Evolution of the hypoxia-sensitive cells involved in amniote respiratory reflexes
Figures
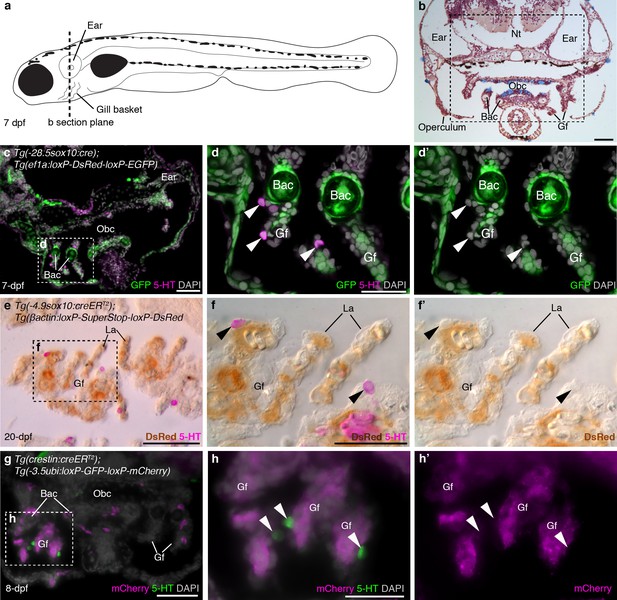
Zebrafish NECs are not neural crest-derived: genetic lineage-tracing data.
(a) Schematic of a 7–8 dpf zebrafish; dotted line indicates section plane in b-f’. (b) Hematoxylin and eosin staining at 7-dpf reveals gill filaments branching from branchial arch cartilages, and the orobranchial cavity. Dashed box indicates approximate region in c,g. (c–d’) In 7-dpf Tg(-28.5sox10:cre);Tg(ef1a:loxP-DsRed-loxP-EGFP) zebrafish, GFP labels neural crest-derived branchial arch cartilage and mesenchyme, but not NECs in the gill filaments (identified by immunoreactivity for serotonin, 5-HT; arrowheads). (e–f’) Horizontal section through the gills of a 20-dpf Tg(-4.9sox10:creERT2);Tg(βactin:loxP-SuperStop-loxP-DsRed) zebrafish. DsRed (brown precipitate) labels neural crest-derived gill pillar cells, but not NECs (arrowheads; inverted fluorescent image overlaid on bright-field image). (g–h’) In 8-dpf Tg(crestin:creERT2);Tg(-3.5ubi:loxP-GFP-loxP-mCherry) zebrafish, mCherry labels gill pillar cells but not NECs (arrowheads). 5-HT, serotonin; Bac, branchial arch cartilage; Gf, gill filament; La, lamellae; Nt, neural tube; Obc, orobranchial cavity. Scale-bars: 50 μm in b,c,e,g; 25 μm in d,f,h.
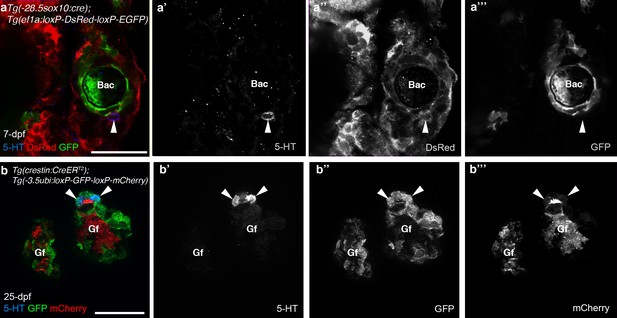
The promoters driving the Cre-switchable reporter cassettes are active in NECs.
In 7-dpf Tg(-28.5sox10:cre);Tg(ef1a:loxP-DsRed-loxP-EGFP) (a–a’’’) and 25-dpf Tg(crestin:creERT2);Tg(-3.5ubi:loxP-GFP-loxP-mCherry) (b–b’’’) zebrafish, NECs in the gill filaments (identified by immunoreactivity for serotonin, 5-HT; arrowheads) express the un-switched reporter gene. 5-HT, serotonin; Bac, branchial arch cartilage; Gf, gill filament. Scale-bar: 25 μm.
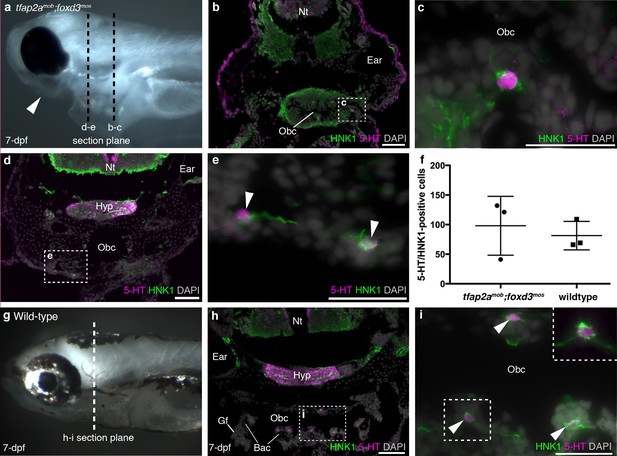
Zebrafish NECs are not neural crest-derived: analysis of neural crest-deficient zebrafish mutants.
(a) 7-dpf tfap2amob;foxd3mos zebrafish lack all neural crest derivatives, including melanophores and jaw skeleton (arrowhead). Dotted lines: section planes in b-e. (b,c) At 7-dpf, tfap2amob;foxd3mos orobranchial epithelium retains innervated serotonergic (5-HT+) cells (putative NECs), identified by cytoplasmic serotonin surrounded by a ring of HNK1 epitope-immunoreactive neurites. (d,e) At 7-dpf, putative NECs (arrowheads) persist in tfap2amob;foxd3mos zebrafish in the region ventral to the orobranchial cavity where the pharyngeal arches would be located in wild-type fish. (NB The hypothalamus [Hyp] extends caudally beneath the midbrain and rostral hindbrain, and often separates from the overlying brain on sections, as here.) (f) The mean number per 7-dpf larva of putative NECs in the orobranchial epithelium does not differ between tfap2amob;foxd3mos (98.0 ± 49.7 s.d.; n = 3) and wild-type zebrafish (81.3 ± 24.0 s.d.; n = 3) (p=0.63, unpaired two-tailed Student’s t-test). All such cells in the orobranchial epithelium were counted for each embryo. Error bars indicate s.d. (g–i) Wild-type sibling at 7-dpf. Dotted line: section plane in h-i. Putative NECs are present in the orobranchial epithelium (arrowheads; dashed box in i, magnified without DAPI in top right corner). 5-HT, serotonin; Gf, gill filament; Hyp, hypothalamus; Nt, neural tube; Obc, orobranchial cavity. Scale-bars: 50 μm in b,d,h; 25 μm in c,e,i.
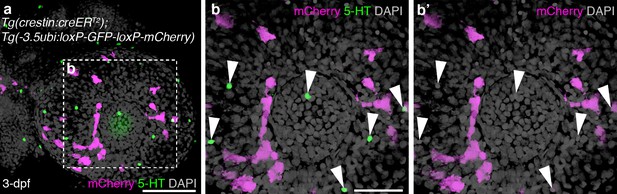
Putative NECs in the skin of embryonic zebrafish are not neural crest-derived.
(a–b’) Three-dimensional rendering of the eye of a 3-dpf Tg(crestin:creERT2);Tg(-3.5ubi:loxP-GFP-loxP-mCherry) embryo, immunostained in whole-mount for serotonin and mCherry. Serotonergic cells are scattered in the epidermis over the eye, as reported (Coccimiglio and Jonz, 2012), but none is mCherry-positive, i.e., neural crest-derived (arrowheads). For associated z-stack movies, see Videos 1 and 2. Scale-bars: 100 μm in a; 50 μm in b.
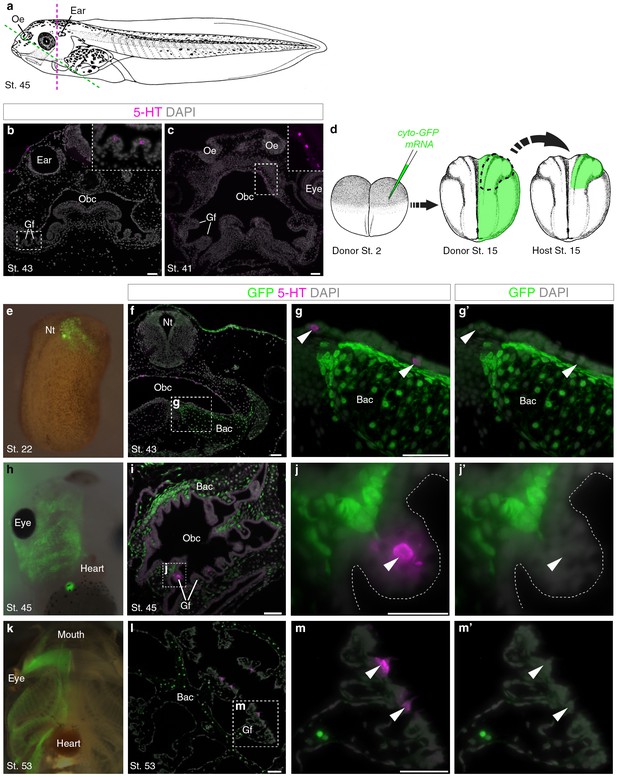
Putative Xenopus NECs are not neural crest-derived.
(a) Schematic of a stage 45 tadpole (Nieuwkoop and Faber, 1967). Dotted lines: section planes in b,f–g’ (magenta, transverse) and in c,i-j’,l-m’ (green, oblique). (b) Serotonergic cells in gill filament epithelium (putative NECs) are first detected at stage 43 (inset shows higher-power view). (c) Similar serotonergic cells are scattered in the orobranchial epithelium from stage 41 (inset shows higher-power view). (d) Schematic (modified from Nieuwkoop and Faber, 1967) showing neural crest labelling: GFP-donors, created by injecting cyto-GFP mRNA into one cell at the two-cell stage, were grown to stages 13–17, and neural folds grafted unilaterally to wild-type hosts. For grafted embryos grown to stage 53, donors were transgenic CMV-GFP embryos. (e) At stage 22, GFP labels neural crest cells migrating towards the branchial arches. (f–g’) At stage 43, GFP labels branchial arch cartilage and surrounding mesenchyme, but not putative NECs (arrowheads) in the orobranchial epithelium. (h–j’) At stage 45, GFP-positive neural crest cells are visible in the branchial arches (whole-mount and section from different embryos). GFP labels branchial arch cartilage and mesenchyme, but not putative NECs (arrowheads) in the gill filaments. (k–m’) At stage 53 (transgenic CMV-GFP donors; whole-mount and section from different embryos), GFP labels branchial arch cartilage and mesenchyme, but not putative NECs (arrowheads) in the gill filaments. 5-HT, serotonin; Bac, branchial arch cartilage; Gf, gill filament; Nt, neural tube; Obc, orobranchial cavity; Oe, olfactory epithelium. Scale-bar: 50 μm.
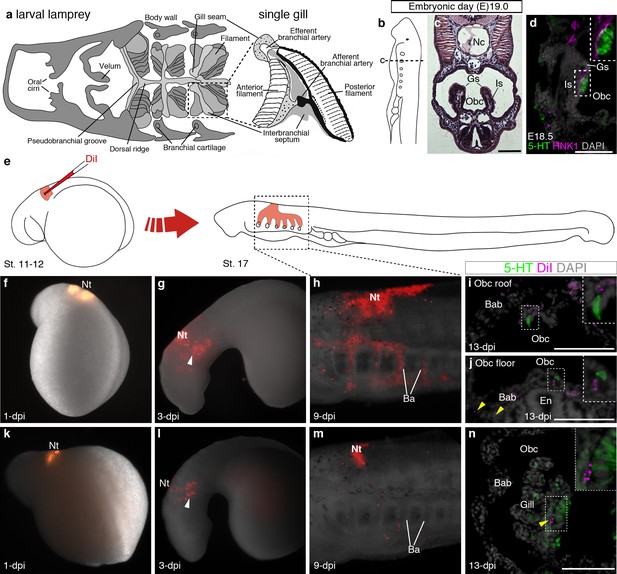
Putative lamprey NECs are not neural crest-derived.
(a) Schematic larval lamprey section (modified from Barreiro-Iglesias et al., 2009) showing gill pairs in the orobranchial cavity, supported by an interbranchial septum, and a single gill at higher power. (b) Schematic Piavis-stage 17 (E19) lamprey (modified from Tahara,1988). Dotted line shows section plane in c,d,i,j,n. (c) Hematoxylin and eosin staining at E19 shows internal gills as ‘stalks’ within the orobranchial cavity. (d) Putative NECs (serotonergic cells associated with HNK1 epitope-immunoreactive neurites) are first visible at E18.5 in the medial gill epithelium. (e) Schematic (modified from Tahara,1988) showing neural crest labelling by DiI injection at E5 (Piavis stages 11–12). (f–n) Two different embryos (f–j, k–n), showing DiI-labeled neural crest cells migrating ventrally (arrowheads, g,l) into the branchial arches, contributing to branchial arch basket and gill supporting cells (arrowheads, j,n), but not serotonergic cells (putative NECs) in the orobranchial epithelium (i,j) or gills (n). 5-HT, serotonin; Ba, branchial arch; Bab, branchial arch basket; En, endostyle; Gs, gill seam; Is, interbranchial septum; Nc, notochord; Nt, neural tube; Obc, orobranchial cavity. Scale-bar: 50 μm.
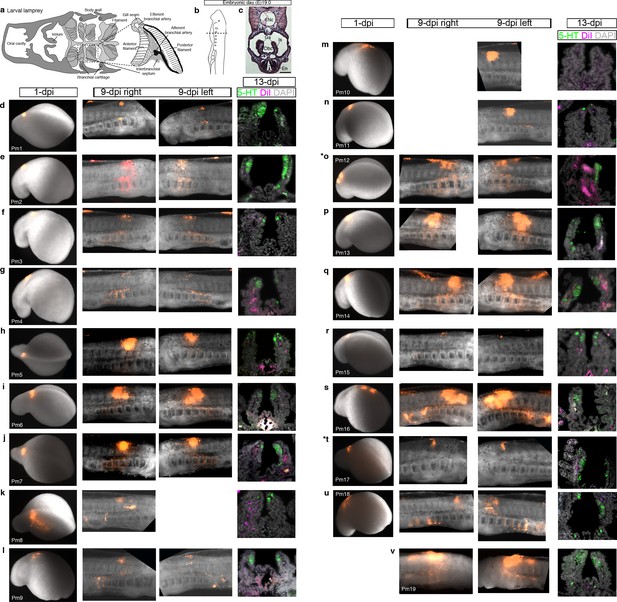
Confirmation of successful targeting of pharyngeal arch-destined neural crest cells in all lamprey embryos analyzed for neural crest contribution to putative NECs.
For orientation, the first three panels are repeated from Figure 4. (a) Schematic larval lamprey section (modified from Barreiro-Iglesias et al., 2009) showing gill pairs in the orobranchial cavity, supported byan interbranchial septum. (b) Schematic Piavis-stage 17 (E19) lamprey (modified from Tahara,1988). Dotted line: section plane. (c) Hematoxylin and eosin staining shows internal gills as ‘stalks’ within the orobranchial cavity. (d–v) Each row shows one of the 19 lamprey embryos with DiI-labeled neural crest derivatives near serotonergic putative NECs at the final analysis. The first panel shows the site of DiI injection at one day post-injection (dpi) (missing for embryo Pm19); the second and third panels show DiI-labeled neural crest cells in the branchial arches in right and/or left-side views at 9-dpi; the fourth panel shows a view of the internal gills in section at 13-dpi (for section plane, see dotted line in panel a), showing DiI contribution to gill support cells, but not to the putative NECs (serotonergic cells) in the gill epithelium. Asterisks indicate the embryos from which data are shown in Figure 4 (Pm12 and Pm17). 5-HT, serotonin; En, endostyle; Gs, gill seam; Is, interbranchial septum; Nc, notochord; Obc, orobranchial cavity.
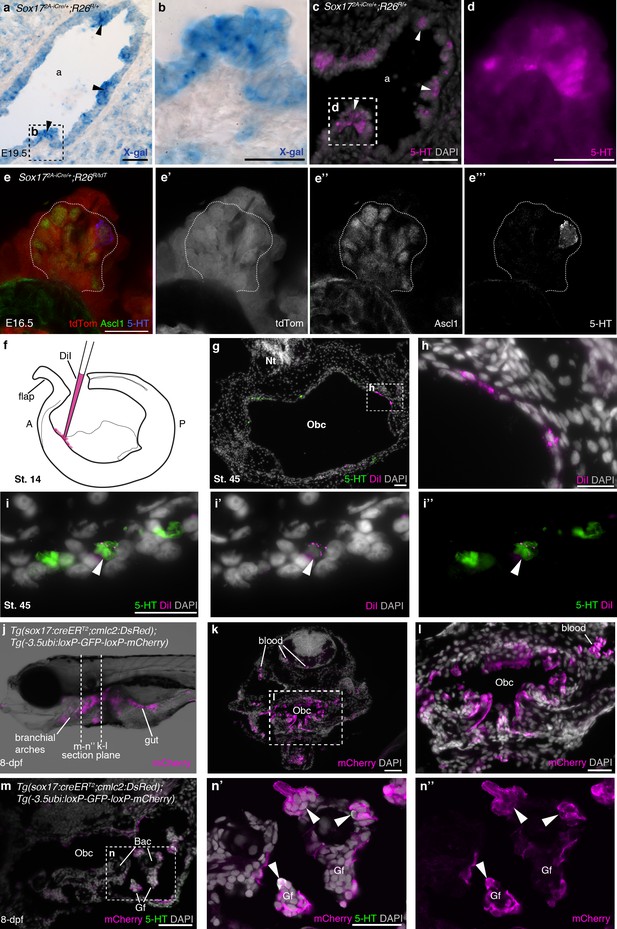
NECs are endoderm-derived, like PNECs.
(a–d) In Sox172A-iCre/+;R26R/+ mice, all endoderm-derived lineages, as well as vascular endothelial cells and the hematopoietic system, constitutively express β-galactosidase (Engert et al., 2009). Serial sections of an E19.5 Sox172A-iCre/+;R26R/+ mouse lung show that X-gal labels PNECs (a,b; black arrowheads), whose identity is confirmed by serotonin expression (c,d; white arrowheads). The serotonin-positive cells are clearly all in the epithelium, which is entirely X-gal-positive, although there is some variation in staining level from cell to cell. (e–e’’’) A high-power view of a cluster of Ascl1-expressing PNECs in a section of an E16.5 Sox172A-iCre/+;R26RtdTomato mouse lung, in which endoderm-derived lineages express tdTomato. Only the occasional PNEC is serotonin-positive at this stage. The Ascl1-expressing PNECs are tdTomato-positive, i.e., endoderm-derived. (f–i’’) An endodermal contribution to putative NECs in Xenopus was investigated by performing focal DiI injections into the anterior endoderm at stage 14 (f), as described in Chalmers and Slack (2000). At stage 45, DiI labels the endoderm lining the orobranchial cavity (g,h), and serotonergic cells (putative NECs, arrowheads) in the orobranchial epithelium (i–i’’). (j–l) In Tg(sox17:creERT2;cmlc2:DsRed);Tg(-3.5ubi:loxP-GFP-loxP-mCherry) zebrafish, the endoderm is labeled with mCherry and (m–n’’) NECs in the gill filaments are mCherry-positive (arrowheads). 5-HT, serotonin; A, anterior; a, airway; Bac, branchial arch cartilage; Gf, gill filament; Obc, orobranchial cavity; P, posterior; tdTom, tdTomato. Scale-bars: 50 μm in a,c,g,k,m; 25 μm in b,d,h,i,l,n; 20 μm in e.
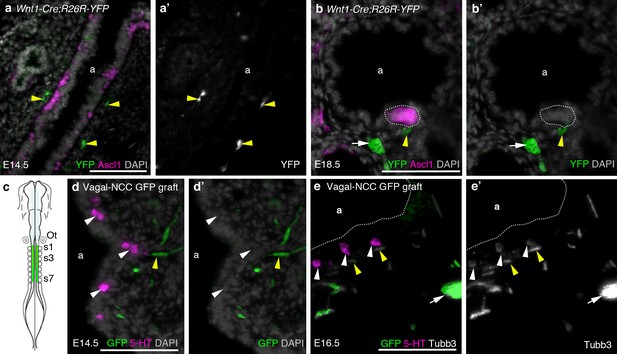
The neural crest does not contribute to amniote PNECs.
(a–b’) Transverse sections through the lungs of Wnt1-Cre;R26R-YFP mouse embryos, in which neural crest cells are permanently labeled with YFP (Danielian et al., 1998; Srinivas et al., 2001), at E14.5 (a,a’) and E18.5 (b,b’). PNECs (Ascl1 [Mash1]-positive cells in the airway epithelium; Ito et al., 2000) are unlabeled, whether solitary or clustered (white dotted line in b,b’), although nearby neural crest-derived cells in the subjacent mesenchyme (yellow arrowheads) are YFP-positive, including putative Schwann cells on a nerve innervating the PNECs (yellow arrowheads in b,b’) and an intrinsic pulmonary ganglion (white arrow in b,b’), as expected (Freem et al., 2010). (In a,a’, the two fainter, out-of-focus green spots within the epithelium are background artefacts from the anti-GFP immunostaining.) (c) The vagal neural crest was labeled in the chicken using GFP-transgenic to wild-type neural tube grafts at E1.5 (schematic modified from Le Douarin, 2004). (d–e’) Transverse sections through the lungs of grafted embryos at E14.5 (d,d’) and E16.5 (e,e’). PNECs (serotonergic cells in the lung airway epithelium) are unlabeled (white arrowheads), while putative Schwann cells (elongated cells, yellow arrowheads) and a nearby intrinsic pulmonary ganglion (white arrow) are GFP-positive, as expected (Burns and Delalande, 2005). 5-HT, serotonin; a, airway; Ot, otic vesicle; s, somite. Scale-bars: 10 μm in a; 50 μm in b,d,e.
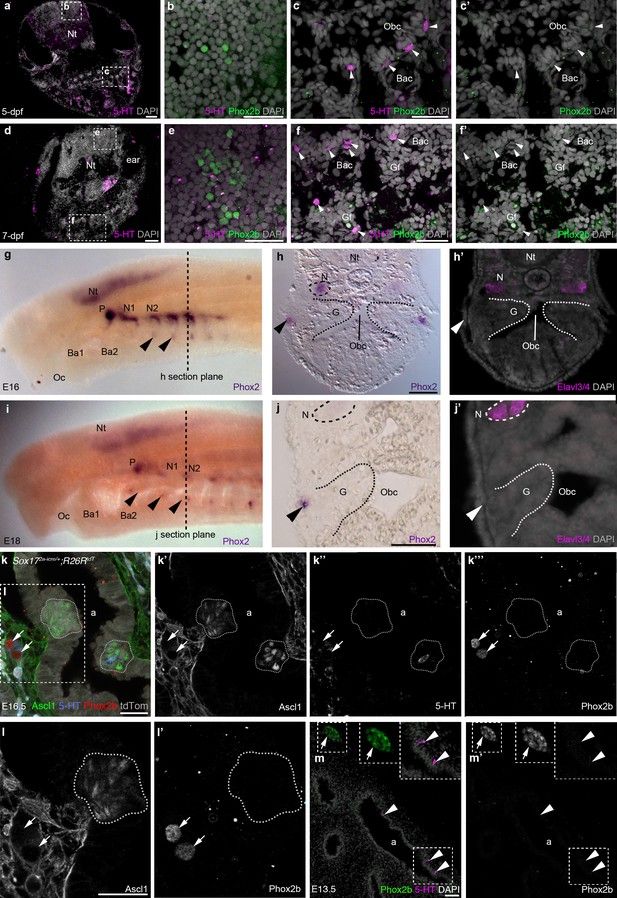
Phox2b expression is absent from gill and lung epithelia.
(a–f’) In wild-type zebrafish at 5- and 7-dpf, Phox2b is expressed by a subset of cells in the hindbrain (b,e), but not by gill NECs or putative NECs in the orobranchial epithelium (arrowheads; c–c’,f–f’). (g–j’) At E16 (g–h’) and E18 (i–j’) in the sea lamprey, Phox2 expression is seen in the neural tube, the epibranchial (petrosal and nodose) ganglia (identified in section by the neuronal marker Elavl3/4), and in patches of ectoderm and subjacent mesenchyme ventral to the epibranchial ganglia (arrowheads). However, Phox2 expression is absent from the gill epithelium, where putative NECS would be located. Dotted lines in panels g and i indicate the section plane in h and j. (k–l’) In a section of an E16.5 Sox172A-iCre/+;R26tdTomatomouse lung, Phox2b expression is seen in intrinsic pulmonary ganglia (arrows), but not in Ascl1/serotonin-positive PNECs located in the lung airway epithelium (dotted lines outline clusters of PNECs). (m,m’) In a section of an E13.5 chicken lung, Phox2b expression is seen in an intrinsic pulmonary ganglion (arrow), but not in serotonin-positive PNECs scattered in the lung airway epithelium (arrowheads). Insets show higher power views. a, airway; Ba, branchial arch; Bac, branchial arch cartilage; G, gill; Gf, gill filament; N, nodose ganglion; Nt, neural tube; Obc, orobranchial cavity; Oc, oral cavity; P, petrosal ganglion. Scale bars: 50 μm in a,d,h,j,m; 25 μm in b,c,e,f,k,l.
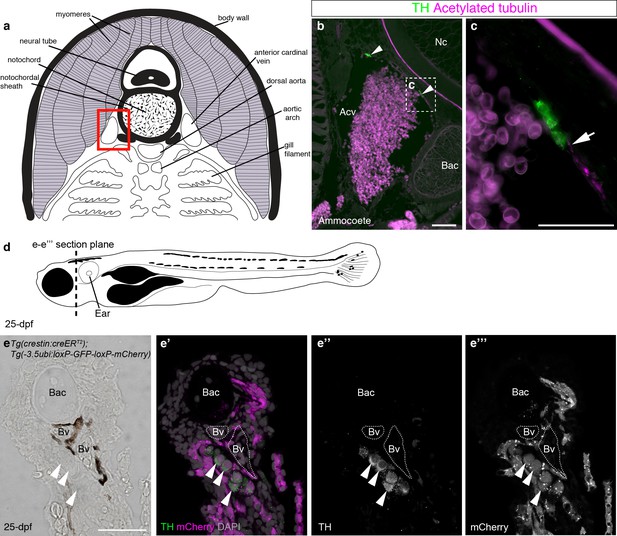
Catecholaminergic cells associated with gill arch blood vessels are neural crest-derived in zebrafish.
(a) Schematic transverse section through ammocoete-stage lamprey gill arch (modified from Ruppert et al., 2003). Red box indicates region shown in b. (b,c) Tyrosine hydroxylase-positive (catecholaminergic) cells are present in the wall of the anterior cardinal vein (arrowheads), closely associated with acetylated tubulin-immunoreactive neurites (arrow). (d) Schematic 25-dpf zebrafish. Dotted line indicates transverse section plane through the gill basket in e-e’’’. (e–e’’’) Tyrosine hydroxylase-positive (catecholaminergic) cells (arrowheads) seen adjacent to melanocyte-covered gill-filament blood vessels (dotted lines), are neural crest-derived (mCherry-positive) in 25-dpf Tg(crestin:creERT2);Tg(-3.5ubi:loxP-GFP-loxP-mCherry) zebrafish. Acv, anterior cardinal vein; Bac, branchial arch cartilage; Bv, blood vessel; Nc, notochord; TH; tyrosine hydroxylase. Scale-bars: 50 μm in b; 25 μm in c,e.
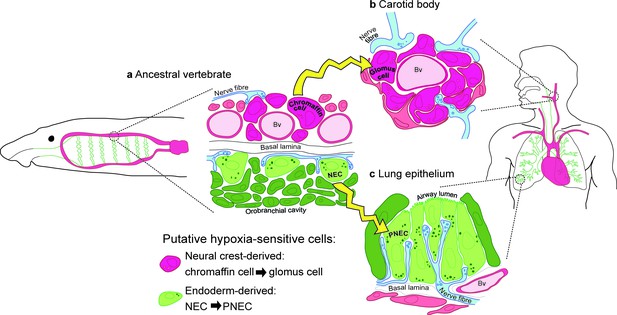
Model for the evolution of the hypoxia-sensitive cells involved in amniote respiratory reflexes.
(a) Schematic ancestral vertebrate with internal gills. Neural crest-derived (magenta) chromaffin cells are associated with large blood vessels in the pharyngeal arches, while NECs differentiate within the endoderm-derived (green) epithelium lining the gills and orobranchial cavity. (b) During the transition to terrestrial life, the glomus cells of the carotid body evolved via the aggregation of neural crest-derived chromaffin cells (which must also have acquired serotonergic properties), while (c) NECs persisted as PNECs in lung airway epithelia. Yellow arrows indicate shared ancestry. Bv, blood vessel; NEC, neuroepithelial cell; PNEC, pulmonary neuroendocrine cell.
Videos
Putative NECs in the skin of embryonic zebrafish are not neural crest-derived.
Z-stack movie showing a whole-mount view of the eye region of a 3-dpf Tg(crestin:creERT2);Tg(-3.5ubi:loxP-GFP-loxP-mCherry) embryo, immunostained for serotonin and mCherry. Serotonergic cells are scattered in the epidermis over the eye, as reported (Coccimiglio and Jonz, 2012), but none is mCherry-positive, i.e., neural crest-derived. Video 2 shows a higher-power movie. A three-dimensional rendering for the eye is shown in Figure 2—figure supplement 1.
Putative NECs in the skin of embryonic zebrafish are not neural crest-derived.
Z-stack movie at higher powerthan Video 1, showing a whole-mount view of the eye region of a 3-dpfTg(crestin:creERT2);Tg(-3.5ubi:loxP-GFP-loxP-mCherry)embryo, immunostained for serotonin and mCherry. Serotonergic cells (arrowheads) are scattered in the epidermis over the eye, as reported (Coccimiglio and Jonz, 2012), but none is mCherry-positive, i.e., neural crest-derived. A 3-dimensional rendering for the eye is shown in Figure 2—figure supplement 1.





