquitous (E, Tek−/iUCKO: 61.95 ± 10.79, n = 12; Control: 100.0 ± 10.94, n = 12; p<0.0001) or EC-specific deletion (F, Tek-/iECKO: 80.69 ± 11.13, n = 5; Control: 100.0 ± 3.77, n = 7; p<0.0015). (G–J) Visualization of retinal blood vessels (G, H, P7) by immunostaining for PECAM-1 (green) and EphB4 (red). Arrows point to veins (v) and arrowheads to arteries (a). Quantification of blood vessel density in the distal retinal venous (I, X 104 μm2/grid; Tek−/iUCKO: 16.91 ± 0.77, n = 6; Control: 14.07 ± 1.54, n = 8; p=0.0014) and arterial segments (J, X 104 μm2/grid; Tek−/iUCKO: 10.45 ± 2.47, n = 6; Control: 10.16 ± 0.76, n = 8; p=0.7571) in Tek mutants compared with control mice. (K, L) Analysis of Dok-2 and ERK1/2 phosphorylation. Total Dok-2, ERK1/2 and beta-actin were used as loading controls. The bands were quantified and normalized against the control group (L). Scale bar: 200 μm in C, D, G; 100 μm in H.
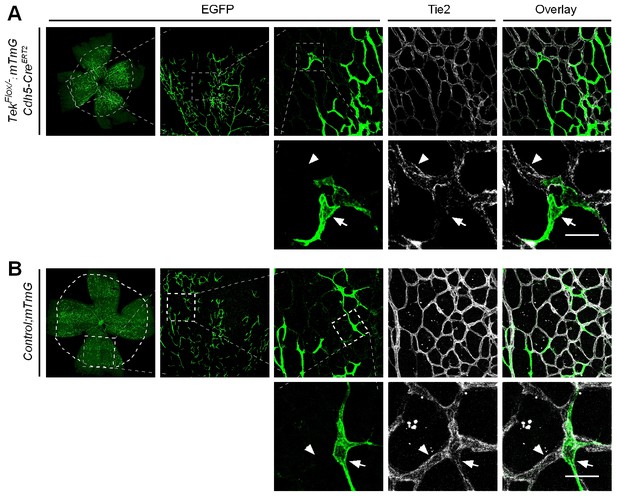
Analysis of Tie2 deletion in Tek−/iECKO;mTmG mice.
The triply compound genetic mouse model (Tek−/Flox;mTmG;Cdh5-CreERT2, A; and control, B) was generated by three rounds of mating, and tamoxifen intragastric (i.g.) administration was performed as described in Figure 2A. The partial Tie2 deletion was indicated by the mosaic GFP expression (arrow) together with the immunostaining for Tie2 (arrowhead). Scale bar: 25 μm.
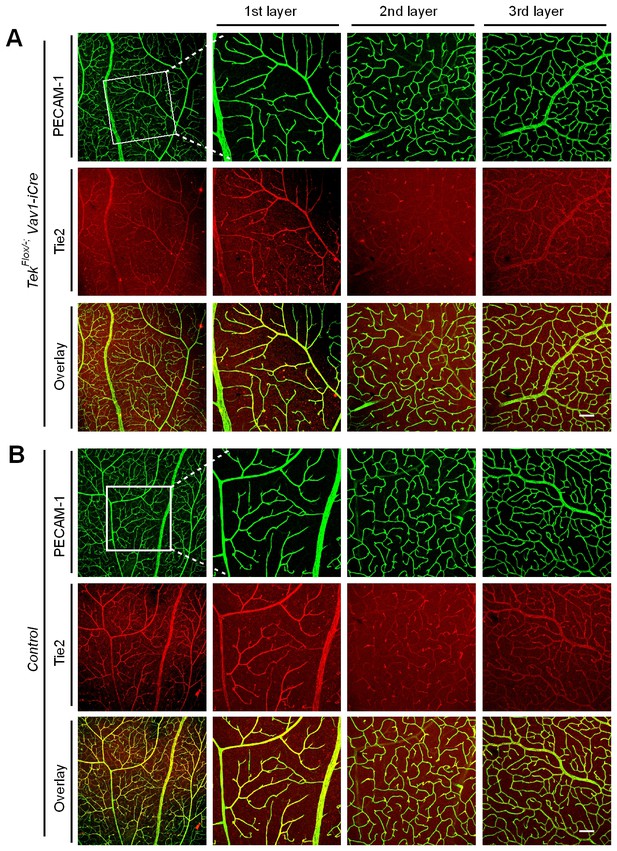
Tie2 deletion in hematopoietic cells does not affect retinal blood vessel growth.
(A, B) Analysis of retinal blood vessels by immunostaining for PECAM-1 (green) and Tie2 (red) in TekFlox/−;Vav-iCre (A) and TekFlox/+;Vav-iCre mice (control, B) at P21. Scale bar: 100 μm in A, B.
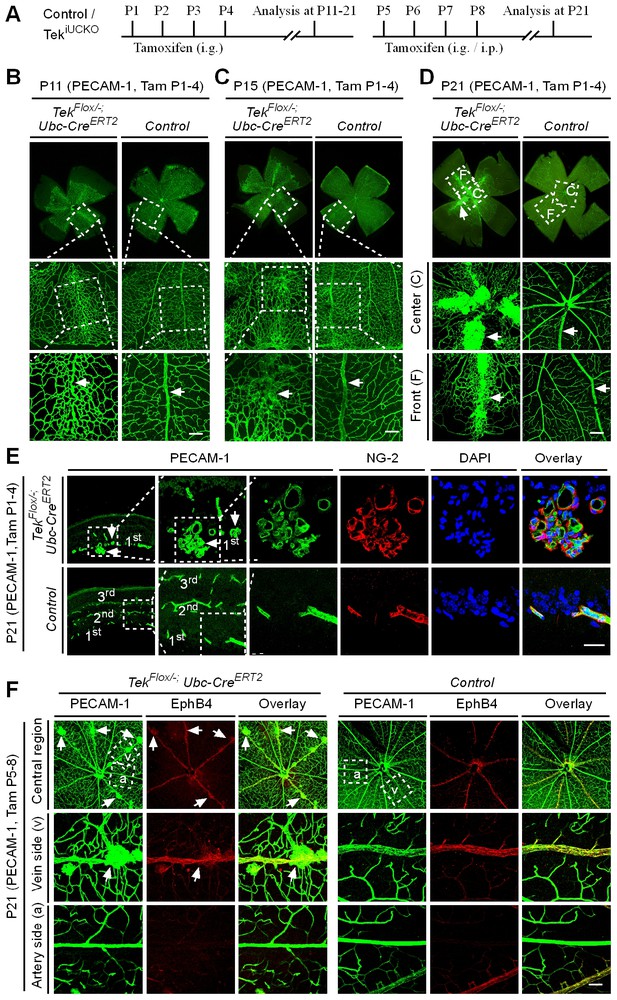
Tie2 attenuation leads to vascular tuft formation along retinal veins.
(A) Tamoxifen intragastric (i.g.) administration and analysis scheme. (B–D) Analysis of blood vessels in the retinas of Tek deleted (P1–4) and control mice at P11 (B), P15 (C), and P21 (D). (E) Cross-sectional analysis of the three layers of retinal blood vessels in Tek mutant and control mice. (F) Tie2 deletion was induced at P5–8, and analysis of retinal blood vessels was performed at P21. Arrows point to haemangioma-like vascular tufts. The experiments at each time point were repeated for at least three times. Scale bar: 100 μm in B–D and F, 25 μm in E.
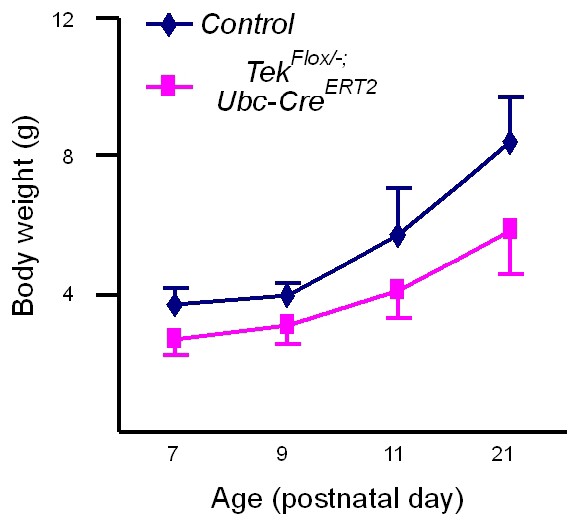
Growth curves of Tek−/iUCKO mutant and littermate control mice.
Tie2 attenuation (Tamoxifen treatment at P1–4) led to a significant decrease in body weight gain as measured until three weeks postnatally: control mice: P7, 3.68 ± 0.48 g (n = 24); P9, 3.97 ± 0.34 g (n = 6); P11, 5.73 ± 1.37 g (n = 16); P21, 8.40 ± 1.29 g (n = 8); and Tek−/iUCKO mutants: P7, 2.71 ± 0.51 g (n = 24; p<0.0001); P9, 3.13 ± 0.57 g (n = 3; p=0.0252); P11, 4.09 ± 0.81 g (n = 11; p=0.0015); P21, 5.87 ± 1.30 g (n = 6; p=0.0035).
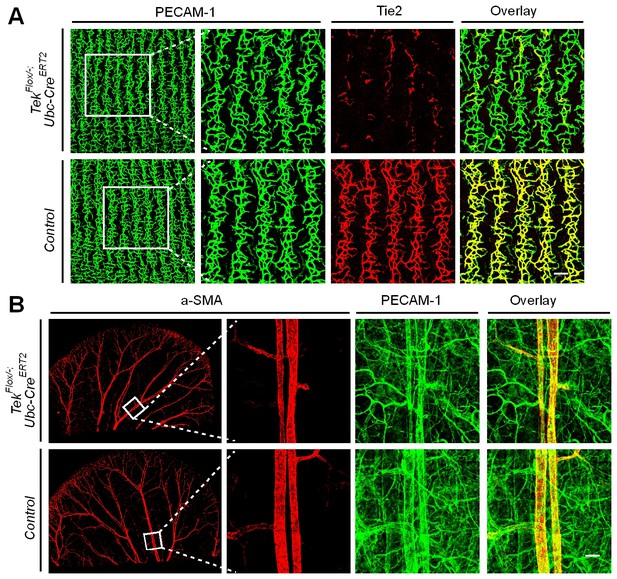
Analysis of cutaneous blood vessels at P7 and P21, after Tie2 deletion at P1-4.
(A, B) Blood vessels were visualized by immunostaining for PECAM-1 (green) and αSMA (red), and the efficiency of gene deletion was analyzed by Tie2 staining (red). No obvious defects were observed in tail skin (P7, A) or ear skin (P21, B) of Tek−/iUCKO mice compared with the littermate controls. Scale bar: 100 μm in A; 200 μm in B.
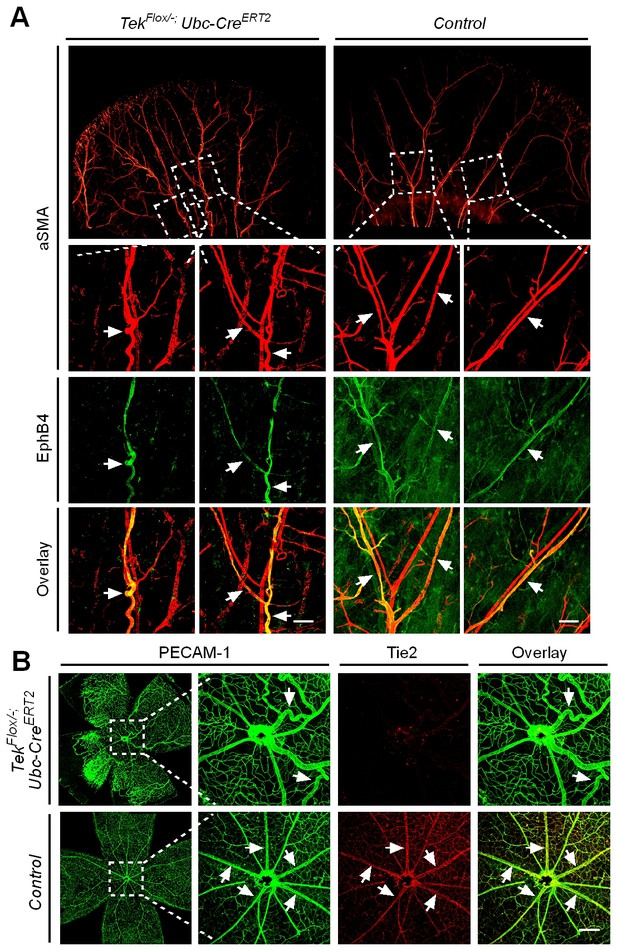
Effect of long-term postnatal attenuation of Tie2 on cutaneous and retinal veins.
(A) Analysis of blood vessels in the ear skin of Tek mutant and control mice by immunostaining for αSMA (red) and EphB4 (green). (B) Visualization of retinal blood vessels by immunostaining for Tie2 (red) and PECAM-1 (green). Note tortuous veins in ear skin and retinas in 2.5 month old Tek−/iUCKO mice after Tie2 deletion at P1–4. Arrows point to veins. The histological analysis for both groups was repeated for at least three times. Scale bar: 200 μm in A, B.
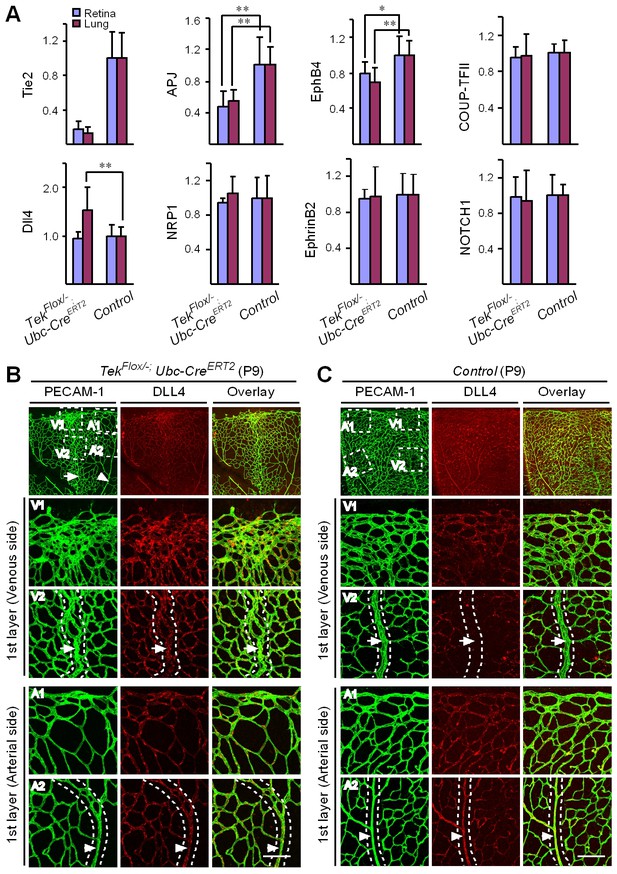
Requirement of Tie2 for the maintenance of venous EC identity.
(A) Quantitative expression analysis of venous markers including EphB4, APJ and COUP-TFII, and arterial markers including EphrinB2, NRP1, NOTCH1 and Dll4 in lung and retina tissues of Tek mutant and control mice (P7). (B, C) Immunostaining for Dll4 with retinas of Tek mutant and control mice (P9). Scale bar: 100 μm in B and C.
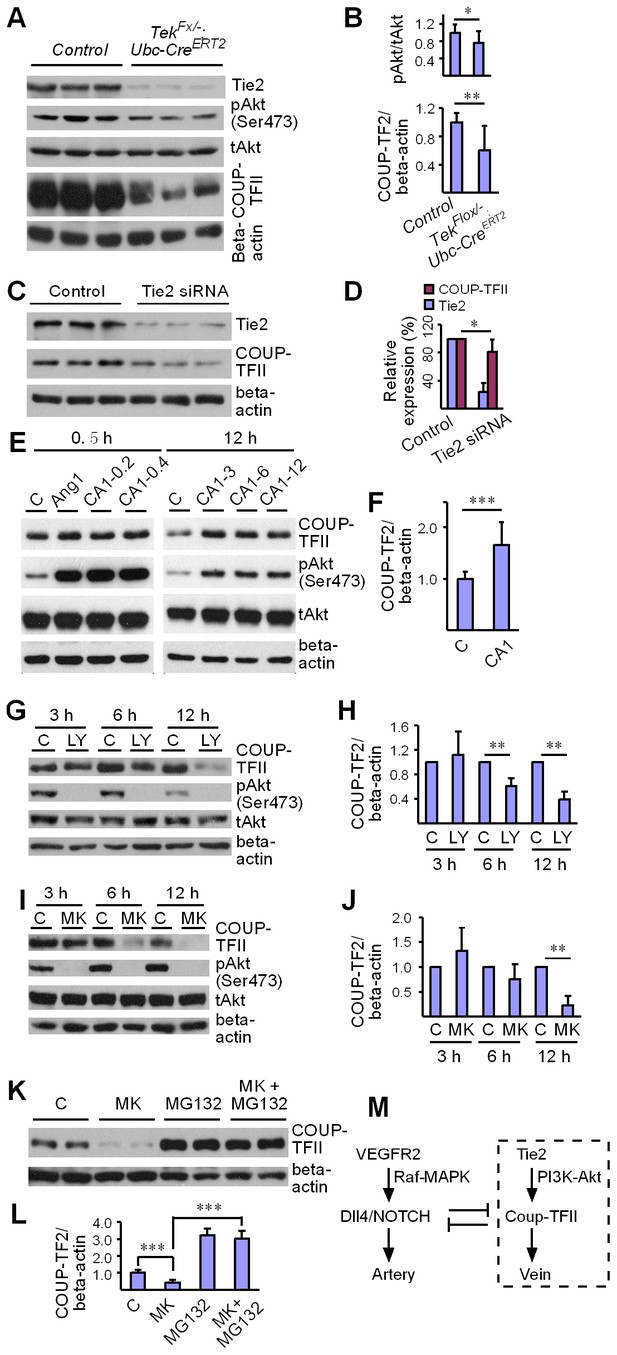
Regulation of COUP-TFII protein level by Tie2 pathway.
(A, B) Analysis and quantification of Akt (Ser473) phosphorylation (pAkt / tAkt, Tek−/iUCKO: 0.76 ± 0.27, n = 10; Control: 1.0 ± 0.19, n = 9; p<0.0407) and COUP-TFII protein in lungs of Tek mutant and control mice, and total Akt and beta-actin as loading controls (COUP-TFII/beta-actin; Tek−/iUCKO: 0.60 ± 0.35, n = 12; control: 1.0 ± 0.13, n = 12; p<0.01). (C, D) Western blotting of COUP-TFII protein in HUVECs after siRNA mediated Tie2 knockdown. COUP-TFII protein level decreased to 80.55 ± 17.79% of the control when Tie2 expression was reduced to 24.33 ± 13.05% of control (normalized by beta-actin; values from six independent experiments). (E, F) Increase of COUP-TFII (COUP-TFII/beta-actin; COMP-Ang1: 1.66 ± 0.45, n = 12; Control: 1.0 ± 0.15, n = 10; p<0.001; values from five independent experiments) by COMP-Ang1 activation of the Tie2/Akt pathway for 12 hr with the recombinant protein added every 4 hr (CA1–3, 0.2–0.4 μg/ml). Note that COMP-Ang1 added every 1 hr (CA1–12) or 2 hr (CA1–6) did not further increase the level of COUP-TFII, and that treatment with Ang1 or COMP-Ang1 for half an hour did not produce an obvious difference in the COUP-TFII protein level. (G–J) Analysis of COUP-TFII in HUVECs after treatment with LY294002 or MK2206 to inhibit the PI3K/Akt signaling pathway for 3 hr, 6 hr or 12 hr. (K, L) Decrease of COUP-TFII by the Akt inhibitor MK2206 was blocked by the proteasome inhibitor MG132 when analyzed 12 hr after the treatment (COUP-TFII/beta-actin; Control: 1.0 ± 0.16, n = 5; MK2206: 0.42 ± 0.17, n = 5; MG132: 3.20 ± 0.38, n = 7; MK2206 + MG132: 3.01 ± 0.46, n = 7; p<0.001; values from three independent experiments). (M) Schematic model of Tie2 mediated signaling in vein specification via the Akt mediated regulation of COUP-TFII. The Tie2/Akt pathway may counterbalance VEGFR2/MAPK signaling during arteriovenous specification.
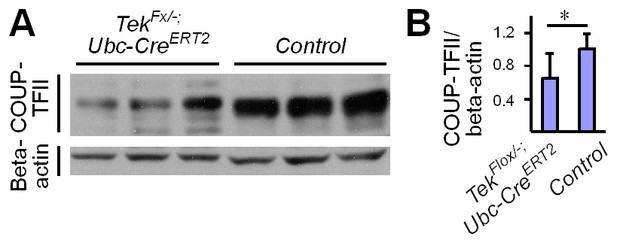
Analysis of COUP-TFII in the liver.
(A) Western blot analysis of COUP-TFII in liver lysates from Tek mutant (Tek−/iUCKO) and control mice. (B) Quantification of COUP-TFII normalized against beta-actin (Tek−/iUCKO: 0.65 ± 0.30; Control: 1.0 ± 0.19, n = 9 for each group; p=0.0093).
Tables
Retina transcript levels of venous and arterial markers.
The mRNA expression level of venous and arterial endothelial cell markers | |||||
|---|---|---|---|---|---|
Venous and arterial markers | mRNA expression level (retina) | n (Tek−/UCKO) | n (Control) | p value | |
Tek−/UCKO | Control (Tek+/UCKO) | ||||
Tie2 | 0.18 ± 0.09 | 1.0 ± 0.31 | 7 | 7 | <0.0001 |
APJ | 0.47 ± 0.20 | 1.0 ± 0.36 | 7 | 7 | 0.00488 |
EphB4 | 0.79 ± 0.12 | 1.0 ± 0.20 | 7 | 7 | 0.0404 |
COUP-TFII | 0.96 ± 0.11 | 1.00 ± 0.10 | 7 | 7 | 0.449 |
Dll4 | 0.94 ± 0.16 | 1.0 ± 0.22 | 7 | 7 | 0.565 |
EphrinB2 | 0.96 ± 0.10 | 1.0 ± 0.24 | 6 | 7 | 0.699 |
NRP1 | 0.94 ± 0.06 | 1.0 ± 0.24 | 6 | 7 | 0.542 |
NOTCH1 | 0.98 ± 0.23 | 1.0 ± 0.22 | 6 | 7 | 0.889 |
Lung transcript levels of venous and arterial markers | |||||
|---|---|---|---|---|---|
Venous and arterial markers | mRNA expression level (lung) | n (Tek−/UCKO) | n (Control) | p value | |
Tek−/UCKO | Control (Tek+/UCKO) | ||||
Tie2 | 0.13 ± 0.06 | 1.0 ± 0.29 | 11 | 11 | <0.0001 |
APJ | 0.55 ± 0.14 | 1.0 ± 0.24 | 11 | 11 | <0.0001 |
EphB4 | 0.69 ± 0.16 | 1.0 ± 0.16 | 11 | 11 | 0.00026 |
COUP-TFII | 0.98 ± 0.24 | 1.0 ± 0.14 | 11 | 11 | 0.734 |
Dll4 | 1.53 ± 0.46 | 1.0 ± 0.19 | 11 | 11 | 0.00207 |
EphrinB2 | 0.98 ± 0.33 | 1.0 ± 0.23 | 11 | 11 | 0.856 |
NRP1 | 1.05 ± 0.19 | 1.0 ± 0.26 | 11 | 11 | 0.607 |
NOTCH1 | 0.93 ± 0.34 | 1.0 ± 0.12 | 11 | 11 | 0.549 |





